Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Tiziana Cillari | + 1901 word(s) | 1901 | 2022-02-22 06:50:39 | | | |
| 2 | Vivi Li | Meta information modification | 1901 | 2022-02-28 02:38:44 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Cillari, T.; Berto, D.; Bosch-Belmar, M.D.M.; Falautano, M.; Maggio, T.; Milisenda, G.; Perzia, P.; Mauro, S.; Castriota, L. Jellyfish Cassiopea andromeda. Encyclopedia. Available online: https://encyclopedia.pub/entry/19936 (accessed on 08 February 2026).
Cillari T, Berto D, Bosch-Belmar MDM, Falautano M, Maggio T, Milisenda G, et al. Jellyfish Cassiopea andromeda. Encyclopedia. Available at: https://encyclopedia.pub/entry/19936. Accessed February 08, 2026.
Cillari, Tiziana, Daniela Berto, Maria Del Mar Bosch-Belmar, Manuela Falautano, Teresa Maggio, Giacomo Milisenda, Patrizia Perzia, Sinopoli Mauro, Luca Castriota. "Jellyfish Cassiopea andromeda" Encyclopedia, https://encyclopedia.pub/entry/19936 (accessed February 08, 2026).
Cillari, T., Berto, D., Bosch-Belmar, M.D.M., Falautano, M., Maggio, T., Milisenda, G., Perzia, P., Mauro, S., & Castriota, L. (2022, February 26). Jellyfish Cassiopea andromeda. In Encyclopedia. https://encyclopedia.pub/entry/19936
Cillari, Tiziana, et al. "Jellyfish Cassiopea andromeda." Encyclopedia. Web. 26 February, 2022.
Copy Citation
Cassiopea andromeda entered the Mediterranean from the Red Sea through the Suez Canal and colonized several areas of the basin. This species is an epibenthic scyphozoan with a maximum umbrella diameter of about 30 cm commonly found in tropical and subtropical shallow coastal ecosystems such as mangroves, estuaries, and sandy mudflats. This species has a metagenetic cycle with the following phases: planula, benthic polyp, ephyra, and adult medusa. The symbiotic relationship with dinoflagellates allows the jellyfish species to feed via direct predation and through photosynthesis by the zooxanthellae (mixotrophy).
non-indigenous species
upside-down jellyfish
Megabenthos Underwater Video
species distribution
stable isotopes
mixotrophic behavior
1. Introduction
The presence of non-indigenous species (NIS), also called alien species, is considered an important cause of biodiversity loss and changes in an ecosystem [1]. In the Mediterranean Sea, NIS can also be introduced via maritime traffic, by means of ballast waters and hull fouling. The diffusion of NIS has also been correlated with climate changes, which allows tropical and subtropical species to expand their distribution to other habitats [2][3][4]. For these reasons, harbors are hotspots for the introduction of NIS, and, usually, the host populations need to be studied to fill the knowledge gap in their pathways of invasion and in their impacts on the surrounding ecosystems. In 2014, the Lessepsian upside-down jellyfish Cassiopea andromeda (Forsskål, 1775) (Cnidaria, Rhizostomeae) invaded a touristic harbour of Palermo (Sicily, Italy) named Cala. Over the years, its abundance within Cala has increased [5].
C. andromeda (Figure 1) entered the Mediterranean from the Red Sea through the Suez Canal [5][6] and colonized several areas of the basin. This species is an epibenthic scyphozoan with a maximum umbrella diameter of about 30 cm commonly found in tropical and subtropical shallow coastal ecosystems such as mangroves, estuaries, and sandy mudflats. This species has a metagenetic cycle with the following phases: planula, benthic polyp, ephyra, and adult medusa [7].
