Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Aleksandr Kazachenko | + 1460 word(s) | 1460 | 2022-02-24 10:01:30 | | | |
| 2 | Catherine Yang | -4 word(s) | 1456 | 2022-02-28 03:33:39 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Kazachenko, A. Betulin. Encyclopedia. Available online: https://encyclopedia.pub/entry/19963 (accessed on 08 February 2026).
Kazachenko A. Betulin. Encyclopedia. Available at: https://encyclopedia.pub/entry/19963. Accessed February 08, 2026.
Kazachenko, Aleksandr. "Betulin" Encyclopedia, https://encyclopedia.pub/entry/19963 (accessed February 08, 2026).
Kazachenko, A. (2022, February 28). Betulin. In Encyclopedia. https://encyclopedia.pub/entry/19963
Kazachenko, Aleksandr. "Betulin." Encyclopedia. Web. 28 February, 2022.
Copy Citation
Betulin is an important triterpenoid substance isolated from birch bark, which, together with its sulfates, exhibits important bioactive properties. Using the potentiometric titration method, the product of acidity constants K1 and K2 of a solution of the betulin disulfate H+ form has been found to be 3.86 × 10–6 ± 0.004. It has been demonstrated by the thermal analysis that betulin and the betulin disulfate sodium salt are stable at temperatures of up to 240 and 220 °C, respectively.
betulin
sulfation
catalysis
amberlyst (R)-15
1. Introduction
Plant biomass is an important feedstock for a wide range of valuable chemicals [1][2][3][4][5]. Catalytic processing of plant lignocellulosic biomass is an urgent task [6]. Birch biomass can serve as a source of various extractive substances. Birch bark is characterized by a particularly high content of extractive substances, which include mono- and triterpenoids, hydrocarbons, alcohols, fatty and resin acids, and phenolic compounds [7][8][9]
Triterpene compounds represent the most important class of bioactive substances promising for use as pharmaceutical active ingredients, drugs, and phytopreparations [10][11][12][13][14][15][16][17]. The betulin derivatives hold a special place in a triterpenoid series. A rich source of betulin is the Betulaceae family, especially Betula alba, Betula pubescens, Betula platyphylla, and Betula pendula [12][14][18]. The betulin content in the birch outer bark ranges within 10–35%, depending on a type of birch, an area and conditions of its growth, an age of the tree, and other factors [15][18]. In the in vitro and in vivo experiments, betulin and its derivatives exhibit the anti-inflammatory, anticonvulsant, antibacterial, antiviral, anti-HIV, antitumor, and other types of biological activity [11][12][13][14][15][16][17][19][20][21][22][23].
Owing to its availability and bioactivity, betulin is well-known as a valuable natural substance, both in its native state and in various modifications. Meanwhile, the low solubility of betulin in water limits its application in medicine, cosmetics, etc. To enhance the water solubility of triterpenoids, different methods are used, including the salt formation, the use of special dosage forms, which ensure the vector delivery of poorly soluble compounds, and nano- and biotechnological techniques [13][16][17][19][20][24][25]. The solubility of triterpenoids can be increased via their chemical modification. In particular, the solubility and bioavailability of triterpenoids can be improved by the complexation with γ-cyclodextrin and other compounds that can form inclusion complexes via the hydrophobic binding [13][17][19][20][24][25][26][27]. Sulfation of betulin improves its water solubility. Sulfuric esters of betulin and betulinic acid were shown to be bioactive [27][28].
The triterpenoid sulfation methods proposed previously [28][29] are based on the use of sulfuric acid and complexes resulting from the interaction of sulfuric anhydride with pyridine or dimethyl sulfoxide. Thus, the synthesis of betulin disulfate and betulinic acid 3-sulfate is carried out via sulfation of betulin and betulinic acid with sulfuric acid in pyridine in the presence of acetic anhydride [28].
Sulfation of triterpenoids with chlorosulfonic or sulfamic acid in an environment of weak bases, e.g., dimethylformamide or dioxane, not only can be accompanied by isomerization of initial betulin [23], but the sulfated triterpenoid can be formed initially in a slightly stable H-form [30]. As is known, the sulfur trioxide pyridine complex is a milder sulfating reagent than the SO3–1,4-dioxane complex; it attacks exclusively alcohol groups and does not affect the double bond [31]. The use of pyridine in betulin sulfation instead of 1,4-dioxane and N, N-dimethylformamide excludes the formation of sulfated betulin in a slightly stable H-form [30]; sulfated betulin has a form of a stable salt.
