
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Tanmay Sarkar | + 4623 word(s) | 4623 | 2022-03-01 09:02:48 | | | |
| 2 | Lindsay Dong | + 2 word(s) | 4625 | 2022-03-02 03:00:21 | | |
Video Upload Options
The healing of chronic wound infections, especially cutaneous wounds, involves a complex cascade of events demanding mutual interaction between immunity and other natural host processes. Wound infections are caused by the consortia of microbial species that keep on proliferating and produce various types of virulence factors that cause the development of chronic infections. The mono- or polymicrobial nature of surface wound infections is best characterized by its ability to form biofilm that renders antimicrobial resistance to commonly administered drugs due to poor biofilm matrix permeability. For the treatment of chronic wounds, extensive research is ongoing to explore a variety of nanoplatforms, including metallic and nonmetallic NPs, nanofibers and self-accumulating nanocarriers. As the use of the magnetic nanoparticle (MNP)-entrenched pre-designed hydrogel sheet (MPS) is found to enhance wound healing, the bio-nanocomposites consisting of bacterial cellulose and magnetic nanoparticles (magnetite) are now successfully used for the healing of chronic wounds. With the objective of precise targeting, some kinds of “intelligent” nanoparticles are constructed to react according to the required environment, which are later incorporated in the dressings, so that the wound can be treated with nano-impregnated dressing material in situ. For the effective healing of skin wounds, high-expressing, transiently modified stem cells, controlled by nano 3D architectures, have been developed to encourage angiogenesis and tissue regeneration. In order to overcome the challenge of time and dose constraints during drug administration, the approach of combinatorial nano therapy is adopted, whereby AI will help to exploit the full potential of nanomedicine to treat chronic wounds.
1. Introduction
2. Wound-Healing Paradigm
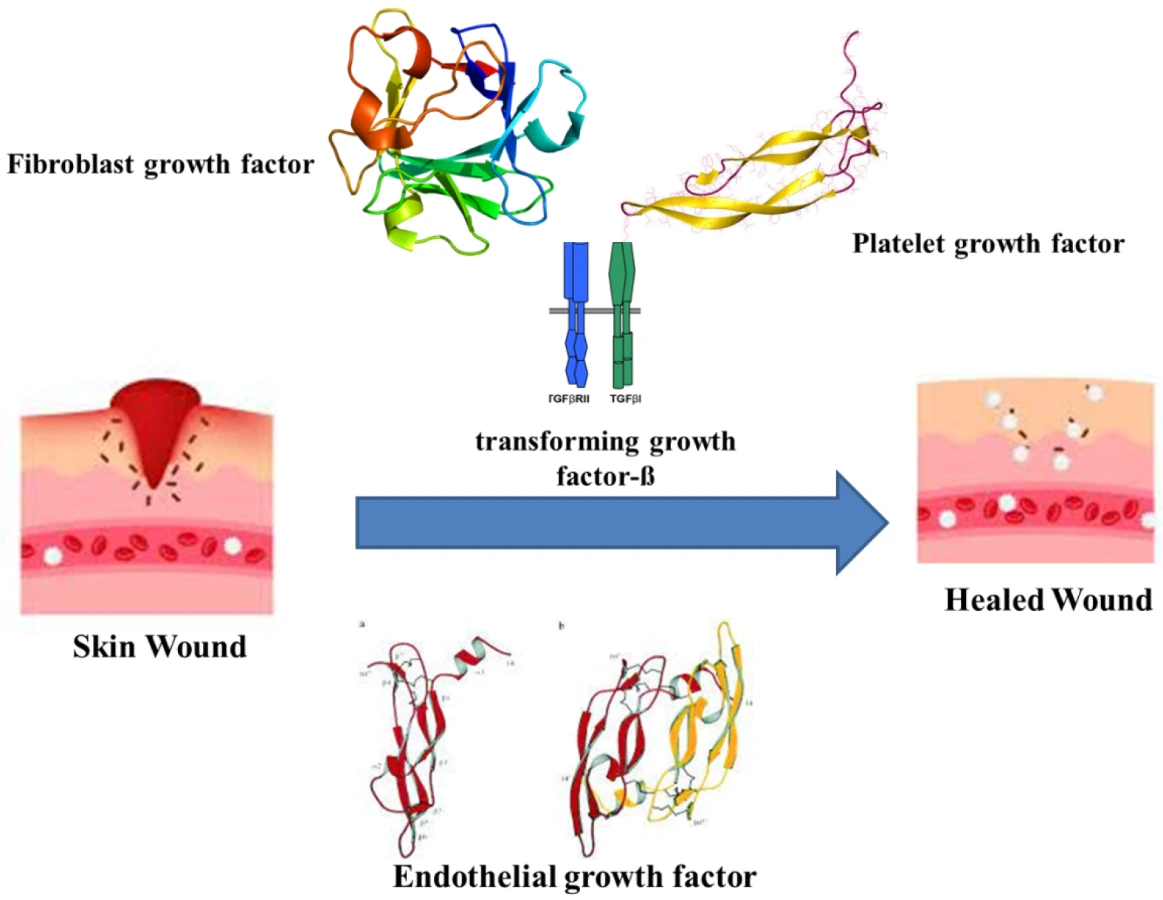 Figure 1. Mechanism of wound healing in the presence of various factors.