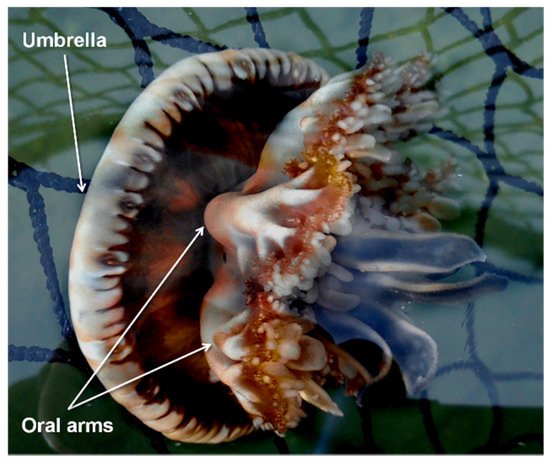
Figure 1. Cassiopea andromeda (Forsskål, 1775).
Contrary to jellyfish with pelagic behaviors, these benthic jellyfish prefer habitats with less water movements [8][9][10]. They often lay down on their umbrella, exposing their oral arms to the sun. Similar to other Rhizostomeae jellyfish, they frequently exhibit a symbiotic relationship with dinoflagellates, such as Symbiodinium spp., which are present in the tentacular tissue on their oral arms [11]. This relationship allows the jellyfish species to feed via direct predation and through photosynthesis by the zooxanthellae [12][13]; this mixed feeding of the jellyfish is called mixotrophy (concurrent autotrophy and heterotrophy).
In this symbiotic interaction, the carbon needed for the basal metabolism of the host is provided by the photosynthetic process [14][15][16][17], whereas nitrogen is taken up from the environment through the digestive processes [12][13]. However, this amount of nutrient intake is not fixed but rather depends on the species and the environments in which they grow (see, e.g., [16][17][18][19][20]). As with other symbiotic cnidarians, Cassiopea jellyfish may acquire dissolved nutrients from the surrounding environment to meet the energetic needs of their photosynthetic partners [21], while zooxanthellae may provide much of the carbon requirements to the host, which are critical to the metamorphosis of ephyrae and to the survival of the jellyfish [8]. Stable carbon and nitrogen isotopes might be valuable indicators of the relative importance between the autotrophy and heterotrophy pathways [22][23][24]. Subsequent studies have found that several factors such as inorganic nitrogen uptake [25], terrestrial nitrogen loads [26], eutrophication [26][27][28][29], zooxanthellae population dynamics [30], light [31], and bleaching [32] may affect the nitrogen isotopic (δ15N) signature of mixotrophic coral organisms with zooxanthellae symbionts.
Some authors have demonstrated the success of a mutualistic interaction with C. andromeda under stressful environmental conditions in shallow waters, i.e., high temperatures, high levels of irradiation, eutrophic conditions, and changes in salinity [33]. These life history traits also permitted this species to become an invader in the Mediterranean Sea, where, being one of the earliest Lessepsian migrants, C. andromeda has spread, reaching the western Mar Menor in Spain, being randomly spotted in the Levant Sea, Aegean Sea, and Strait of Sicily [6][34][35][36]. Mediterranean C. andromeda populations form short-term outbreaks up to 20 individuals m−2 in semi-enclosed human-impacted coastal systems with eutrophic waters and low hydrodynamics [5]. Stoner et al. [8] observed that Cassiopea spp. populations were significantly denser, and that individuals from these populations were larger in areas with high human population densities (a proxy of nutrient enrichment) with respect to more natural sites. Equally, The et al. [37] reported high densities of Cassiopea jellyfish within shrimp farms where environmental conditions were stable and the concentrations of nutrients and organic matter were high.
2. Distribution of Cassiopea andromeda
Studying NIS in harbor environments is not always easy due to the presence of obstacles (e.g., floating docks, anchored or moving nautical vehicles, and ropes) which hinder the use of standard visual methods and tools. A Megabenthos Underwater Video (MUV) device specially designed and built to overcome these sampling difficulties [38] allowed recording several C. andromeda specimens of different sizes in the different sub-areas of Cala. Their abundance and density varied across the four sampling dates. The large number of individuals of small sizes observed in February 2018 compared with that during previous months, when the intermediate size was the most abundant, suggests a previous reproductive event and indicates that the population of jellyfish in this study area is quite established.