2. Fourier-Transform Infrared Spectroscopy Study
The introduction of the sulfate group into the betulin molecule was confirmed by IR spectroscopy (Figure 1).
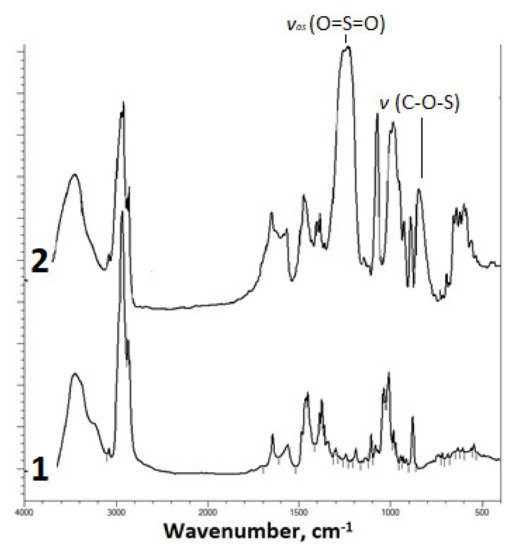
Figure 1. FTIR spectra of (1) betulin and (2) sulfated betulin in the sodium form.
The FTIR spectrum of the betulin disulfate sodium salt contains, along with the absorption bands characteristic of the initial betulin, a high-intensity band of asymmetric stretching vibrations υas (O=S=O) at 1249 cm−1 and an absorption band of stretching vibrations υ (C–O–S) [32][33] in the range of 835–841 cm−1.
3. Ultraviolet—Visible Spectroscopy Study
The initial and sulfated betulin was examined by UV-Vis spectroscopy (Figure 2).
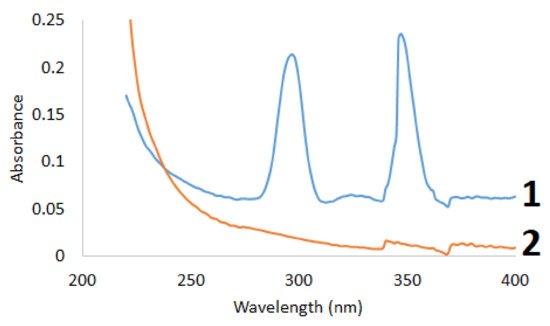
Figure 2. UV spectra of (1) betulin and (2) sulfated betulin.
To qualitatively identify the presence of sulfo groups in the betulin sulfation products, the electronic absorption spectra in the range of 220–400 nm were reproduced in the alcohol solution. It can be seen in Figure 2 that the UV spectra of the initial and sulfated betulin have different profiles, which is indicative of the presence of new functional groups in the sulfated products [34]. A factor indicating the introduction of a sulfo group into the betulin structure is a decrease in the total intensity of the sulfation products. The introduction of an additional functional group with an increase in the average molecular weight leads to a decrease in the intensity in the UV range.
4. Nuclear Magnetic Resonance Study
The composition and structure of the betulin disulfate sodium salt was confirmed by 13C NMR spectroscopy. According to the literature data [35], the chemical shift of the secondary C3 carbon atom bonded to the hydroxyl group is observed at 78–79 ppm and the chemical shift of the primary C28 carbon atom, at 59–60 ppm. An analysis of the 13C NMR spectra of the initial betulin and the betulin disulfate sodium salt showed that the chemical shift of the C3 carbon atom in the initial betulin is observed at 78.25 ppm and the chemical shift of the C28 carbon atom, at 58.96 ppm. In the synthesized betulin disulfate, the chemical shifts of the С3 and С28 carbons in comparison with betulin are completely shifted to the low-field region (to 88.21 and 67.32 ppm, respectively). This proves the complete replacement of the betulin hydroxyl groups by the sulfate.
5. X-Ray Diffraction Study
The initial betulin and betulin sulfate were analyzed by X-ray diffractometry (Figure 3). The initial betulin has a crystalline structure with the high-intensity bands [36]. During sulfation, a decrease in the crystallinity of betulin is observed, as is the case with its chemical modification by other methods [37].