Figure 1. Mechanism of wound healing in the presence of various factors.3. Impact of Biofilms on Wounds
4. Nanoparticles as Antimicrobials
5. Nanoparticles in Chronic Wound Treatment
6. Nanoplatforms against Wound Biofilms
6.1. Organic Nanoplatforms
6.1.1. Nanoemulsions
6.1.2. Biopolymer NPs
6.1.3. Synthetic Polymer NPs
6.2. Inorganic Nanoplatforms
6.2.1. Metal NPs
6.2.2. Non-Metallic
7. Nanotechnology in Regeneration
7.1. Intrinsic Regenerative Properties
7.2. Transdermal Nanocarriers
7.3. Nano Scaffold Tissue Engineering
7.4. Nanotopography in Prevention of Biofilm
8. Intelligent Nanotechnology
8.1. Wound Healing
8.1.1. Physically Responsive Nanomaterials
8.1.2. Chemically Responsive Nanomaterials
8.1.3. Bio-Responsive Nanomaterial
8.2. Anti-Microbial Wound Care
9. Advanced Nanotechnology
9.1. Wireless Monitoring
9.2. Artificial Intelligence
References
- Balas, M.; Popescu Din, I.M.; Hermenean, A.; Cinteza, L.O.; Dinischiotu, A. Exposure to Iron Oxide Nanoparticles Coated with Phospholipid-Based Polymeric Micelles Induces Renal Transitory Biochemical and Histopathological Changes in Mice. Materials 2021, 14, 2605.
- Hudita, A.; Radu, I.C.; Galateanu, B.; Ginghina, O.; Herman, H.; Balta, C.; Rosu, M.; Zaharia, C.; Costache, M.; Tanasa, E.; et al. Bioinspired silk fibroin nano-delivery systems protect against 5-FU induced gastrointestinal mucositis in a mouse model and display antitumor effects on HT-29 colorectal cancer cells in vitro. Nanotoxicology 2021, 15, 973–994.
- Vicas, S.I.; Laslo, V.; Timar, A.V.; Balta, C.; Herman, H.; Ciceu, A.; Gharbia, S.; Rosu, M.; Mladin, B.; Fritea, L.; et al. Functional Food Product Based on Nanoselenium-Enriched Lactobacillus casei against Cadmium Kidney Toxicity. Appl. Sci. 2021, 11, 4220.
- Gurdasani, K.; Li, L.; Rafter, M.A.; Daglish, G.J.; Walter, G.H. Nanoparticles as potential external markers for mark–release–recapture studies on Tribolium castaneum. Entomol. Exp. Appl. 2021, 169, 575–581.
- Cao, Z.; Li, B.; Sun, L.; Li, L.; Xu, Z.P.; Gu, Z. 2D Layered Double Hydroxide Nanoparticles: Recent Progress toward Preclinical/Clinical Nanomedicine. Small Methods 2020, 4, 1900343.
- Malekkhaiat Häffner, S.; Nyström, L.; Strömstedt, A.A.; Li, L.; van der Plas, M.J.A.; Malmsten, M. Nanoclay-induced bacterial flocculation for infection confinement. J. Colloid Interface Sci. 2020, 562, 71–80.
- Bharadwaj, K.K.; Rabha, B.; Pati, S.; Choudhury, B.K.; Sarkar, T.; Gogoi, S.K.; Kakati, N.; Baishya, D.; Kari, Z.A.; Edinur, H.A. Green Synthesis of Silver Nanoparticles Using Diospyros malabarica Fruit Extract and Assessments of Their Antimicrobial, Anticancer and Catalytic Reduction of 4-Nitrophenol (4-NP). Nanomater. 2021, 11, 1999.
- Sood, A.; Granick, M.S.; Tomaselli, N.L. Wound Dressings and Comparative Effectiveness Data. Adv. Wound Care 2014, 3, 511–529.
- Proksch, E.; Brandner, J.M.; Jensen, J.-M. The skin: An indispensable barrier. Exp. Dermatol. 2008, 17, 1063–1072.
- Madison, K.C. Barrier function of the skin: “la raison d’être” of the epidermis. J. Investig. Dermatol. 2003, 121, 231–241.
- Nethi, S.K.; Das, S.; Patra, C.R.; Mukherjee, S. Recent advances in inorganic nanomaterials for wound-healing applications. Biomater. Sci. 2019, 7, 2652–2674.
- Crovetti, G.; Martinelli, G.; Issi, M.; Barone, M.; Guizzardi, M.; Campanati, B.; Moroni, M.; Carabelli, A. Platelet gel for healing cutaneous chronic wounds. Transfus. Apher. Sci. Off. J. World Apher. Assoc. Off. J. Eur. Soc. Haemapheresis 2004, 30, 145–151.
- Lazarus, G.S.; Cooper, D.M.; Knighton, D.R.; Margolis, D.J.; Pecoraro, R.E.; Rodeheaver, G.; Robson, M.C. Definitions and guidelines for assessment of wounds and evaluation of healing. Arch. Dermatol. 1994, 130, 489–493.
