The healing of chronic wound infections, especially cutaneous wounds, involves a complex cascade of events demanding mutual interaction between immunity and other natural host processes. Wound infections are caused by the consortia of microbial species that keep on proliferating and produce various types of virulence factors that cause the development of chronic infections. The mono- or polymicrobial nature of surface wound infections is best characterized by its ability to form biofilm that renders antimicrobial resistance to commonly administered drugs due to poor biofilm matrix permeability. For the treatment of chronic wounds, extensive research is ongoing to explore a variety of nanoplatforms, including metallic and nonmetallic NPs, nanofibers and self-accumulating nanocarriers. As the use of the magnetic nanoparticle (MNP)-entrenched pre-designed hydrogel sheet (MPS) is found to enhance wound healing, the bio-nanocomposites consisting of bacterial cellulose and magnetic nanoparticles (magnetite) are now successfully used for the healing of chronic wounds. With the objective of precise targeting, some kinds of “intelligent” nanoparticles are constructed to react according to the required environment, which are later incorporated in the dressings, so that the wound can be treated with nano-impregnated dressing material in situ. For the effective healing of skin wounds, high-expressing, transiently modified stem cells, controlled by nano 3D architectures, have been developed to encourage angiogenesis and tissue regeneration. In order to overcome the challenge of time and dose constraints during drug administration, the approach of combinatorial nano therapy is adopted, whereby AI will help to exploit the full potential of nanomedicine to treat chronic wounds.
- nanocomposite
- nanoparticle
- artificial intelligence
- chronic wound
- biofilm
1. Introduction
2. Wound-Healing Paradigm
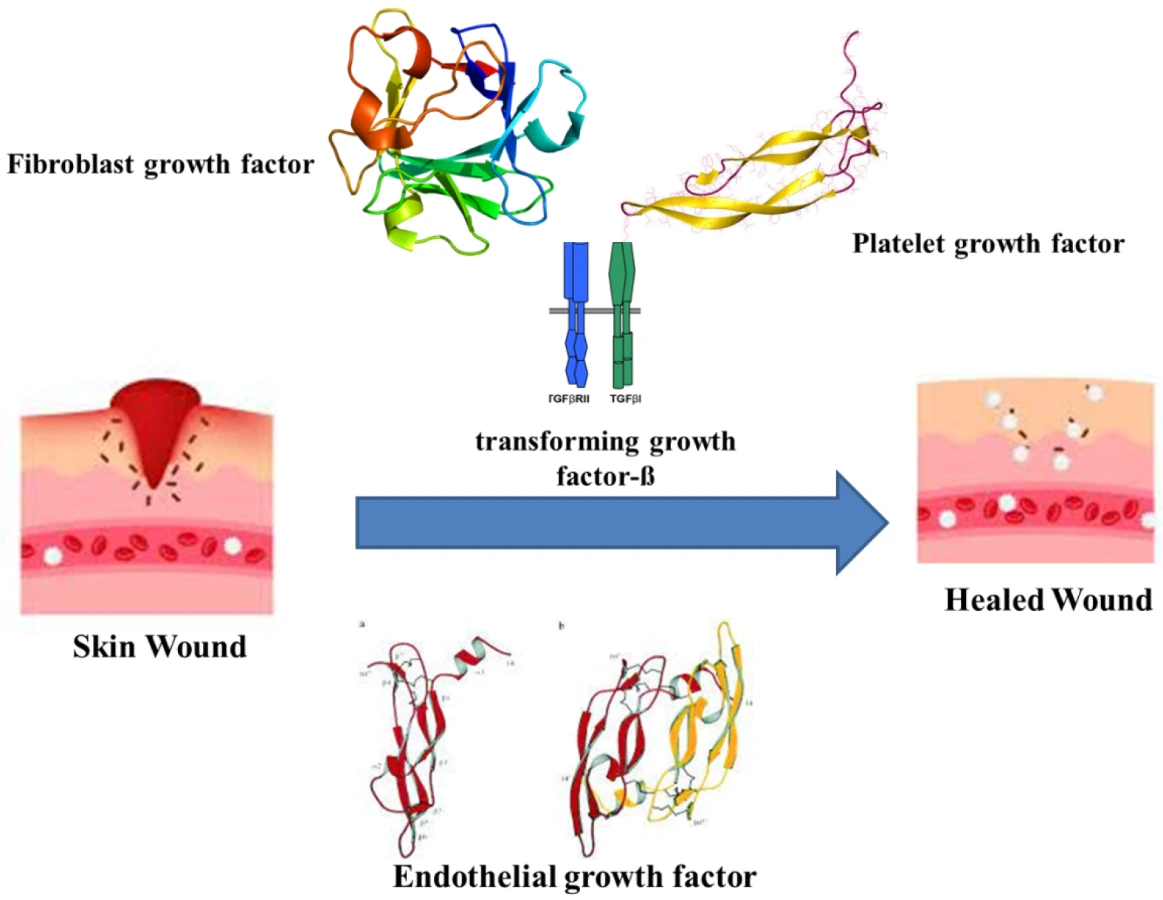
The healing of wounds combines the indulgence of various intricate factors and cell types, connective tissue, cytokines and the vascular system, along with growth and coagulation factors. As wound healing recasts the skin, it undergoes orderly meticulous phases. The major phases are hemostasis, inflammatory, proliferative and maturation [24][28]. Haemostasis occurs immediately after an injury by forming a blood clot to restrict bleeding. The clotting is assisted by thrombin activation, which in turn activates intravascular platelets [25][29]. The platelets eventually release growth factors, cytokines and vasoactive substances. The releasates, such as the fibroblast growth factor (FGF), platelet-derived growth factor (PDGF), platelet-derived angiogenesis factor, transforming growth factor-ß (TGF-ß), endothelial growth factor (EGF), bradykinin, thromboxane A2, platelet factor IV, platelet-activating factor, histamine, serotonin and prostaglandins, initiate the early wound-healing processes (Figure 1) [26][27][30,31].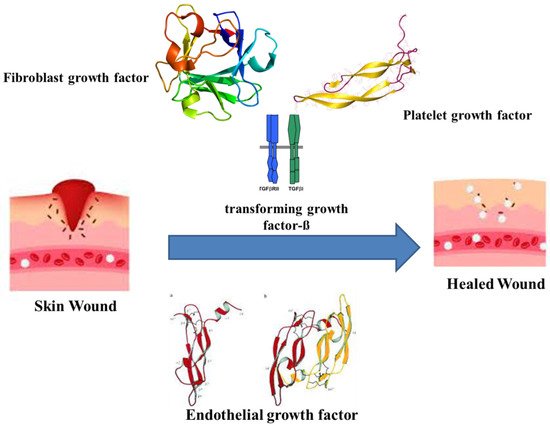
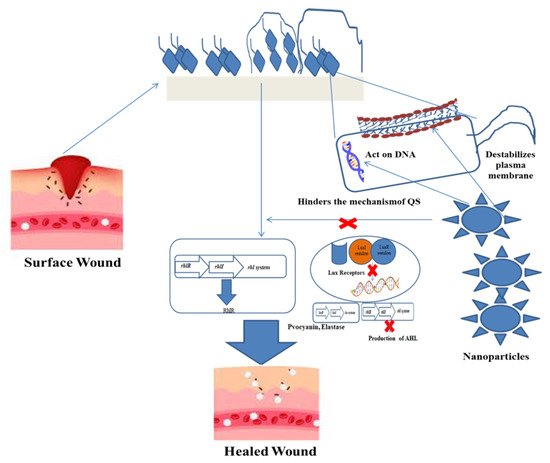
3. Impact of Biofilms on Wounds
3. Impact of Biofilms on Wounds
4. Nanoparticles as Antimicrobials