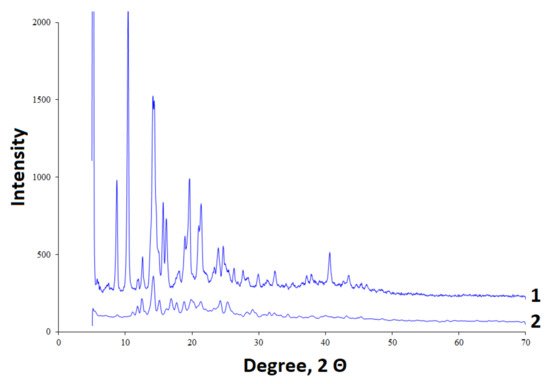
Figure 3. XRD diffraction patterns of (1) betulin and (2) betulin sulfate.
6. Thermal Analysis
Figure 4 shows a thermogram of betulin and the betulin disulfate sodium salt obtained upon heating in the argon atmosphere.
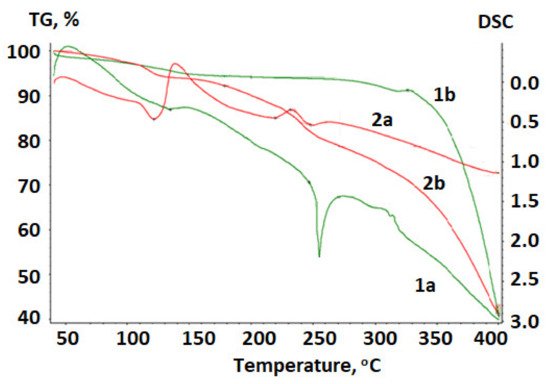
Figure 4. Thermogram of (1) betulin and (2) betulin disulfate sodium salt: (a) DTA and (b) TG curves.
The temperature dependence of the weight loss (the TG curve) for betulin has a plateau; the horizontal section is indicative of the stability of the chemical compound in the investigated temperature range at the absence of chemical transformations. A vertical step in the curve is indicative of the chemical decomposition of the material [38].
At a temperature of 134.5 °C, a loss of water contained in betulin is observed. The weight loss is 5.83%. The TG curve reflects an intense loss in the sample mass above 260 °C.
To determine the transformation temperatures more accurately, a differential notation was used. A peak in the DTA curve in the range of 240–260 °C is indicative of a phase transformation in betulin, which is accompanied by the endothermic effect. This is a first-order phase transition; in this region, betulin melts with the subsequent decomposition.
In contrast to initial betulin, in the betulin disulfate sodium salt the water loss occurs earlier and the weight loss is 7.76%.
In the DTA curve at temperatures of 220–230 °C, the exothermic effect is reflected, which corresponds to the decomposition of the betulin disulfate sodium salt, apparently with the SO2 release. The weight loss is 15.5%, according to the sulfur content in betulin disulfate. The degree of decomposition of betulin disulfate with the SO2 release in this region is 79%, which is consistent with the data reported in [39][40][41]. Betulin and the betulin disulfate sodium salt are stable at temperatures of up to 240 and 220 °C, respectively.
7. Acidity Constants
The product of acidity constants K1 and K2 of the solution of the betulin disulfate H + form was determined by a potentiometric titration (Figure 5). The average product of the first and second dissociation constants K1 and K2 was found to be 3.86 × 10−6 ± 0.004. Figure 5 shows the dependence of pH of the solution of the betulin disulfate H + form on the sodium hydroxide volume.
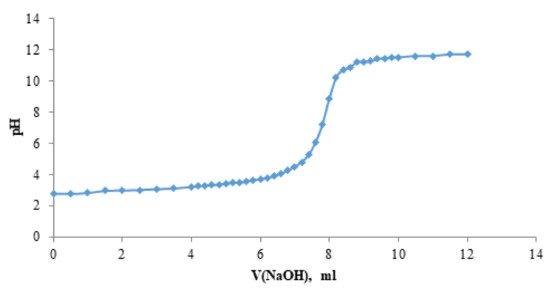
Figure 5. Dependence of pH of the solution of the H + form of betulin disulfate on the sodium hydroxide volume.
Since the titration curve contains only one jump, it is obvious that the H + form of betulin disulfate has similar values of the first and second dissociation constants K1 and K2. This, most likely, originates from the betulin disulfate structure, in which sulfate groups are distant from each other.
This conclusion does not contradict the data on dissociation constants of the known dicarboxylic acids with carboxyl groups significantly distant from each other [42]; therefore, for example, in adipic acid, the K1 and K2 values are of the same order of magnitude: 3.7 × 10−5 and 1.93 × 10−5, respectively.