- Sen, C.K. Human Wounds and Its Burden: An Updated Compendium of Estimates. Adv. wound care 2019, 8, 39–48.
- Khorasani, M.T.; Joorabloo, A.; Moghaddam, A.; Shamsi, H.; MansooriMoghadam, Z. Incorporation of ZnO nanoparticles into heparinised polyvinyl alcohol/chitosan hydrogels for wound dressing application. Int. J. Biol. Macromol. 2018, 114, 1203–1215.
- Das, U.; Behera, S.S.; Singh, S.; Rizvi, S.I.; Singh, A.K. Progress in the Development and Applicability of Potential Medicinal Plant Extract-Conjugated Polymeric Constructs for Wound Healing and Tissue Regeneration. Phytother. Res. 2016, 30, 1895–1904.
- Attinger, C.; Wolcott, R. Clinically Addressing Biofilm in Chronic Wounds. Adv. Wound Care 2012, 1, 127–132.
- Pastar, I.; Stojadinovic, O.; Yin, N.C.; Ramirez, H.; Nusbaum, A.G.; Sawaya, A.; Patel, S.B.; Khalid, L.; Isseroff, R.R.; Tomic-Canic, M. Epithelialization in Wound Healing: A Comprehensive Review. Adv. Wound Care 2014, 3, 445–464.
- Kirker, K.R.; Secor, P.R.; James, G.A.; Fleckman, P.; Olerud, J.E.; Stewart, P.S. Loss of viability and induction of apoptosis in human keratinocytes exposed to Staphylococcus aureus biofilms in vitro. Wound Repair Regen. 2009, 17, 690–699.
- Leung, K.P.; D’Arpa, P.; Seth, A.K.; Geringer, M.R.; Jett, M.; Xu, W.; Hong, S.J.; Galiano, R.D.; Chen, T.; Mustoe, T.A. Dermal wound transcriptomic responses to Infection with Pseudomonas aeruginosa versus Klebsiella pneumoniae in a rabbit ear wound model. BMC Clin. Pathol. 2014, 14, 20.
- Kirker, K.R.; James, G.A. In vitro studies evaluating the effects of biofilms on wound-healing cells: A review. APMIS 2017, 125, 344–352.
- Fazli, M.; Bjarnsholt, T.; Kirketerp-Møller, K.; Jørgensen, B.; Andersen, A.S.; Krogfelt, K.A.; Givskov, M.; Tolker-Nielsen, T. Nonrandom distribution of Pseudomonas aeruginosa and Staphylococcus aureus in chronic wounds. J. Clin. Microbiol. 2009, 47, 4084–4089.
- Kalashnikova, I.; Das, S.; Seal, S. Nanomaterials for wound healing: Scope and advancement. Nanomedicine 2015, 10, 2593–2612.
- Patil, S.; Chandrasekaran, R. Biogenic nanoparticles: A comprehensive perspective in synthesis, characterization, application and its challenges. J. Genet. Eng. Biotechnol. 2020, 18, 67.
- Martin, P. Wound healing--aiming for perfect skin regeneration. Science 1997, 276, 75–81.
- Stadelmann, W.K.; Digenis, A.G.; Tobin, G.R. Physiology and healing dynamics of chronic cutaneous wounds. Am. J. Surg. 1998, 176, 26S–38S.
- Greenhalgh, D.G. The Role of Growth Factors in Wound Healing. J. Trauma Acute Care Surg. 1996, 41, 159–167.
- Adler, A.I.; Boyko, E.J.; Ahroni, J.H.; Smith, D.G. Lower-extremity amputation in diabetes. The independent effects of peripheral vascular disease, sensory neuropathy, and foot ulcers. Diabetes Care 1999, 22, 1029–1035.
- James, G.A.; Swogger, E.; Wolcott, R.; deLancey Pulcini, E.; Secor, P.; Sestrich, J.; Costerton, J.W.; Stewart, P.S. Biofilms in chronic wounds. Wound Repair Regen. 2008, 16, 37–44.
- Kirketerp-Møller, K.; Jensen, P.Ø.; Fazli, M.; Madsen, K.G.; Pedersen, J.; Moser, C.; Tolker-Nielsen, T.; Høiby, N.; Givskov, M.; Bjarnsholt, T. Distribution, organization, and ecology of bacteria in chronic wounds. J. Clin. Microbiol. 2008, 46, 2717–2722.
- Malone, M.; Bjarnsholt, T.; McBain, A.J.; James, G.A.; Stoodley, P.; Leaper, D.; Tachi, M.; Schultz, G.; Swanson, T.; Wolcott, R.D. The prevalence of biofilms in chronic wounds: A systematic review and meta-analysis of published data. J. Wound Care 2017, 26, 20–25.
- Gurjala, A.N.; Geringer, M.R.; Seth, A.K.; Hong, S.J.; Smeltzer, M.S.; Galiano, R.D.; Leung, K.P.; Mustoe, T.A. Development of a novel, highly quantitative in vivo model for the study of biofilm-impaired cutaneous wound healing. Wound Repair Regen. 2011, 19, 400–410.
