Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Aleksandr Kazachenko and Version 2 by Catherine Yang.
Betulin is an important triterpenoid substance isolated from birch bark, which, together with its sulfates, exhibits important bioactive properties. Using the potentiometric titration method, the product of acidity constants K1 and K2 of a solution of the betulin disulfate H+ form has been found to be 3.86 × 10–6 ± 0.004. It has been demonstrated by the thermal analysis that betulin and the betulin disulfate sodium salt are stable at temperatures of up to 240 and 220 °C, respectively.
- betulin
- sulfation
- catalysis
- amberlyst (R)-15
1. Introduction
Plant biomass is an important feedstock for a wide range of valuable chemicals [1][2][3][4][5][1,2,3,4,5]. Catalytic processing of plant lignocellulosic biomass is an urgent task [6]. Birch biomass can serve as a source of various extractive substances. Birch bark is characterized by a particularly high content of extractive substances, which include mono- and triterpenoids, hydrocarbons, alcohols, fatty and resin acids, and phenolic compounds [7][8][9][7,8,9]
Triterpene compounds represent the most important class of bioactive substances promising for use as pharmaceutical active ingredients, drugs, and phytopreparations [10][11][12][13][14][15][16][17][10,11,12,13,14,15,16,17]. The betulin derivatives hold a special place in a triterpenoid series. A rich source of betulin is the Betulaceae family, especially Betula alba, Betula pubescens, Betula platyphylla, and Betula pendula [12][14][18][12,14,18]. The betulin content in the birch outer bark ranges within 10–35%, depending on a type of birch, an area and conditions of its growth, an age of the tree, and other factors [15][18][15,18]. In the in vitro and in vivo experiments, betulin and its derivatives exhibit the anti-inflammatory, anticonvulsant, antibacterial, antiviral, anti-HIV, antitumor, and other types of biological activity [11][12][13][14][15][16][17][19][20][21][22][23][11,12,13,14,15,16,17,19,20,21,22,23].
Owing to its availability and bioactivity, betulin is well-known as a valuable natural substance, both in its native state and in various modifications. Meanwhile, the low solubility of betulin in water limits its application in medicine, cosmetics, etc. To enhance the water solubility of triterpenoids, different methods are used, including the salt formation, the use of special dosage forms, which ensure the vector delivery of poorly soluble compounds, and nano- and biotechnological techniques [13][16][17][19][20][24][25][13,16,17,19,20,24,25]. The solubility of triterpenoids can be increased via their chemical modification. In particular, the solubility and bioavailability of triterpenoids can be improved by the complexation with γ-cyclodextrin and other compounds that can form inclusion complexes via the hydrophobic binding [13][17][19][20][24][25][26][27][13,17,19,20,24,25,26,27]. Sulfation of betulin improves its water solubility. Sulfuric esters of betulin and betulinic acid were shown to be bioactive [27][28][27,28].
The triterpenoid sulfation methods proposed previously [28][29][28,29] are based on the use of sulfuric acid and complexes resulting from the interaction of sulfuric anhydride with pyridine or dimethyl sulfoxide. Thus, the synthesis of betulin disulfate and betulinic acid 3-sulfate is carried out via sulfation of betulin and betulinic acid with sulfuric acid in pyridine in the presence of acetic anhydride [28].
Sulfation of triterpenoids with chlorosulfonic or sulfamic acid in an environment of weak bases, e.g., dimethylformamide or dioxane, not only can be accompanied by isomerization of initial betulin [23], but the sulfated triterpenoid can be formed initially in a slightly stable H-form [30]. As is known, the sulfur trioxide pyridine complex is a milder sulfating reagent than the SO3–1,4-dioxane complex; it attacks exclusively alcohol groups and does not affect the double bond [31]. The use of pyridine in betulin sulfation instead of 1,4-dioxane and N, N-dimethylformamide excludes the formation of sulfated betulin in a slightly stable H-form [30]; sulfated betulin has a form of a stable salt.
2. Fourier-Transform Infrared Spectroscopy Study
The introduction of the sulfate group into the betulin molecule was confirmed by IR spectroscopy (Figure 1).
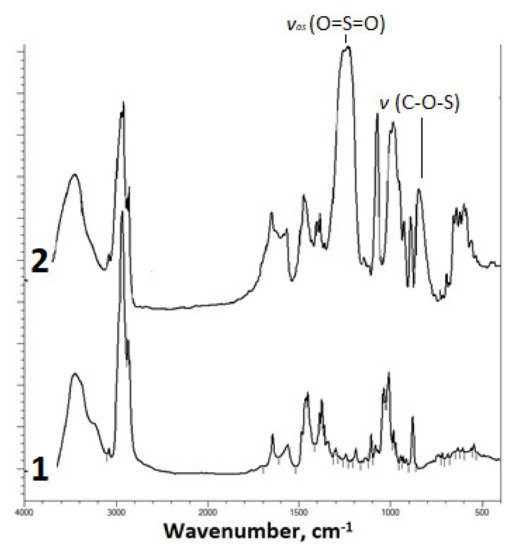