According to the obtained acidity constant, the H + form of betulin disulfate is an acid stronger than carboxylic acids, but weaker than sulfonic acids. Basing on the determined constant, the H + form of betulin disulfate was confirmed, which can be obtained by adding a mineral acid salt to the aqueous solution.
References
- Kazachenko, A.S.; Miroshnikova, A.V.; Tarabanko, V.E.; Skripnikov, A.M.; Malyar, Y.N.; Borovkova, V.S.; Sychev, V.V.; Taran, O.P. Thermal Conversion of Flax Shives in Sub- and Supercritical Ethanol in the Presence of Ru/C Catalyst. Catalysts 2021, 11, 970.
- Kuznetsov, B.N.; Baryshnikov, S.V.; Miroshnikova, A.V.; Kazachenko, A.S.; Malyar, Y.N.; Skripnikov, A.M.; Taran, O.P. Fractionation of Birch Wood by Integrating Alkaline-Acid Treatments and Hydrogenation in Ethanol over a Bifunctional Ruthenium Catalyst. Catalysts 2021, 11, 1362.
- Kazachenko, A.S.; Tarabanko, V.E.; Miroshnikova, A.V.; Sychev, V.V.; Skripnikov, A.M.; Malyar, Y.N.; Mikhlin, Y.L.; Baryshnikov, S.V.; Taran, O.P. Reductive Catalytic Fractionation of Flax Shive over Ru/C Catalysts. Catalysts 2021, 11, 42.
- Gallezot, P. Conversion of biomass to selected chemical products. Chem. Soc. Rev. 2012, 41, 1538–1558.
- Zhao, X.; Zhou, H.; Sikarwar, V.S.; Zhao, M.; Park, A.-H.A.; Fennell, P.S.; Shen, L.; Fan, L.-S. Biomass-based chemical looping technologies: The good, the bad and the future. Energy Environ. Sci. 2017, 10, 1885–1910.
- Borovkova, V.S.; Malyar, Y.N.; Sudakova, I.G.; Chudina, A.I.; Skripnikov, A.M.; Fetisova, O.Y.; Kazachenko, A.S.; Miroshnikova, A.V.; Zimonin, D.V.; Ionin, V.A.; et al. Molecular Characteristics and Antioxidant Activity of Spruce (Picea abies) Hemicelluloses Isolated by Catalytic Oxidative Delignification. Molecules 2022, 27, 266.
- Lachowicz, H.; Wróblewska, H.; Sajdak, M.; Komorowicz, M.; Wojtan, R. The chemical composition of silver birch (Betula pendula Roth.) wood in Poland depending on forest stand location and forest habitat type. Cellulose 2019, 26, 3047–3067.
- Mononen, K.; Jääskeläinen, A.-S.; Alvila, L.; Pakkanen, T.T.; Vuorinen, T. Chemical changes in silver birch (Betula pendula Roth) wood caused by hydrogen peroxide bleaching and monitored by color measurement (CIELab) and UV-Vis, FTIR and UVRR spectroscopy. Holzforschung 2005, 59, 381–388.
- Hiltunen, E.; Mononen, K.; Alvila, L.; Pakkanen, T.T. Discolouration of birch wood: Analysis of extractives from discoloured surface of vacuum-dried European white birch (Betula pubescens) board. Wood Sci. Technol. 2007, 42, 103.
- Ashour, M.; Wink, M.; Gershenzon, J. Biochemistry of Terpenoids: Monoterpenes, Sesquiterpenes and Diterpenes. In Annual Plant Reviews Volume 40: Biochemistry of Plant Secondary Metabolism; Blackwell Publishing Ltd.: Hoboken, NJ, USA, 2010; pp. 258–303.
- Tolstikova, T.G.; Sorokina, I.V.; Tolstikov, G.A.; Tolstikov, A.G.; Flekhter, O.B. Biological activity and pharmacological prospects of lupane terpenoids: I. natural lupane derivatives. Russ. J. Bioorg. Chem. 2006, 32, 37–49.
- Šiman, P.; Filipová, A.; Tichá, A.; Niang, M.; Bezrouk, A.; Havelek, R. Effective Method of Purification of Betulin from Birch Bark: The Importance of Its Purity for Scientific and Medicinal Use. PLoS ONE 2016, 11, e0154933.
- Lou, H.; Li, H.; Zhang, S.; Lu, H.; Chen, Q. A Review on Preparation of Betulinic Acid and Its Biological Activities. Molecules 2021, 26, 5583.