- Trengove, N.J.; Stacey, M.C.; McGechie, D.F.; Mata, S. Qualitative bacteriology and leg ulcer healing. J. Wound Care 1996, 5, 277–280.
- Davies, C.E.; Hill, K.E.; Newcombe, R.G.; Stephens, P.; Wilson, M.J.; Harding, K.G.; Thomas, D.W. A prospective study of the microbiology of chronic venous leg ulcers to reevaluate the clinical predictive value of tissue biopsies and swabs. Wound Repair Regen. 2007, 15, 17–22.
- Dalton, T.; Dowd, S.E.; Wolcott, R.D.; Sun, Y.; Watters, C.; Griswold, J.A.; Rumbaugh, K.P. An in vivo polymicrobial biofilm wound infection model to study interspecies interactions. PLoS ONE 2011, 6, e27317.
- Woo, K.Y.; Sibbald, R.G. The improvement of wound-associated pain and healing trajectory with a comprehensive foot and leg ulcer care model. J. Wound Ostomy Cont. Nurs. Off. Publ. Wound Ostomy Cont. Nurses Soc. 2009, 36, 183–184.
- Dow, G.; Browne, A.; Sibbald, R.G. Infection in chronic wounds: Controversies in diagnosis and treatment. Ostomy. Wound. Manage. 1999, 45, 29–40.
- Sharma, A.K.; Dhasmana, N.; Dubey, N.; Kumar, N.; Gangwal, A.; Gupta, M.; Singh, Y. Bacterial Virulence Factors: Secreted for Survival. Indian J. Microbiol. 2017, 57, 1–10.
- Kintarak, S.; Nair, S.P.; Speight, P.M.; Whawell, S.A. A recombinant fragment of the fibronectin-binding protein of Staphylococcus aureus inhibits keratinocyte migration. Arch. Dermatol. Res. 2004, 296, 250–257.
- Bjarnsholt, T.; Jensen, P.Ø.; Burmølle, M.; Hentzer, M.; Haagensen, J.A.J.; Hougen, H.P.; Calum, H.; Madsen, K.G.; Moser, C.; Molin, S.; et al. Pseudomonas aeruginosa tolerance to tobramycin, hydrogen peroxide and polymorphonuclear leukocytes is quorum-sensing dependent. Microbiology 2005, 151, 373–383.
- Lee, N.-Y.; Ko, W.-C.; Hsueh, P.-R. Nanoparticles in the Treatment of Infections Caused by Multidrug-Resistant Organisms. Front. Pharmacol. 2019, 10, 1153.
- Mihai, M.M.; Dima, M.B.; Dima, B.; Holban, A.M. Nanomaterials for Wound Healing and Infection Control. Materials 2019, 12, 2176.
- Losi, P.; Briganti, E.; Magera, A.; Spiller, D.; Ristori, C.; Battolla, B.; Balderi, M.; Kull, S.; Balbarini, A.; Di Stefano, R.; et al. Tissue response to poly(ether)urethane-polydimethylsiloxane-fibrin composite scaffolds for controlled delivery of pro-angiogenic growth factors. Biomaterials 2010, 31, 5336–5344.
- Bartolucci, C.; Antonacci, A.; Arduini, F.; Moscone, D.; Fraceto, L.; Campos, E.; Attaallah, R.; Amine, A.; Zanardi, C.; Cubillana-Aguilera, L.M.; et al. Green nanomaterials fostering agrifood sustainability. TrAC Trends Anal. Chem. 2020, 125, 115840.
- Hamdan, S.; Pastar, I.; Drakulich, S.; Dikici, E.; Tomic-Canic, M.; Deo, S.; Daunert, S. Nanotechnology-Driven Therapeutic Interventions in Wound Healing: Potential Uses and Applications. ACS Cent. Sci. 2017, 3, 163–175.
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, applications and toxicities. Arab. J. Chem. 2019, 12, 908–931.
- Huh, A.J.; Kwon, Y.J. “Nanoantibiotics”: A new paradigm for treating infectious diseases using nanomaterials in the antibiotics resistant era. J. Control. Release 2011, 156, 128–145.
- Peulen, T.-O.; Wilkinson, K.J. Diffusion of Nanoparticles in a Biofilm. Environ. Sci. Technol. 2011, 45, 3367–3373.
- Slomberg, D.L.; Lu, Y.; Broadnax, A.D.; Hunter, R.A.; Carpenter, A.W.; Schoenfisch, M.H. Role of Size and Shape on Biofilm Eradication for Nitric Oxide-Releasing Silica Nanoparticles. ACS Appl. Mater. Interfaces 2013, 5, 9322–9329.
- Penders, J.; Stolzoff, M.; Hickey, D.J.; Andersson, M.; Webster, T.J. Shape-dependent antibacterial effects of non-cytotoxic gold nanoparticles. Int. J. Nanomedicine 2017, 12, 2457–2468.
- Fraser, J.F.; Cuttle, L.; Kempf, M.; Kimble, R.M. Cytotoxicity of topical antimicrobial agents used in burn wounds in Australasia. ANZ J. Surg. 2004, 74, 139–142.
