Cassiopea andromeda entered the Mediterranean from the Red Sea through the Suez Canal and colonized several areas of the basin. This species is an epibenthic scyphozoan with a maximum umbrella diameter of about 30 cm commonly found in tropical and subtropical shallow coastal ecosystems such as mangroves, estuaries, and sandy mudflats. This species has a metagenetic cycle with the following phases: planula, benthic polyp, ephyra, and adult medusa. The symbiotic relationship with dinoflagellates allows the jellyfish species to feed via direct predation and through photosynthesis by the zooxanthellae (mixotrophy).
- non-indigenous species
- upside-down jellyfish
- Megabenthos Underwater Video
- species distribution
- stable isotopes
- mixotrophic behavior
1. Introduction
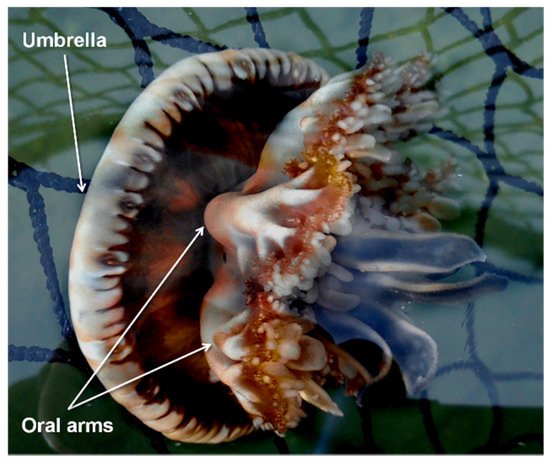
2. Distribution of Cassiopea andromeda
3. Trophic Behavior of Cassiopea andromeda
This entry is adapted from the peer-reviewed paper 10.3390/biology11020319
References
- Katsanevakis, S.; Wallentinus, I.; Zenetos, A.; Leppäkoski, E.; Çinar, M.E.; Oztürk, B.; Grabowski, M.; Golani, D.; Cardoso, A.N. Impacts of Invasive Alien Marine Species on Ecosystem Services and Biodiversity: A Pan-European Review. Aquat. Invasions 2014, 9, 391–423.
- Bianchi, C.N. Biodiversity issues for the forthcoming tropical Mediterranean Sea. Hydrobiologia 2007, 580, 7.
- Parravicini, V.; Mangialajo, L.; Mousseau, L.; Peirano, A.; Morri, C.; Montefalcone, M.; Francour, P.; Kulbicki, M.; Bianchi, C.N. Climate change and warm-water species at the north-western boundary of the Mediterranean Sea. Mar. Ecol. 2015, 36, 897–909.
- Lasram, B.F.; Tomasini, J.A.; Guilhaumon, F.; Romdhane, M.S.; Do Chi, T.; Mouillot, D. Ecological correlates of dispersal success of Lessepsian fishes. Mar. Ecol. Prog. Ser. 2008, 363, 273–286.
- Maggio, T.; Allegra, A.; Bosch-Belmar, M.; Cillari, T.; Cuttitta, A.; Falautano, M.; Milisenda, G.; Nicosia, A.; Perzia, P.; Sinopoli, M.; et al. Molecular identity of the non-indigenous species Cassiopea sp. from Palermo Harbour (central Mediterranean Sea). J. Mar. Biolog. Assoc. UK 2019, 99, 1765–1773.
- Maas, O. Die Scyphomedusen der Siboga-Expedition; Siboga-Expeditie, Buchhandlung und Druckerei Vormals, EJ Brill: Leiden, The Netherlands, 1903; Volume 11, pp. 1–91.
- Stampar, S.N.; Gamero-Mora, E.; Maronna, M.M.; Fritscher, J.M.; Oliveira, B.S.P.; Sampaio, C.L.S.; Morandini, A.C. The puzzling occurrence of the upside-down jellyfish Cassiopea (Cnidaria: Scyphozoa) along the Brazilian coast: A result of several invasion events? Zoologia 2020, 37, 1–10.
- Stoner, E.W.; Layman, C.A.; Yeager, L.A.; Hassett, H.M. Effects of anthropogenic disturbance on the abundance and size of epibenthic jellyfish Cassiopea spp. Mar. Pollut. Bull. 2011, 62, 1109–1114.
- Heins, A.; Glatzel, T.; Holst, S. Revised descriptions of the nematocysts and the asexual reproduction modes of the scyphozoan jellyfish Cassiopea andromeda (Forsskål, 1775). Zoomorphology 2015, 134, 351–366.