- Hordyjewska, A.; Ostapiuk, A.; Horecka, A.; Kurzepa, J. Betulin and betulinic acid: Triterpenoids derivatives with a powerful biological potential. Phytochem. Rev. 2019, 18, 929–951.
- Svetlana, A.K.; Tatyana, P.S.; Mikhail, A.M.; Yury, N.M.; Anna, S.K.; Valeri, A.D.; Boris, N.K. Preparation and Antitumor Activity of Betulin Dipropionate and its Composites: A minireview. Biointerface Res. Appl. Chem. 2022, 12, 6873–6894.
- Alakurtti, S.; Mäkelä, T.; Koskimies, S.; Yli-Kauhaluoma, J. Pharmacological properties of the ubiquitous natural product betulin. Eur. J. Pharm. Sci. 2006, 29, 1–13.
- Amiri, S.; Dastghaib, S.; Ahmadi, M.; Mehrbod, P.; Khadem, F.; Behrouj, H.; Aghanoori, M.-R.; Machaj, F.; Ghamsari, M.; Rosik, J.; et al. Betulin and its derivatives as novel compounds with different pharmacological effects. Biotechnol. Adv. 2020, 38, 107409.
- Ressmann, A.K.; Strassl, K.; Gaertner, P.; Zhao, B.; Greiner, L.; Bica, K. New aspects for biomass processing with ionic liquids: Towards the isolation of pharmaceutically active betulin. Green Chem. 2012, 14, 940–944.
- Myszka, H.; Grzywacz, D.; Zdrowowicz, M.; Spisz, P.; Butowska, K.; Rak, J.; Piosik, J.; Jaśkiewicz, M.; Kamysz, W.; Liberek, B. Design, synthesis and biological evaluation of betulin-3-yl 2-amino-2-deoxy-β-D-glycopyranosides. Bioorg. Chem. 2020, 96, 103568.
- Król, S.K.; Kiełbus, M.; Rivero-Müller, A.; Stepulak, A. Comprehensive Review on Betulin as a Potent Anticancer Agent. BioMed Res. Int. 2015, 2015, 584189.
- Heidary Navid, M.; Laszczyk-Lauer, M.N.; Reichling, J.; Schnitzler, P. Pentacyclic triterpenes in birch bark extract inhibit early step of herpes simplex virus type 1 replication. Phytomedicine 2014, 21, 1273–1280.
- Shikov, A.N.; Djachuk, G.I.; Sergeev, D.V.; Pozharitskaya, O.N.; Esaulenko, E.V.; Kosman, V.M.; Makarov, V.G. Birch bark extract as therapy for chronic hepatitis C—A pilot study. Phytomedicine 2011, 18, 807–810.
- Dehaen, W.; Mashentseva, A.A.; Seitembetov, T.S. Allobetulin and Its Derivatives: Synthesis and Biological Activity. Molecules 2011, 16, 2443.
- Kaplun, A.; Bezrukov, D.A.; Popenko, V.I.; Shvets, V.I. Spherical amorphous nanoparticles from birch bark triterpenoids-A novel type of submicronic vehicle for drug delivery. Russ. J. Biopharm. 2011, 3, 28–40.
- Wang, H.M.; Şoica, C.M.; Wenz, G. A Comparison Investigation on the Solubilization of Betulin and Betulinic Acid in Cyclodextrin Derivatives. Nat. Prod. Commun. 2012, 7, 1934578X1200700304.
- Şoica, C.; Dehelean, C.; Danciu, C.; Wang, H.M.; Wenz, G.; Ambrus, R.; Bojin, F.; Anghel, M. Betulin Complex in γ-Cyclodextrin Derivatives: Properties and Antineoplasic Activities in In Vitro and In Vivo Tumor Models. Int. J. Mol. Sci. 2012, 13, 4992.
- Vorobyova, O.; Deryabina, O.; Malygina, D.; Plotnikova, N.; Solovyeva, A.; Belyaeva, K.; Melnikova, N. Betulin-3,28-diphosphate as a Component of Combination Cytostatic Drugs for the Treatment of Ehrlich Ascites Carcinoma In Vitro and In Vivo Experiments. Sci. Pharm. 2018, 86, 17.
- Bureeva, S.; Andia-Pravdivy, J.; Symon, A.; Bichucher, A.; Moskaleva, V.; Popenko, V.; Shpak, A.; Shvets, V.; Kozlov, L.; Kaplun, A. Selective inhibition of the interaction of C1q with immunoglobulins and the classical pathway of complement activation by steroids and triterpenoids sulfates. Bioorg. Med. Chem. 2007, 15, 3489–3498.