- Chaby, G.; Viseux, V.; Poulain, J.-F.; De Cagny, B.; Denoeux, J.-P.; Lok, C. Topical silver sulfadiazine-induced acute renal failure. Ann. Dermatol. Venereol. 2005, 132, 891–893.
- Karakoti, A.S.; Hench, L.L.; Seal, S. The potential toxicity of nanomaterials—The role of surfaces. JOM 2006, 58, 77–82.
- Sankar, R.; Baskaran, A.; Shivashangari, K.S.; Ravikumar, V. Inhibition of pathogenic bacterial growth on excision wound by green synthesized copper oxide nanoparticles leads to accelerated wound healing activity in Wistar Albino rats. J. Mater. Sci. Mater. Med. 2015, 26, 214.
- Ismail, N.A.; Razali, M.H.; Amin, K.A.M. Mechanical and physicochemical properties study on gellan gum thin film prepared using film casting method. AIP Conf. Proc. 2017, 1885, 20045.
- Oliveira, J.T.; Martins, L.; Picciochi, R.; Malafaya, P.B.; Sousa, R.A.; Neves, N.M.; Mano, J.F.; Reis, R.L. Gellan gum: A new biomaterial for cartilage tissue engineering applications. J. Biomed. Mater. Res. A 2010, 93, 852–863.
- Iram, N.E.; Khan, M.S.; Jolly, R.; Arshad, M.; Alam, M.; Alam, P.; Khan, R.H.; Firdaus, F. Interaction mode of polycarbazole-titanium dioxide nanocomposite with DNA: Molecular docking simulation and in-vitro antimicrobial study. J. Photochem. Photobiol. B 2015, 153, 20–32.
- Ismail, N.A.; Amin, K.A.M.; Majid, F.A.A.; Razali, M.H. Gellan gum incorporating titanium dioxide nanoparticles biofilm as wound dressing: Physicochemical, mechanical, antibacterial properties and wound healing studies. Mater. Sci. Eng. C 2019, 103, 109770.
- Dowd, S.E.; Wolcott, R.D.; Sun, Y.; McKeehan, T.; Smith, E.; Rhoads, D. Polymicrobial nature of chronic diabetic foot ulcer biofilm infections determined using bacterial tag encoded FLX amplicon pyrosequencing (bTEFAP). PLoS ONE 2008, 3, e3326.
- Bjarnsholt, T.; Kirketerp-Møller, K.; Jensen, P.Ø.; Madsen, K.G.; Phipps, R.; Krogfelt, K.; Høiby, N.; Givskov, M. Why chronic wounds will not heal: A novel hypothesis. Wound Repair Regen. 2008, 16, 2–10.
- Ikuma, K.; Decho, A.W.; Lau, B.L.T. When nanoparticles meet biofilms-interactions guiding the environmental fate and accumulation of nanoparticles. Front. Microbiol. 2015, 6, 591.
- Dryden, M.S. Complicated skin and soft tissue infection. J. Antimicrob. Chemother. 2010, 65 (Suppl. 3), iii35–iii44.
- Galanty, A.; Paśko, P.; Podolak, I. Enantioselective activity of usnic acid: A comprehensive review and future perspectives. Phytochem. Rev. 2019, 18, 527–548.
- Nunes, P.S.; Rabelo, A.S.; Souza, J.C.; Santana, B.V.; da Silva, T.M.; Serafini, M.R.; Dos, P.M.P.; Dos Santos, L.B.; Cardoso, J.C.; Alves, J.C.; et al. Gelatin-based membrane containing usnic acid-loaded liposome improves dermal burn healing in a porcine model. Int. J. Pharm. 2016, 513, 473–482.
- Grumezescu, A.M.; Holban, A.M.; Andronescu, E.; Mogoşanu, G.D.; Vasile, B.S.; Chifiriuc, M.C.; Lazar, V.; Andrei, E.; Constantinescu, A.; Maniu, H. Anionic polymers and 10 nm Fe3O4@UA wound dressings support human faetal stem cells normal development and exhibit great antimicrobial properties. Int. J. Pharm. 2014, 463, 146–154.
- Furneri, P.M.; Fuochi, V.; Pignatello, R. Lipid-based Nanosized Delivery Systems for Fluoroquinolones: A Review. Curr. Pharm. Des. 2017, 23, 6696–6704.
- Allen, T.M.; Cullis, P.R. Liposomal drug delivery systems: From concept to clinical applications. Adv. Drug Deliv. Rev. 2013, 65, 36–48.
- Fenske, D.B.; Cullis, P.R. Liposomal nanomedicines. Expert Opin. Drug Deliv. 2008, 5, 25–44.
- Rommasi, F.; Esfandiari, N. Liposomal Nanomedicine: Applications for Drug Delivery in Cancer Therapy. Nanoscale Res. Lett. 2021, 16, 95.
- Li, C.; Zhang, X.; Huang, X.; Wang, X.; Liao, G.; Chen, Z. Preparation and characterization of flexible nanoliposomes loaded with daptomycin, a novel antibiotic, for topical skin therapy. Int. J. Nanomed. 2013, 8, 1285.