The lowest values of abundance and density were recorded in April 2018, when the medusa stage population was clearly in decline. Since then, the medusa stage disappeared for two years and was subsequently spotted in October 2020 and in November 2021 (unpublished data from the authors’ on-site visual inspections), when a few small and medium-sized specimens were observed. The period of apparent absence of the species in this harbour of Palermo could correspond to the polyp phase, which is not macroscopically visible. After a long period of two years, the transition from the polyp to medusa (strobilation) phases could have been triggered by exogenous factors (according to [11]), which led to the reappearance of a visible population.
The presence of C. andromeda down to 7.5 m depths may be due to the jellyfish’s photosynthetic symbionts needing light, and its presence in the internal and intermediate zones of Cala could be due to the more eutrophic conditions [8]. Moreover, the dense packing of the smallest individuals in the internal zone could depend on the hypothetical presence of a close polyp population due to the lower hydrodynamics and the presence of many artificial substrates in this area of the harbour. Equally, the lack of C. andromeda observed at deeper and external sites (i.e., at the Cala mouth) may also be due to the greater amount of water movement typically caused by the continuous passage of boats.
From the results of the GIS-based statistical analyses, the jellyfish aggregation seen during June and November in different sub-areas and with different orientations suggests that the C. andromeda population is established in various zones of Cala. This is probably due to the environmental variability of the harbour, which is severely influenced by various factors (e.g., terrigenous and anthropogenic inputs) heterogeneously distributed over time and space, as well as by the variation in some environmental parameters (e.g., water turbidity due to vessel movements and external inputs).
The ranges of the environmental parameters measured in the study area (temperature, salinity, and transparency) were consistent with the conditions necessary to maintain a Cassiopean population [37] and did not influence the distribution of specimens, which clustered around both low and high parameter values. In fact, Cassiopea spp. tolerate a wide range of temperatures (up to about 29 °C), salinities (up to 36), and levels of light exposure (from 200 to 500 μmol photons m−1 s−1) [10][39]. The cluster behavior is possibly generated by other factors, such as a greater concentration of nutrients or organic matter in these areas, which would attract jellyfish to specific areas, as hypothesized by [37].
3. Trophic Behavior of Cassiopea andromeda
In the literature, the use of δ15N from aquatic autotrophic organisms and consumers to trace anthropogenic sources of N has often been reported [40][41][42][43][44]. The generally most useful observation is that, in nutrient-rich environments, the δ15N of algae can track the δ15N from nitrate. The transformation of inorganic nitrogen compounds into an organic form during biosynthesis by living autotrophic organisms influences the reduction of oxidized forms of N to NH4+ and, then, its assimilation into organic matter. This process generally prefers the incorporation of an isotope with a lower mass. Some authors [45][46] measured a large range of N fractionations (−30 to 0‰) for nitrate and ammonium assimilation by algae and bacteria. The atmospheric N2 bacterial fixation by the enzyme nitrogenase is reflected in organic material having δ15N values slightly less than 0‰ and lower than the environmental values for organic materials produced by other mechanisms [47]. For this reason, low δ15N values in organic matter are generally thought to indicate N2 fixation. Assimilation produces isotope fractionation by favoring the incorporation of lighter isotopes. Several authors measured a wide range of N fractionations (−30 to 0‰) in field studies [45][46] and in laboratory experiments for nitrate and ammonium assimilation by algae [48][49][50]. For ammonium assimilation, the authors of [47] reported a range of fractionation from −4 to −27‰, depending on whether the algae cells were nitrogen limited, enzyme limited, or diffusion limited.
The Cassiopea andromeda trophic position and energy assimilation strategy may vary according to the availability of food sources, allowing the species to subsist in water masses ranging from eutrophic to oligotrophic conditions [51][52][53].