Figure 1.
FTIR spectra of (
1
) betulin and (
2
) sulfated betulin in the sodium form.
The FTIR spectrum of the betulin disulfate sodium salt contains, along with the absorption bands characteristic of the initial betulin, a high-intensity band of asymmetric stretching vibrations υas (O=S=O) at 1249 cm−1 and an absorption band of stretching vibrations υ (C–O–S) [32][33][56,57] in the range of 835–841 cm−1.
3. Ultraviolet—Visible Spectroscopy Study
The initial and sulfated betulin was examined by UV-Vis spectroscopy (Figure 2).
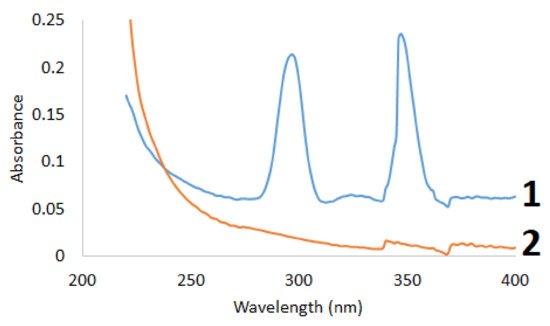

Figure 2.
UV spectra of (
1
) betulin and (
2
) sulfated betulin.
To qualitatively identify the presence of sulfo groups in the betulin sulfation products, the electronic absorption spectra in the range of 220–400 nm were reproduced in the alcohol solution. It can be seen in Figure 2 that the UV spectra of the initial and sulfated betulin have different profiles, which is indicative of the presence of new functional groups in the sulfated products [34][58]. A factor indicating the introduction of a sulfo group into the betulin structure is a decrease in the total intensity of the sulfation products. The introduction of an additional functional group with an increase in the average molecular weight leads to a decrease in the intensity in the UV range.
4. Nuclear Magnetic Resonance Study
The composition and structure of the betulin disulfate sodium salt was confirmed by 13C NMR spectroscopy. According to the literature data [35][59], the chemical shift of the secondary C3 carbon atom bonded to the hydroxyl group is observed at 78–79 ppm and the chemical shift of the primary C28 carbon atom, at 59–60 ppm. An analysis of the 13C NMR spectra of the initial betulin and the betulin disulfate sodium salt showed that the chemical shift of the C3 carbon atom in the initial betulin is observed at 78.25 ppm and the chemical shift of the C28 carbon atom, at 58.96 ppm. In the synthesized betulin disulfate, the chemical shifts of the С3 and С28 carbons in comparison with betulin are completely shifted to the low-field region (to 88.21 and 67.32 ppm, respectively). This proves the complete replacement of the betulin hydroxyl groups by the sulfate.
5. X-Ray Diffraction Study
The initial betulin and betulin sulfate were analyzed by X-ray diffractometry (Figure 3). The initial betulin has a crystalline structure with the high-intensity bands [36][60]. During sulfation, a decrease in the crystallinity of betulin is observed, as is the case with its chemical modification by other methods [37][61].
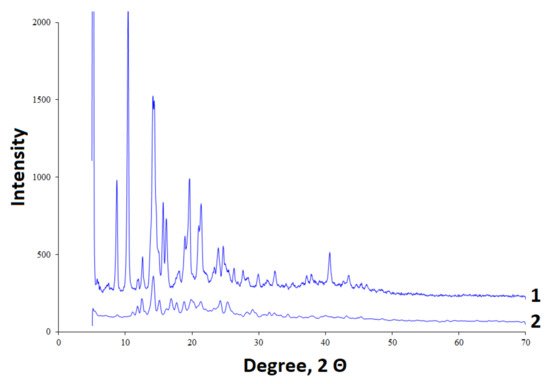