- Khan, F.; Pham, D.T.N.; Oloketuyi, S.F.; Manivasagan, P.; Oh, J.; Kim, Y.M. Chitosan and their derivatives: Antibiofilm drugs against pathogenic bacteria. Colloids Surfaces B Biointerfaces 2020, 185, 110627.
- Kamoun, E.A.; Kenawy, E.R.S.; Chen, X. A review on polymeric hydrogel membranes for wound dressing applications: PVA-based hydrogel dressings. J. Adv. Res. 2017, 8, 217–233.
- Miguel, S.P.; Figueira, D.R.; Simões, D.; Ribeiro, M.P.; Coutinho, P.; Ferreira, P.; Correia, I.J. Electrospun polymeric nanofibres as wound dressings: A review. Colloids Surfaces B Biointerfaces 2018, 169, 60–71.
- Liu, H.; Slamovich, E.B.; Webster, T.J. Less harmful acidic degradation of poly(lactic-co-glycolic acid) bone tissue engineering scaffolds through titania nanoparticle addition. Int. J. Nanomed. 2006, 1, 541.
- Hasan, N.; Cao, J.; Lee, J.; Hlaing, S.P.; Oshi, M.A.; Naeem, M.; Ki, M.H.; Lee, B.L.; Jung, Y.; Yoo, J.W. Bacteria-Targeted Clindamycin Loaded Polymeric Nanoparticles: Effect of Surface Charge on Nanoparticle Adhesion to MRSA, Antibacterial Activity, and Wound Healing. Pharmaceutics 2019, 11, 236.
- Vijayakumar, V.; Samal, S.K.; Mohanty, S.; Nayak, S.K. Recent advancements in biopolymer and metal nanoparticle-based materials in diabetic wound healing management. Int. J. Biol. Macromol. 2019, 122, 137–148.
- Jura, J.; Szmyd, R.; Goralczyk, A.G.; Skalniak, L.; Cierniak, A.; Lipert, B.; Filon, F.L.; Crosera, M.; Borowczyk, J.; Laczna, E.; et al. Effect of silver nanoparticles on human primary keratinocytes. Biol. Chem. 2013, 394, 113–123.
- Zhu, C.; Chen, Z.; Gao, S.; Goh, B.L.; Samsudin, I.B.; Lwe, K.W.; Wu, Y.; Wu, C.; Su, X. Recent advances in non-toxic quantum dots and their biomedical applications. Prog. Nat. Sci. Mater. Int. 2019, 29, 628–640.
- Li, P.; Liu, S.; Yang, X.; Du, S.; Tang, W.; Cao, W.; Zhou, J.; Gong, X.; Xing, X. Low-drug resistance carbon quantum dots decorated injectable self-healing hydrogel with potent antibiofilm property and cutaneous wound healing. Chem. Eng. J. 2021, 403, 126387.
- Darabpour, E.; Doroodmand, M.M.; Halabian, R.; Imani Fooladi, A.A. Sulfur-Functionalized Fullerene Nanoparticle as an Inhibitor and Eliminator Agent on Pseudomonas aeruginosa Biofilm and Expression of toxA Gene. Microb. Drug Resist. 2019, 25, 594–602.
- Lee, I.; Kim, D.; Park, G.-L.; Jeon, T.-J.; Kim, S.M. Investigation of wound healing process guided by nano-scale topographic patterns integrated within a microfluidic system. PLoS ONE 2018, 13, e0201418.
- Yu, Z.; Meng, X.; Zhang, S.; Chen, Y.; Zhang, Z.; Zhang, Y. Recent Progress in Transdermal Nanocarriers and Their Surface Modifications. Molecules 2021, 26, 3093.
- Whittam, A.J.; Maan, Z.N.; Duscher, D.; Wong, V.W.; Barrera, J.A.; Januszyk, M.; Gurtner, G.C. Challenges and Opportunities in Drug Delivery for Wound Healing. Adv. Wound Care 2014, 5, 79–88.
- Carney, D.H.; Mann, R.; Redin, W.R.; Pernia, S.D.; Berry, D.; Heggers, J.P.; Hayward, P.G.; Robson, M.C.; Christie, J.; Annable, C.; et al. Enhancement of incisional wound healing and neovascularization in normal rats by thrombin and synthetic thrombin receptor-activating peptides. J. Clin. Investig. 1992, 89, 1469–1477.
- Glenn, K.C.; Frost, G.H.; Bergmann, J.S.; Carney, D.H. Synthetic peptides bind to high-affinity thrombin receptors and modulate thrombin mitogenesis. Pept. Res. 1988, 1, 65–73.
- Kim, J.; Bae, W.-G.; Kim, Y.J.; Seonwoo, H.; Choung, H.-W.; Jang, K.-J.; Park, S.; Kim, B.H.; Kim, H.-N.; Choi, K.S.; et al. Directional Matrix Nanotopography with Varied Sizes for Engineering Wound Healing. Adv. Healthc. Mater. 2017, 6, 1700297.
- Ojeh, N.; Pastar, I.; Tomic-Canic, M.; Stojadinovic, O. Stem Cells in Skin Regeneration, Wound Healing, and Their Clinical Applications. Int. J. Mol. Sci. 2015, 16, 25476–25501.