In the study area, the lower values of δ15N for C. andromeda, indicating a depletion of 15N compared with 14N, were unexpected, but they could be the result of the availability of nitrogen sources rich in 14N, such as those from untreated urban waste [54]. These lower values could be more affected by the characteristic of the two sampling sites of Cala (Calamida and Canottieri), which are confined and feature low hydrodynamics. The stable isotope values in C. andromeda were consistently lower than those in the other community components, suggesting the production of organic components based on the metabolism of associated symbionts.
Septic tank effluent contains predominantly organic and inorganic carbon, organic nitrogen, and ammonium. The septic sludge δ15N reported by [54] had low values (–2.1‰), whereas the corresponding particulate and dissolved fractions generally had different nitrogen and carbon ratios as a result of fractionation in the anaerobic or aerobic processes. Nevertheless, an influence of atmospheric precipitation was not excluded given that the δ15N of nitrate reported for a wet deposition showed values ranging from −11‰ to +3.5‰, with a mean value of −3.1‰ [55][56][57].
For both sampling sites, the lower δ15N values observed for the oral arms of jellyfish suggest a lower trophic level with respect to the umbrella, highlighting the higher concentration of autotrophic symbionts in these tissues [11][58]. Indeed, the pattern suggested is that the zooxanthellae, which colonize the oral arms of C. andromeda, uptake the dissolved inorganic nitrogen with a low δ15N value from the environment [52][53][59], while the umbrella uses a mix of the uptake of nitrogen with higher δ15N values, due to fractionation along the food web through predation [24][51][60], and degradation of the colonizers.
Less negative δ13C values (typically from −10‰ to −14‰) than those of particulate organic matter and plankton (ca. −20‰ [51]) are typical for the uptakes of dissolved inorganic carbon by zooxanthellae [20][22][61][62]. The δ13C values obtained for the umbrella in this entry were higher than those obtained for the oral arms, in particular at the Calamida site, suggesting less translocation of the metabolites derived from symbionts and greater dependence on the heterotrophic metabolism of the jellyfish [52][53].
During synthesis, the isotopic signature evidenced that the metabolites derived from inorganic nutrient uptake and predation are then exchanged and recycled between the zooxanthellae and the host (e.g., [63]). The photosynthesis that occurs in the oral arms could involve processes depleting the nutrient pools derived from untreated discharge civil effluents and mixed with the discharge of wet depositions.
References
- Katsanevakis, S.; Wallentinus, I.; Zenetos, A.; Leppäkoski, E.; Çinar, M.E.; Oztürk, B.; Grabowski, M.; Golani, D.; Cardoso, A.N. Impacts of Invasive Alien Marine Species on Ecosystem Services and Biodiversity: A Pan-European Review. Aquat. Invasions 2014, 9, 391–423.
- Bianchi, C.N. Biodiversity issues for the forthcoming tropical Mediterranean Sea. Hydrobiologia 2007, 580, 7.
- Parravicini, V.; Mangialajo, L.; Mousseau, L.; Peirano, A.; Morri, C.; Montefalcone, M.; Francour, P.; Kulbicki, M.; Bianchi, C.N. Climate change and warm-water species at the north-western boundary of the Mediterranean Sea. Mar. Ecol. 2015, 36, 897–909.
- Lasram, B.F.; Tomasini, J.A.; Guilhaumon, F.; Romdhane, M.S.; Do Chi, T.; Mouillot, D. Ecological correlates of dispersal success of Lessepsian fishes. Mar. Ecol. Prog. Ser. 2008, 363, 273–286.
- Maggio, T.; Allegra, A.; Bosch-Belmar, M.; Cillari, T.; Cuttitta, A.; Falautano, M.; Milisenda, G.; Nicosia, A.; Perzia, P.; Sinopoli, M.; et al. Molecular identity of the non-indigenous species Cassiopea sp. from Palermo Harbour (central Mediterranean Sea). J. Mar. Biolog. Assoc. UK 2019, 99, 1765–1773.
- Maas, O. Die Scyphomedusen der Siboga-Expedition; Siboga-Expeditie, Buchhandlung und Druckerei Vormals, EJ Brill: Leiden, The Netherlands, 1903; Volume 11, pp. 1–91.