Nanoparticles (NP) are observed to be efficient employees for escaping AMR [41][92], in addition to delivering antimicrobial agents [42][93] and growth promoters [43][94]. The generation of cost-effective biocompatible and biodegradable NPs and the route of biogenic synthesis is being encouraged [44][95]. The only setback of NPs is the compromised efficiency caused by the pre-mature or unintended release of drugs and promoters. Hence, the cultivation of localized response-inclusive strategies is necessary to attend this challenge. Engineering NPs with an inherent capability to activate in relation to their microenvironment or on being triggered by an external stimulus has surfaced as a remedy. Other nanoscale establishments also supplement benefits to wound reformation and biofilm extirpation. The undertone of nanomaterial-assisted wound therapy usually entails one of the two routes, one that possesses intrinsic properties that aid in wound closure and the other being delivery vehicles for therapeutic agents [45][96]. As described earlier, wound closure is at the mercy of microbial incompetence at the site. Hence, overviewing the key strategies of nanotechnology to obstruct microbial growth and encourage wound healing is imperative. Typically, NPs are <100 nm in size [46][97]. This size renders them novel properties due to a large surface area–mass ratio [47][98]. Researchers have reported the rapid diffusion of smaller NPs through the pores of P. fluorescens biofilm, further stating an exponential decrease in the relative self-diffusion coefficients with an increase in the square of the radius of the nanoparticle [48][99]. Better prevention of biofilms is achieved with shapes delivering a higher surface area-to-mass ratio. For example; rod-like NPs destruct biofilms more effectively than spherical NPs [49][100]. Researchers have compared gold NP with different shapes to check their antibiofilm activity against S. aureus. The gold nano-stars and nanoflowers had higher antimicrobial activity than gold nanospheres. The authors attributed the difference to increased surface area, which generated a greater probability of interactions with the bacterial cell and biofilm components [50][101]. The mere availability of an alternative antimicrobial is not enough to consider against available agents. Silver sulfadiazine is a standard antimicrobial for burn wounds. However, this topical agent can cause adverse effects, such as leucopenia, argyria, renal and hepatic toxicity, making this unfit for long-term applications [51][52][102,103]. The major advantage of NPs against classical treatments is the ability to generate superior antimicrobial as well as healing properties. Another route adopted by nanoparticles is to trigger the production of reactive oxygen species (ROS), succeeded by the phospholipid oxidation damaging the cell wall integrity before finally collapsing the internal nucleic acids and/or proteins, thereby staging a bactericidal impact [53][109]. Considering the ease of cost-effectiveness, nanoparticles can also be synthesized via the eco-friendly route. The topical application of green synthesized copper oxide NPs revealed antibacterial activity against S. dysenteriae, K. pneumoniae, S. aureus, S. typhimurium and E. coli through accelerating wound healing among Wistar Albino rats [54][119], thereby illustrating the antimicrobial effectivity of metallic and non-metallic nano topical agents. Another mode of tackling injuries is by