- Li, M.; Zhao, Y.; Hao, H.; Han, W.; Fu, X. Mesenchymal stem cell-based therapy for nonhealing wounds: Today and tomorrow. Wound Repair Regen. 2015, 23, 465–482.
- Ma, K.; Liao, S.; He, L.; Lu, J.; Ramakrishna, S.; Chan, C.K. Effects of nanofiber/stem cell composite on wound healing in acute full-thickness skin wounds. Tissue Eng. Part A 2011, 17, 1413–1424.
- Cheng, Y.; Feng, G.; Moraru, C.I. Micro- and Nanotopography Sensitive Bacterial Attachment Mechanisms: A Review. Front. Microbiol. 2019, 10, 191.
- Beltrán-Partida, E.; Valdez-Salas, B.; Curiel-Álvarez, M.; Castillo-Uribe, S.; Escamilla, A.; Nedev, N. Enhanced antifungal activity by disinfected titanium dioxide nanotubes via reduced nano-adhesion bonds. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 76, 59–65.
- Percival, S.L.; McCarty, S.; Hunt, J.A.; Woods, E.J. The effects of pH on wound healing, biofilms, and antimicrobial efficacy. Wound Repair Regen. 2014, 22, 174–186.
- Jones, E.M.; Cochrane, C.A.; Percival, S.L. The Effect of pH on the Extracellular Matrix and Biofilms. Adv. Wound Care 2015, 4, 431–439.
- Galiano, R.D.; Tepper, O.M.; Pelo, C.R.; Bhatt, K.A.; Callaghan, M.; Bastidas, N.; Bunting, S.; Steinmetz, H.G.; Gurtner, G.C. Topical vascular endothelial growth factor accelerates diabetic wound healing through increased angiogenesis and by mobilizing and recruiting bone marrow-derived cells. Am. J. Pathol. 2004, 164, 1935–1947.
- Drinkwater, S.L.; Burnand, K.G.; Ding, R.; Smith, A. Increased but ineffectual angiogenic drive in nonhealing venous leg ulcers. J. Vasc. Surg. 2003, 38, 1106–1112.
- Auf dem Keller, U.; Kümin, A.; Braun, S.; Werner, S. Reactive Oxygen Species and Their Detoxification in Healing Skin Wounds. J. Investig. Dermatol. Symp. Proc. 2006, 11, 106–111.
- Tang, T.; Jiang, H.; Yu, Y.; He, F.; Ji, S.; Liu, Y.; Wang, Z.; Xiao, S.; Tang, C.; Wang, G.-Y.; et al. A new method of wound treatment: Targeted therapy of skin wounds with reactive oxygen species-responsive nanoparticles containing SDF-1α. Int. J. Nanomed. 2015, 10, 6571–6585.
- Bhakdi, S.; Tranum-Jensen, J. Alpha-toxin of Staphylococcus aureus. Microbiol. Rev. 1991, 55, 733–751.
- Wu, Y.; Song, Z.; Wang, H.; Han, H. Endogenous stimulus-powered antibiotic release from nanoreactors for a combination therapy of bacterial infections. Nat. Commun. 2019, 10, 4464.
- Tyner, H.; Patel, R. Hyaluronidase in Clinical Isolates of Propionibacterium acnes. Int. J. Bacteriol. 2015, 2015, 218918.
- Liu, Y.; Lin, A.; Liu, J.; Chen, X.; Zhu, X.; Gong, Y.; Yuan, G.; Chen, L.; Liu, J. Enzyme-Responsive Mesoporous Ruthenium for Combined Chemo-Photothermal Therapy of Drug-Resistant Bacteria. ACS Appl. Mater. Interfaces 2019, 11, 26590–26606.
- Zhou, J.; Yao, D.; Qian, Z.; Hou, S.; Li, L.; Jenkins, A.T.A.; Fan, Y. Bacteria-responsive intelligent wound dressing: Simultaneous In situ detection and inhibition of bacterial infection for accelerated wound healing. Biomaterials 2018, 161, 11–23.
- Thet, N.T.; Alves, D.R.; Bean, J.E.; Booth, S.; Nzakizwanayo, J.; Young, A.E.R.; Jones, B.V.; Jenkins, A.T.A. Prototype Development of the Intelligent Hydrogel Wound Dressing and Its Efficacy in the Detection of Model Pathogenic Wound Biofilms. ACS Appl. Mater. Interfaces 2016, 8, 14909–14919.
- Sun, L.; Jiang, W.; Zhang, H.; Guo, Y.; Chen, W.; Jin, Y.; Chen, H.; Du, K.; Dai, H.; Ji, J.; et al. Photosensitizer-Loaded Multifunctional Chitosan Nanoparticles for Simultaneous in Situ Imaging, Highly Efficient Bacterial Biofilm Eradication, and Tumor Ablation. ACS Appl. Mater. Interfaces 2019, 11, 2302–2316.