- Stampar, S.N.; Gamero-Mora, E.; Maronna, M.M.; Fritscher, J.M.; Oliveira, B.S.P.; Sampaio, C.L.S.; Morandini, A.C. The puzzling occurrence of the upside-down jellyfish Cassiopea (Cnidaria: Scyphozoa) along the Brazilian coast: A result of several invasion events? Zoologia 2020, 37, 1–10.
- Stoner, E.W.; Layman, C.A.; Yeager, L.A.; Hassett, H.M. Effects of anthropogenic disturbance on the abundance and size of epibenthic jellyfish Cassiopea spp. Mar. Pollut. Bull. 2011, 62, 1109–1114.
- Heins, A.; Glatzel, T.; Holst, S. Revised descriptions of the nematocysts and the asexual reproduction modes of the scyphozoan jellyfish Cassiopea andromeda (Forsskål, 1775). Zoomorphology 2015, 134, 351–366.
- Morandini, A.C.; Stampar, S.N.; Maronna, M.M.; Da Silveira, F.L. All non-indigenous species were introduced recently? The case study of Cassiopea (Cnidaria: Scyphozoa) in Brazilian waters. J. Mar. Biol. Assoc. UK 2017, 97, 321–328.
- Ohdera, A.H.; Abrams, M.J.; Ames, C.L.; Baker, D.M.; Suescún-Bolívar, L.P.; Collins, A.G.; Freeman, C.J.; Gamero-Mora, E.; Goulet, T.L.; Hofmann, D.K.; et al. Upside-down but headed in the right direction: Review of the highly versatile Cassiopea xamachana system. Front. Ecol. Evol. 2018, 6, 1–15.
- Kremer, P. Ingestion and elemental budgets for Linuche unguiculata, a scyphomedusa with zooxanthellae. J. Mar. Biol. Assoc. UK 2005, 85, 613–625.
- Welsh, D.T.; Dunn, R.J.; Meziane, T. Oxygen and nutrient dynamics of the upside down jellyfish (Cassiopea sp.) and its influence on benthic nutrient exchanges and primary production. Hydrobiologia 2009, 635, 351–362.
- Kremer, P.; Costello, J.; Kremer, J.; Canino, M. Significance of photosynthetic endosymbionts to the carbon budget of the scyphomedus Linuche unguiculata. Limnol. Oceanogr. 1990, 35, 609–624.
- Kikinger, R. Cotylorhiza tuberculata (Cnidaria: Scyphozoa)—Life history of a stationary population. Mar. Ecol. 1992, 13, 333–362.
- McCloskey, L.R.; Muscatine, L.; Wilkerson, F.P. Daily photosynthesis, respiration, and carbon budgets in a tropical marine jellyfish (Mastigias sp.). Mar. Biol. 1994, 119, 13–22.
- Verde, E.A.; McCloskey, L.R. Production, respiration, and photophysiology of the mangrove jellyfish Cassiopea xamachana symbiotic with zooxanthellae: Effect of jellyfish size and season. Mar. Ecol. Prog. Ser. 1998, 168, 147–162.
- Sugiura, Y. On the life-history of Rhizostome medusae V. On the relation between zooxanthellae and the strobilation of Cephea cephea. Bull. Mar. Biol. Stn. Asamushi 1969, 13, 227–233.
- Bolton, T.F.; Graham, W.M. Morphological variation among populations of an invasive jellyfish. Mar. Ecol. Prog. Ser. 2004, 278, 125–139.
- Djeghri, N.; Stibor, H.; Lebeau, O.; Pondaven, P. δ13C, δ15N, and C:N ratios as nutrition indicators of zooxanthellate jellyfishes: Insights from an experimental approach. J. Exp. Mar. Biol. Ecol. 2020, 522, 151257.
- Todd, B.D.; Thornhill, D.J.; Fitt, W.K. Patterns of inorganic phosphate uptake in Cassiopea xamachana: A bioindicator species. Mar. Pollut. Bull. 2006, 52, 515–521.