- Grishkovets, V.I. Synthesis of triterpenoid sulfates using the SO3—dimethyl sulfoxide complex. Chem. Nat. Compd. 1999, 35, 73–74.
- Levdanskii, V.A.; Levdanskii, A.V.; Kuznetsov, B.N. Sulfation of Betulin by Sulfamic Acid in DMF and Dioxane. Chem. Nat. Compd. 2014, 50, 1029–1031.
- Dzhil’bert, E.E. Sulfonation of Organic Compounds; Himiya: Moscow, Russia, 1969; p. 415. (In Russian)
- Vasilyeva, N.Y.; Kazachenko, A.S.; Malyar, Y.; Kuznetsov, B.N. Sulfation of betulin with chlorosulfonic acid in pyridine. J. Sib. Fed. Univ. Chem. 2020, 13, 447–459.
- Cabassi, F.; Casu, B.; Perlin, A.S. Infrared absorption and raman scattering of sulfate groups of heparin and related glycosaminoglycans in aqueous solution. Carbohydr. Res. 1978, 63, 1–11.
- Krichen, F.; Bougatef, H.; Capitani, F.; Ben Amor, I.; Koubaa, I.; Gargouri, J.; Maccari, F.; Mantovani, V.; Galeotti, F.; Volpi, N.; et al. Purification and structural elucidation of chondroitin sulfate/dermatan sulfate from Atlantic bluefin tuna (Thunnus thynnus) skins and their anticoagulant and ACE inhibitory activities. RSC Adv. 2018, 8, 37965–37975.
- Levdanskii, V.A.; Levdanskii, A.V.; Kuznetsov, B.N. Sulfonation of Betulinic Acid by Sulfamic Acid. Chem. Nat. Compd. 2015, 51, 894–896.
- Drebushchak, T.N.; Mikhailovskaya, A.V.; Drebushchak, V.A.; Mikhailenko, M.A.; Myz’, S.A.; Shakhtshneider, T.P.; Kuznetsova, S.A. Crystalline forms of betulin: Polymorphism or pseudopolymorphism? J. Struct. Chem. 2020, 61, 1260–1266.
- Melnikova, N.B.; Malygina, D.S.; Klabukova, I.N.; Belov, D.V.; Vasin, V.A.; Petrov, P.S.; Knyazev, A.V.; Markin, A.V. Betulin-3,28-diphosphate. Physico-Chemical Properties and In Vitro Biological Activity Experiments. Molecules 2018, 23, 1175.
- Shakhtshneider, T.; Mikhailenko, M.; Drebushchak, V.; Drebushchak, T.; Malyar, Y.; Kuznetsova, S. Effect of ball-milling on the formation of betulin and betulin diacetate composites with polyethylene glycol. Mater. Today Proc. 2019, 12, 78–81.
- Kazachenko, A.S.; Malyar, Y.N.; Vasilyeva, N.Y.; Bondarenko, G.N.; Korolkova, I.V.; Antonov, A.V.; Karacharov, A.A.; Fetisova, O.Y.; Skvortsova, G.P. «Green» synthesis and characterization of galactomannan sulfates obtained using sulfamic acid. Biomass Convers. Biorefin. 2020, 1–10.
- Malyar, Y.N.; Kazachenko, A.S.; Vasilyeva, N.Y.; Fetisova, O.Y.; Borovkova, V.S.; Miroshnikova, A.V.; Levdansky, A.V.; Skripnikov, A.M. Sulfation of wheat straw soda lignin: Role of solvents and catalysts. Catal. Today 2021, in press.
- Yang, D.; Gong, N.; Zhang, L.; Lu, Y.; Du, G. Structural and Computational Study of 4 New Solvatomorphs of Betulin: A Combined X-Ray, Hirshfeld Surface, and Thermal Analysis. J. Pharm. Sci. 2017, 106, 826–834.
- Morales, J.E.T.; Pedroso, M.T.C.; Siguenza, J.C.; Villavicencio, C.B. Evaluation of Reactivity (pKa) of Substituted Aromatic Diamines, Monomers for Polyamides Synthesis, via Nuclear Magnetic Resonance, NMR-1H. Chem. Proc. 2021, 3, 8436.
More
Information
Subjects:
Biochemical Research Methods
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.8K
Entry Collection:
Organic Synthesis
Revisions:
2 times
(View History)
Update Date:
28 Feb 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