the usage of bio-films that keep the toxicity in check. Investigation of the titanium oxide NP seeded in the gallium gum as a wound film dressing projected a versatile antimicrobial and healing potency. The biopolymer matrix of Gallium gum enacts as a suitable structure for skin regeneration, as it enhances cell proliferation and viability [55][56][120,121]. Meanwhile, the nano-titanium dioxide has an affinity for DNA. This was indicated by molecular docking that led to the proposition of TiO2 NPs binding to the G–C bonds of the DNA, creating hindrance in the bacterial multiplication [57][122]. The bio-film dressing was effective against S. aureus and E. coli, with no cytotoxicity on mouse fibroblast cells [58][123]. The in vivo and in vitro evidence declares nanotechnology to have a diversity of antimicrobial weaponry against wound biofilm remediation.5. Nanoparticles in Chronic Wound Treatment
Cutaneous wound management grants biofilm formation as a major ingredient contributing to the chronicity of wounds [29][59][60][57,124,125]. Initially, the interaction of nanoparticles with the biofilm surface occurs at a bulk phase. It interacts with the lipids, lipopolysaccharides (LPS) or cell membrane proteins of the bacteria. The maturity, surface composition and chemistry of the biofilm are primary quotients governing the penetration of the NP. Further, the entry within the biofilm matrix is also dependent upon the physical and chemical characteristics of the NP, such as size, shape, charge, concentration, hydrophobicity and surface chemistry [61][126]. Clinical isolates of S. aureus, P. aeruginosa, S. epidermidis and Enterococcus spp. are the most predominant skin wound infections [62][127]. Thus, nanotechnology has to eradicate multiple species of bacteria at the same time and space. This can be achieved by modulating the wound microenvironment by inducing biophysical and biochemical alterations that can aid the removal of biofilms. For instance, the use of usnic acid derived from lichens is a bioactive compound with numerous biological activities, such as antioxidant, anti-inflammatory, wound healing and antimicrobial properties [63][128]. Clinical evidence has demonstrated accelerated healing and higher collagen deposition in porcine burn wounds by the application of usnic acid/liposomes based on gelatin membrane compared to silver sulfadiazine ointment and duoDerme® dressings [64][129]. Apart from addressing wound recovery, usnic acid demonstrates both a antibiofilm nature and the biocompatibility of nanocomposites. The study used anionic polymer dressings of carboximethylcellulose (CMC) or sodium alginate (AlgNa) with usnic acid-loaded magnetic NPs (Fe3O4@UA) to eradicate S. aureus biofilms. However, the exact mechanism of action by usinc acid on the biofilm is not clear. Further, they demonstrated biocompatibility against human endothelial cell lines and foetal progenitor cells to suggest the tissue regeneration capacity of the dressing [65][130].6. Nanoplatforms against Wound Biofilms
The diversity of nanoplatforms delivers antibiofilm activity, according to the physiological condition of the wound site. The nature, depth, state, exudates, comorbidities and healing pace of the wound will suggest the necessary nanoplatform (Figure 2) to be allocated for infection control.