- Morandini, A.C.; Stampar, S.N.; Maronna, M.M.; Da Silveira, F.L. All non-indigenous species were introduced recently? The case study of Cassiopea (Cnidaria: Scyphozoa) in Brazilian waters. J. Mar. Biol. Assoc. UK 2017, 97, 321–328.
- Ohdera, A.H.; Abrams, M.J.; Ames, C.L.; Baker, D.M.; Suescún-Bolívar, L.P.; Collins, A.G.; Freeman, C.J.; Gamero-Mora, E.; Goulet, T.L.; Hofmann, D.K.; et al. Upside-down but headed in the right direction: Review of the highly versatile Cassiopea xamachana system. Front. Ecol. Evol. 2018, 6, 1–15.
- Kremer, P. Ingestion and elemental budgets for Linuche unguiculata, a scyphomedusa with zooxanthellae. J. Mar. Biol. Assoc. UK 2005, 85, 613–625.
- Welsh, D.T.; Dunn, R.J.; Meziane, T. Oxygen and nutrient dynamics of the upside down jellyfish (Cassiopea sp.) and its influence on benthic nutrient exchanges and primary production. Hydrobiologia 2009, 635, 351–362.
- Kremer, P.; Costello, J.; Kremer, J.; Canino, M. Significance of photosynthetic endosymbionts to the carbon budget of the scyphomedus Linuche unguiculata. Limnol. Oceanogr. 1990, 35, 609–624.
- Kikinger, R. Cotylorhiza tuberculata (Cnidaria: Scyphozoa)—Life history of a stationary population. Mar. Ecol. 1992, 13, 333–362.
- McCloskey, L.R.; Muscatine, L.; Wilkerson, F.P. Daily photosynthesis, respiration, and carbon budgets in a tropical marine jellyfish (Mastigias sp.). Mar. Biol. 1994, 119, 13–22.
- Verde, E.A.; McCloskey, L.R. Production, respiration, and photophysiology of the mangrove jellyfish Cassiopea xamachana symbiotic with zooxanthellae: Effect of jellyfish size and season. Mar. Ecol. Prog. Ser. 1998, 168, 147–162.
- Sugiura, Y. On the life-history of Rhizostome medusae V. On the relation between zooxanthellae and the strobilation of Cephea cephea. Bull. Mar. Biol. Stn. Asamushi 1969, 13, 227–233.
- Bolton, T.F.; Graham, W.M. Morphological variation among populations of an invasive jellyfish. Mar. Ecol. Prog. Ser. 2004, 278, 125–139.
- Djeghri, N.; Stibor, H.; Lebeau, O.; Pondaven, P. δ13C, δ15N, and C:N ratios as nutrition indicators of zooxanthellate jellyfishes: Insights from an experimental approach. J. Exp. Mar. Biol. Ecol. 2020, 522, 151257.
- Todd, B.D.; Thornhill, D.J.; Fitt, W.K. Patterns of inorganic phosphate uptake in Cassiopea xamachana: A bioindicator species. Mar. Pollut. Bull. 2006, 52, 515–521.
- Muscatine, L.; Porter, J.W.; Kaplan, I.R. Resource partitioning by reef corals as determined from stable isotope composition I. δ13C of zooxanthellae and animal tissue versus depth. Mar. Biol. 1989, 100, 185–193.
- Muscatine, L.; Kaplan, I.R. Resource partitioning by reef corals as determined from stable isotope composition II. δ15N of zooxanthellae and animal tissue versus depth. Pac. Sci. 1994, 48, 304–312.
- Ferrier-Pagès, C.; Peirano, A.; Abbate, M.; Cocito, S.; Negri, A.; Rottier, C.; Riera, P.; Metalpa, R.; Reynaud, S. Summer autotrophy and winter heterotrophy in the temperate symbiotic coral Cladocora caespitosa. Limnol. Oceanogr. 2011, 56, 1429–1438.
- Yamamuro, M.; Kayanne, H.; Minagawa, M. Carbon and nitrogen stable isotopes of primary producers in coral reef ecosystems. Limnol. Oceanogr. 1995, 40, 617–621.
- Sammarco, P.W.; Risk, M.J.; Schwarcz, H.P.; Heikoop, J.M. Cross-continental shelf trend in coral δ15N on the Great Barrier Reef: Further consideration of the reef nutrient paradox. Mar. Ecol. Prog. Ser. 1999, 180, 131–138.
- Heikoop, J.M.; Dun, J.J.; Risk, M.J.; Tomascik, T.; Schwarz, H.P.; Sandeman, I.M.; Sammarco, P.W. Nitrogen-15 signals of anthropogenic nutrient loading in reef corals. Mar. Pollut. Bull. 2000, 40, 628–636.