- Zarrintaj, P.; Moghaddam, A.S.; Manouchehri, S.; Atoufi, Z.; Amiri, A.; Amirkhani, M.A.; Nilforoushzadeh, M.A.; Saeb, M.R.; Hamblin, M.R.; Mozafari, M. Can regenerative medicine and nanotechnology combine to heal wounds? The search for the ideal wound dressing. Nanomedicine 2017, 12, 2403–2422.
- Yue, K.; Trujillo-de Santiago, G.; Alvarez, M.M.; Tamayol, A.; Annabi, N.; Khademhosseini, A. Synthesis, properties, and biomedical applications of gelatin methacryloyl (GelMA) hydrogels. Biomaterials 2015, 73, 254–271.
- Niu, X.; Mo, Z.; Yang, X.; Sun, M.; Zhao, P.; Li, Z.; Ouyang, M.; Liu, Z.; Gao, H.; Guo, R.; et al. Advances in the use of functional composites of β-cyclodextrin in electrochemical sensors. Microchim. Acta 2018, 185, 328.
- Bezerra, F.M.; Lis, M.J.; Firmino, H.B.; Dias da Silva, J.G.; Curto Valle, R.d.C.S.; Borges Valle, J.A.; Scacchetti, F.A.P.; Tessaro, A.L. The Role of β-Cyclodextrin in the Textile Industry-Review. Molecules 2020, 25, 3624.
- Zhang, Y.; Li, T.; Zhao, C.; Li, J.; Huang, R.; Zhang, Q.; Li, Y.; Li, X. An Integrated Smart Sensor Dressing for Real-Time Wound Microenvironment Monitoring and Promoting Angiogenesis and Wound Healing. Front. Cell Dev. Biol. 2021, 9, 2172.
- Williams, S.; Okolie, C.L.; Deshmukh, J.; Hawco, L.; McNeil, J.; Nganou Assonkeng, A.C.; Bennett, C.; Mkandawire, M. Magnetizing Cellulose Fibers with CoFe2O4 Nanoparticles for Smart Wound Dressing for Healing Monitoring Capability. ACS Appl. Bio Mater. 2019, 2, 5653–5662.
- Safaee, M.M.; Gravely, M.; Roxbury, D. A Wearable Optical Microfibrous Biomaterial with Encapsulated Nanosensors Enables Wireless Monitoring of Oxidative Stress. Adv. Funct. Mater. 2021, 31, 2006254.
- Gravely, M.; Safaee, M.M.; Roxbury, D. Biomolecular Functionalization of a Nanomaterial to Control Stability and Retention within Live Cells. Nano Lett. 2019, 19, 6203–6212.
- O’ Connell, M.J.; Eibergen, E.E.; Doorn, S.K. Chiral selectivity in the charge-transfer bleaching of single-walled carbon-nanotube spectra. Nat. Mater. 2005, 4, 412–418.
- Ho, D.; Wang, P.; Kee, T. Artificial intelligence in nanomedicine. Nanoscale Horizons 2019, 4, 365–377.
- Ho, D.; Teo, G. Digital Medicine–The New Frontier for AI in Healthcare. Adv. Ther. 2020, 3, 2000015.
- Secinaro, S.; Calandra, D.; Secinaro, A.; Muthurangu, V.; Biancone, P. The role of artificial intelligence in healthcare: A structured literature review. BMC Med. Inform. Decis. Mak. 2021, 21, 125.
- Tharsanee, R.M.; Soundariya, R.S.; Kumar, A.S.; Karthiga, M.; Sountharrajan, S. Deep convolutional neural network–based image classification for COVID-19 diagnosis. In Data science for COVID-19; Kose, U., Gupta, D., de Albuquerque, V.H.C., Khanna, A., Eds.; Academic Press: Cambridge, MA, USA, 2021; pp. 117–145. ISBN 978-0-12-824536-1.
- Dimauro, G.; Deperte, F.; Maglietta, R.; Bove, M.; La Gioia, F.; Renò, V.; Simone, L.; Gelardi, M. A Novel Approach for Biofilm Detection Based on a Convolutional Neural Network. Electronics 2020, 9, 881.
- Stone, B.; Sapper, E. Machine Learning for the Design and Development of 2 Biofilm Regulators 3. 2018. Available online: https://doi.org/10.20944/preprints201803.0118.v1 (accessed on 6 December 2021).
- Howell, R.S.; Liu, H.H.; Khan, A.A.; Woods, J.S.; Lin, L.J.; Saxena, M.; Saxena, H.; Castellano, M.; Petrone, P.; Slone, E.; et al. Development of a Method for Clinical Evaluation of Artificial Intelligence-Based Digital Wound Assessment Tools. JAMA Netw. Open 2021, 4, e217234.
- Daneshkhah, A.; Siegel, A.P.; Agarwal, M. Volatile organic compounds: Potential biomarkers for improved diagnosis and monitoring of diabetic wounds. In Wound healing, tissue repairing, and regeneration in diabetes; Bagchi, D., Das, A., Roy, S., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 491–512. ISBN 978-0-12-816413-6.
- Wang, B.; Cancilla, J.C.; Torrecilla, J.S.; Haick, H. Artificial sensing intelligence with silicon nanowires for ultraselective detection in the gas phase. Nano Lett. 2014, 14, 933–938.