- Muscatine, L.; Porter, J.W.; Kaplan, I.R. Resource partitioning by reef corals as determined from stable isotope composition I. δ13C of zooxanthellae and animal tissue versus depth. Mar. Biol. 1989, 100, 185–193.
- Muscatine, L.; Kaplan, I.R. Resource partitioning by reef corals as determined from stable isotope composition II. δ15N of zooxanthellae and animal tissue versus depth. Pac. Sci. 1994, 48, 304–312.
- Ferrier-Pagès, C.; Peirano, A.; Abbate, M.; Cocito, S.; Negri, A.; Rottier, C.; Riera, P.; Metalpa, R.; Reynaud, S. Summer autotrophy and winter heterotrophy in the temperate symbiotic coral Cladocora caespitosa. Limnol. Oceanogr. 2011, 56, 1429–1438.
- Yamamuro, M.; Kayanne, H.; Minagawa, M. Carbon and nitrogen stable isotopes of primary producers in coral reef ecosystems. Limnol. Oceanogr. 1995, 40, 617–621.
- Sammarco, P.W.; Risk, M.J.; Schwarcz, H.P.; Heikoop, J.M. Cross-continental shelf trend in coral δ15N on the Great Barrier Reef: Further consideration of the reef nutrient paradox. Mar. Ecol. Prog. Ser. 1999, 180, 131–138.
- Heikoop, J.M.; Dun, J.J.; Risk, M.J.; Tomascik, T.; Schwarz, H.P.; Sandeman, I.M.; Sammarco, P.W. Nitrogen-15 signals of anthropogenic nutrient loading in reef corals. Mar. Pollut. Bull. 2000, 40, 628–636.
- Hoegh-Guldberg, O.; Muscatine, L.; Goiran, C.; Siggaard, D.; Marion, G. Nutrient-induced perturbations to δ13C and δ15N in symbiotic dinoflagellates and their coral hosts. Mar. Ecol. Prog. Ser. 2004, 280, 105–114.
- Marion, G.S.; Hoegh-Guldberg, O.; McCulloch, M.T.; Jupiter, S.D. Coral isotopic records (δ15N) of unprecedented land-use stress in Great Barrier Reef coastal communities. EOS Trans. Am. Geophys. Union Ocean Sci. Meet. Suppl. 2006, 87.
- Heikoop, J.M.; Dun, J.J.; Risk, M.J.; McConnaughey, T.A.; Sandman, I.M. Separation of kinetic and metabolic effect in carbon-13 records preserved in reef coral skeletons. Geochim. Cosmochim. Acta 2000, 64, 975–987.
- Heikoop, J.M.; Dunn, J.J.; Risk, M.J.; Sandeman, I.M.; Schwartz, H.P.; Waltho, N. Relationship between light and the δ15N of coral tissue: Examples from Jamaica and Zanzibar. Limnol. Oceanogr. 1998, 43, 909–920.
- Rodrigues, L.; Grottoli, A. Calcification rate, and the stable carbon, oxygen and nitrogen isotopes in the skeleton, host tissue and zooxanthellae of bleached and recovering Hawaiian corals. Geochim. Cosmochim. Acta 2006, 70, 2781–2789.
- Lampert, K.P. Cassiopea and its zooxanthellae. In The Cnidaria, Past, Present and Future; Springer: Cham, Switzerland, 2016; pp. 415–423.
- Schäfer, W. Eine Qualle aus dem Indischen Ozean in der Agais. Nat. Volk 1955, 85, 241–245.
- Schembri, P.J.; Deidun, A.; Vella, P.J. First record of Cassiopea andromeda (Scyphozoa: Rhizostomeae: Cassiopeidae) from the central Mediterranean Sea. Mar. Biodivers. Rec. 2010, 3, E6.
- Cillari, T.; Andaloro, F.; Castriota, L. First documented record of Cassiopea cfr andromeda (Cnidaria: Scyphozoa) in Italian waters. Cah. Biol. Mar. 2018, 59, 193–195.