- Hoegh-Guldberg, O.; Muscatine, L.; Goiran, C.; Siggaard, D.; Marion, G. Nutrient-induced perturbations to δ13C and δ15N in symbiotic dinoflagellates and their coral hosts. Mar. Ecol. Prog. Ser. 2004, 280, 105–114.
- Marion, G.S.; Hoegh-Guldberg, O.; McCulloch, M.T.; Jupiter, S.D. Coral isotopic records (δ15N) of unprecedented land-use stress in Great Barrier Reef coastal communities. EOS Trans. Am. Geophys. Union Ocean Sci. Meet. Suppl. 2006, 87.
- Heikoop, J.M.; Dun, J.J.; Risk, M.J.; McConnaughey, T.A.; Sandman, I.M. Separation of kinetic and metabolic effect in carbon-13 records preserved in reef coral skeletons. Geochim. Cosmochim. Acta 2000, 64, 975–987.
- Heikoop, J.M.; Dunn, J.J.; Risk, M.J.; Sandeman, I.M.; Schwartz, H.P.; Waltho, N. Relationship between light and the δ15N of coral tissue: Examples from Jamaica and Zanzibar. Limnol. Oceanogr. 1998, 43, 909–920.
- Rodrigues, L.; Grottoli, A. Calcification rate, and the stable carbon, oxygen and nitrogen isotopes in the skeleton, host tissue and zooxanthellae of bleached and recovering Hawaiian corals. Geochim. Cosmochim. Acta 2006, 70, 2781–2789.
- Lampert, K.P. Cassiopea and its zooxanthellae. In The Cnidaria, Past, Present and Future; Springer: Cham, Switzerland, 2016; pp. 415–423.
- Schäfer, W. Eine Qualle aus dem Indischen Ozean in der Agais. Nat. Volk 1955, 85, 241–245.
- Schembri, P.J.; Deidun, A.; Vella, P.J. First record of Cassiopea andromeda (Scyphozoa: Rhizostomeae: Cassiopeidae) from the central Mediterranean Sea. Mar. Biodivers. Rec. 2010, 3, E6.
- Cillari, T.; Andaloro, F.; Castriota, L. First documented record of Cassiopea cfr andromeda (Cnidaria: Scyphozoa) in Italian waters. Cah. Biol. Mar. 2018, 59, 193–195.
- Thé, J.; de Sousa Barroso, H.; Mammone, M.; Viana, M.; Melo, C.S.B.; Mies, M.; Banha, T.N.S.; Morandini, A.C.; Rossi, S.; de Oliveira Soares, M. Aquaculture facilities promote populational stability throughout seasons and increase medusae size for the invasive jellyfish Cassiopea andromeda. Mar. Environ. Res. 2020, 162, 105161.
- Cillari, T.; Allegra, A.; Bosch-Belmar, M.; Castriota, L.; Falautano, M.; Milisenda, G.; Maggio, T.; Perzia, P.; Sinopoli, M. Megabenthos Underwater Video (MUV): A new device to evaluate species distribution in hard-to-reach marine areas. In Proceedings of the International Workshop on Metrology for the Sea, Learning to Measure Sea Health Parameters (MetroSea), Virtual Conference, Milazzo, Italy, 4–6 October 2021; pp. 199–203.
- Mammone, M.; Ferrier-Pagés, C.; Lavorano, S.; Rizzo, L.; Piraino, S.; Rossi, S. High photosynthetic plasticity may reinforce invasiveness of upside-down zooxanthellate jellyfish in Mediterranean coastal waters. PLoS ONE 2021, 16, e0248814.
- Viana, I.G.; Bode, A.; Bartholomew, M.; Valiela, I. Experimental assessment of the macroalgae Ascophyllum nodosum and Fucus vesiculosus for monitoring N sources at different time-scales using stable isotope composition. J. Exp. Mar. Biol. Ecol. 2015, 466, 24–33.
- McClellend, J.; Valiela, I. Linking nitrogen in estuarian producers to land-derived sources. Limnol. Oceanogr. 1998, 43, 577–585.
- Risk, M. The reef crisis and the reef science crisis: Nitrogen isotopic ratios as an objective indicator of stress. Mar. Pollut. Bull. 2009, 58, 787–788.
- Risk, M.J.; Sherwood, O.A.; Nairn, R.; Gibbons, C. Tracking the record of sewage discharge off Jeddah, Saudi Arabia, since 1950, using stable isotope records from antipatharians. Mar. Ecol. Prog. Ser. 2009, 397, 219–226.