Figure 3.
XRD diffraction patterns of (
1
) betulin and (
2
) betulin sulfate.
6. Thermal Analysis
Figure 4 shows a thermogram of betulin and the betulin disulfate sodium salt obtained upon heating in the argon atmosphere.
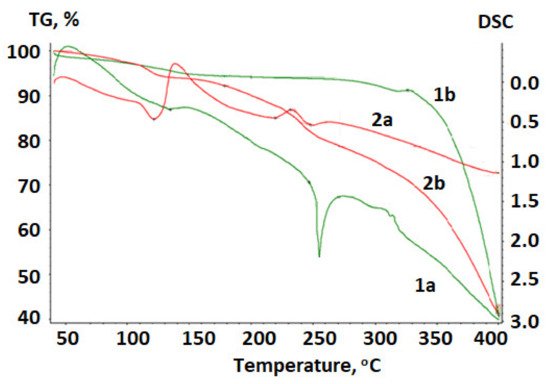

Figure 4.
Thermogram of (
1
) betulin and (
2
) betulin disulfate sodium salt: (
a
) DTA and (
b
) TG curves.
The temperature dependence of the weight loss (the TG curve) for betulin has a plateau; the horizontal section is indicative of the stability of the chemical compound in the investigated temperature range at the absence of chemical transformations. A vertical step in the curve is indicative of the chemical decomposition of the material [38][62].
At a temperature of 134.5 °C, a loss of water contained in betulin is observed. The weight loss is 5.83%. The TG curve reflects an intense loss in the sample mass above 260 °C.
To determine the transformation temperatures more accurately, a differential notation was used. A peak in the DTA curve in the range of 240–260 °C is indicative of a phase transformation in betulin, which is accompanied by the endothermic effect. This is a first-order phase transition; in this region, betulin melts with the subsequent decomposition.
In contrast to initial betulin, in the betulin disulfate sodium salt the water loss occurs earlier and the weight loss is 7.76%.
In the DTA curve at temperatures of 220–230 °C, the exothermic effect is reflected, which corresponds to the decomposition of the betulin disulfate sodium salt, apparently with the SO2 release. The weight loss is 15.5%, according to the sulfur content in betulin disulfate. The degree of decomposition of betulin disulfate with the SO2 release in this region is 79%, which is consistent with the data reported in [39][40][41][43,55,63]. We can Bstate that betulin and the betulin disulfate sodium salt are stable at temperatures of up to 240 and 220 °C, respectively.
7. Acidity Constants
The product of acidity constants K1 and K2 of the solution of the betulin disulfate H + form was determined by a potentiometric titration (Figure 5). The average product of the first and second dissociation constants K1 and K2 was found to be 3.86 × 10−6 ± 0.004. Figure 5 shows the dependence of pH of the solution of the betulin disulfate H + form on the sodium hydroxide volume.
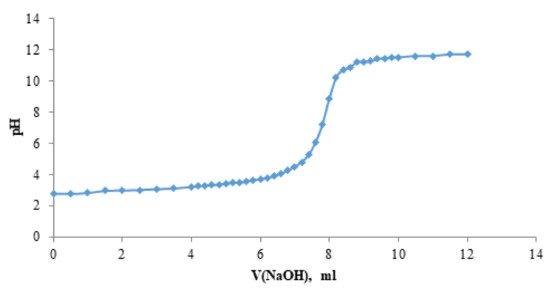

Figure 5.
Dependence of pH of the solution of the H + form of betulin disulfate on the sodium hydroxide volume.
Since the titration curve contains only one jump, it is obvious that the H + form of betulin disulfate has similar values of the first and second dissociation constants K1 and K2. This, most likely, originates from the betulin disulfate structure, in which sulfate groups are distant from each other.
This conclusion does not contradict the data on dissociation constants of the known dicarboxylic acids with carboxyl groups significantly distant from each other [42][64]; therefore, for example, in adipic acid, the K1 and K2 values are of the same order of magnitude: 3.7 × 10−5 and 1.93 × 10−5, respectively.
According to the obtained acidity constant, the H + form of betulin disulfate is an acid stronger than carboxylic acids, but weaker than sulfonic acids. Basing on the determined constant, the H + form of betulin disulfate was confirmed, which can be obtained by adding a mineral acid salt to the aqueous solution.