- Thé, J.; de Sousa Barroso, H.; Mammone, M.; Viana, M.; Melo, C.S.B.; Mies, M.; Banha, T.N.S.; Morandini, A.C.; Rossi, S.; de Oliveira Soares, M. Aquaculture facilities promote populational stability throughout seasons and increase medusae size for the invasive jellyfish Cassiopea andromeda. Mar. Environ. Res. 2020, 162, 105161.
- Cillari, T.; Allegra, A.; Bosch-Belmar, M.; Castriota, L.; Falautano, M.; Milisenda, G.; Maggio, T.; Perzia, P.; Sinopoli, M. Megabenthos Underwater Video (MUV): A new device to evaluate species distribution in hard-to-reach marine areas. In Proceedings of the International Workshop on Metrology for the Sea, Learning to Measure Sea Health Parameters (MetroSea), Virtual Conference, Milazzo, Italy, 4–6 October 2021; pp. 199–203.
- Mammone, M.; Ferrier-Pagés, C.; Lavorano, S.; Rizzo, L.; Piraino, S.; Rossi, S. High photosynthetic plasticity may reinforce invasiveness of upside-down zooxanthellate jellyfish in Mediterranean coastal waters. PLoS ONE 2021, 16, e0248814.
- Viana, I.G.; Bode, A.; Bartholomew, M.; Valiela, I. Experimental assessment of the macroalgae Ascophyllum nodosum and Fucus vesiculosus for monitoring N sources at different time-scales using stable isotope composition. J. Exp. Mar. Biol. Ecol. 2015, 466, 24–33.
- McClellend, J.; Valiela, I. Linking nitrogen in estuarian producers to land-derived sources. Limnol. Oceanogr. 1998, 43, 577–585.
- Risk, M. The reef crisis and the reef science crisis: Nitrogen isotopic ratios as an objective indicator of stress. Mar. Pollut. Bull. 2009, 58, 787–788.
- Risk, M.J.; Sherwood, O.A.; Nairn, R.; Gibbons, C. Tracking the record of sewage discharge off Jeddah, Saudi Arabia, since 1950, using stable isotope records from antipatharians. Mar. Ecol. Prog. Ser. 2009, 397, 219–226.
- Risk, M.J.; Erdmann, M.V. Isotopic composition of nitrogen in stomatopod (Crustacea) tissues as an indicator of human sewage impacts on Indonesian coral reefs. Mar. Pollut. Bull. 2000, 40, 50–58.
- Cifuentes, L.A.; Fogel, M.L.; Pennock, J.R.; Sharp, J.H. Biogeochemical factors that influence the stable nitrogen isotope ratio of dissolved ammonium in the Delaware estuary. Geochim. Cosmochim. Acta 1989, 53, 2713–2721.
- Montoya, P.; Korrigan, S.G.; McCarthy, J.J. Rapid, storm-induced changes in the natural abundance of 15N in a planktonic ecosystem, Chesapeake Bay, USA. Geochim. Cosmochim. Acta 1991, 55, 3627–3638.
- Fogel, M.L.; Cifuentes, L.A. Isotope fractionation during primary production. In Organic Geochemistry; Engel, M.H., Macko, S.A., Eds.; Springer: Boston, MA, USA, 1993; Volume 11, pp. 73–98.
- Pennock, J.R.; Velinsky, D.J.; Ludlam, J.M.; Sharp, J.H.; Fogel, M.L. Isotopic fractionation of ammonium and nitrate during uptake by Skeletonema costatum: Implications for δ15N dynamics under bloom conditions. Limnol. Oceanogr. 1996, 41, 451–459.
- Waser, N.A.D.; Harrison, P.J.; Nielsen, B.; Calvert, S.E.; Turpin, D.H. Nitrogen isotope fractionation during the uptake and assimilation of nitrate, nitrite, ammonium, and urea by a marine diatom. Limnol. Oceanogr. 1998, 43, 215–224.
- Granger, J.; Sigman, D.M.; Needoba, J.A.; Harrison, P.J. Coupled nitrogen and oxygen isotope fractionation of nitrate during assimilation by cultures of marine phytoplankton. Limnol. Oceanogr. 2004, 49, 1763–1773.
- Ferrier-Pagès, C.; Leal, M.C. Stable isotopes as tracers of trophic interactions in marine mutualistic symbioses. Ecol. Evol. 2018, 9, 723–740.
- Freeman, C.J.; Stoner, E.W.; Easson, C.G.; Matterson, K.O.; Baker, D.M. Symbiont carbon and nitrogen assimilation in the Cassiopea–Symbiodinium mutualism. Mar. Ecol. Prog. Ser. 2016, 544, 281–286.
- Freeman, C.J.; Stoner, E.W.; Easson, C.G.; Matterson, K.O.; Baker, D.M. Variation in δ13C and δ15N values suggests a coupling of host and symbiont metabolism in the Symbiodinium-Cassiopea mutualism. Mar. Ecol. Prog. Ser. 2017, 571, 245–251.
- Cravotta, C.A. Use of Stable Isotopes of Carbon, Nitrogen, and Sulfur to Identify Sources of Nitrogen in Surface Waters in the Lower Susquehanna River Basin, Pennsylvania; U.S. Department of the Interior, U.S. Geological Survey: Denver, CO, USA, 2002; p. 99.
- Elliott, E.M.; Kendall, C.; Burns, D.A.; Boyer, E.W.; Harlin, K.; Wankel, S.D.; Butler, T.J.; Carlton, R. Nitrate isotopes in precipitation to distinguish NOx sources, atmospheric processes, and source areas in the United States. In AGU Fall Meeting Abstracts; American Geophysical Union: San Francisco, CA, USA, 2006; Volume 2007, abstract id. H52B-01.
- Hastings, M.G.; Sigman, D.M.; Lipschultz, F. Isotopic evidence for source changes of nitrate in rain at Bermuda. J. Geophys. Res. 2003, 108, 4790.
- Hastings, M.G. Studies of Reactive Nitrogen in the Atmosphere Using Global Modelling and Stable Isotope Measurements. Ph.D. Thesis, Princeton University, Princeton, NJ, USA, 2004; 217p.
- Arai, M.N. A Functional Biology of the Scyphozoa; Chapman & Hall: London, UK, 1997; p. 300.
- Balderston, W.L.; Claus, G. Study of the symbiotic relationship between Symbiodinium microadriaticum Freudenthal, a Zooxanthella and the upside down jellyfish, Cassiopea sp. Nova Hedwigia 1969, 17, 373–382.
- Post, D.M. Using stable isotopes to estimate trophic position: Models, methods, and assumptions. Ecology 2002, 83, 703–718.
- Swart, P.K.; Saied, A.; Lamb, K. Temporal and spatial variation in the δ15N and δ13C of coral tissue and zooxanthellae in Montastraea faveolata collected from the Florida reef tract. Limnol. Oceanogr. 2005, 50, 1049–1058.
- Alamaru, A.; Loya, Y.; Brokovich, E.; Yam, R.; Shemesh, A. Carbon and nitrogen utilization in two species of Red Sea corals along a depth gradient: Insights from stable isotope analysis of total organic material and lipids. Geochim. Cosmochim. Acta 2009, 73, 5333–5342.
- Reynaud, S.; Martinez, P.; Houlbrèque, F.; Billy, I.; Allemand, D.; Ferrier-Pagès, C. Effect of light and feeding on the nitrogen isotopic composition of a zooxanthellate coral: Role of nitrogen recycling. Mar. Ecol. Prog. Ser. 2009, 392, 103–110.
More
Information
Subjects:
Marine & Freshwater Biology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
3.3K
Entry Collection:
Environmental Sciences
Revisions:
2 times
(View History)
Update Date:
28 Feb 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