- Risk, M.J.; Erdmann, M.V. Isotopic composition of nitrogen in stomatopod (Crustacea) tissues as an indicator of human sewage impacts on Indonesian coral reefs. Mar. Pollut. Bull. 2000, 40, 50–58.
- Cifuentes, L.A.; Fogel, M.L.; Pennock, J.R.; Sharp, J.H. Biogeochemical factors that influence the stable nitrogen isotope ratio of dissolved ammonium in the Delaware estuary. Geochim. Cosmochim. Acta 1989, 53, 2713–2721.
- Montoya, P.; Korrigan, S.G.; McCarthy, J.J. Rapid, storm-induced changes in the natural abundance of 15N in a planktonic ecosystem, Chesapeake Bay, USA. Geochim. Cosmochim. Acta 1991, 55, 3627–3638.
- Fogel, M.L.; Cifuentes, L.A. Isotope fractionation during primary production. In Organic Geochemistry; Engel, M.H., Macko, S.A., Eds.; Springer: Boston, MA, USA, 1993; Volume 11, pp. 73–98.
- Pennock, J.R.; Velinsky, D.J.; Ludlam, J.M.; Sharp, J.H.; Fogel, M.L. Isotopic fractionation of ammonium and nitrate during uptake by Skeletonema costatum: Implications for δ15N dynamics under bloom conditions. Limnol. Oceanogr. 1996, 41, 451–459.
- Waser, N.A.D.; Harrison, P.J.; Nielsen, B.; Calvert, S.E.; Turpin, D.H. Nitrogen isotope fractionation during the uptake and assimilation of nitrate, nitrite, ammonium, and urea by a marine diatom. Limnol. Oceanogr. 1998, 43, 215–224.
- Granger, J.; Sigman, D.M.; Needoba, J.A.; Harrison, P.J. Coupled nitrogen and oxygen isotope fractionation of nitrate during assimilation by cultures of marine phytoplankton. Limnol. Oceanogr. 2004, 49, 1763–1773.
- Ferrier-Pagès, C.; Leal, M.C. Stable isotopes as tracers of trophic interactions in marine mutualistic symbioses. Ecol. Evol. 2018, 9, 723–740.
- Freeman, C.J.; Stoner, E.W.; Easson, C.G.; Matterson, K.O.; Baker, D.M. Symbiont carbon and nitrogen assimilation in the Cassiopea–Symbiodinium mutualism. Mar. Ecol. Prog. Ser. 2016, 544, 281–286.
- Freeman, C.J.; Stoner, E.W.; Easson, C.G.; Matterson, K.O.; Baker, D.M. Variation in δ13C and δ15N values suggests a coupling of host and symbiont metabolism in the Symbiodinium-Cassiopea mutualism. Mar. Ecol. Prog. Ser. 2017, 571, 245–251.
- Cravotta, C.A. Use of Stable Isotopes of Carbon, Nitrogen, and Sulfur to Identify Sources of Nitrogen in Surface Waters in the Lower Susquehanna River Basin, Pennsylvania; U.S. Department of the Interior, U.S. Geological Survey: Denver, CO, USA, 2002; p. 99.
- Elliott, E.M.; Kendall, C.; Burns, D.A.; Boyer, E.W.; Harlin, K.; Wankel, S.D.; Butler, T.J.; Carlton, R. Nitrate isotopes in precipitation to distinguish NOx sources, atmospheric processes, and source areas in the United States. In AGU Fall Meeting Abstracts; American Geophysical Union: San Francisco, CA, USA, 2006; Volume 2007, abstract id. H52B-01.
- Hastings, M.G.; Sigman, D.M.; Lipschultz, F. Isotopic evidence for source changes of nitrate in rain at Bermuda. J. Geophys. Res. 2003, 108, 4790.
- Hastings, M.G. Studies of Reactive Nitrogen in the Atmosphere Using Global Modelling and Stable Isotope Measurements. Ph.D. Thesis, Princeton University, Princeton, NJ, USA, 2004; 217p.
- Arai, M.N. A Functional Biology of the Scyphozoa; Chapman & Hall: London, UK, 1997; p. 300.
- Balderston, W.L.; Claus, G. Study of the symbiotic relationship between Symbiodinium microadriaticum Freudenthal, a Zooxanthella and the upside down jellyfish, Cassiopea sp. Nova Hedwigia 1969, 17, 373–382.
- Post, D.M. Using stable isotopes to estimate trophic position: Models, methods, and assumptions. Ecology 2002, 83, 703–718.
- Swart, P.K.; Saied, A.; Lamb, K. Temporal and spatial variation in the δ15N and δ13C of coral tissue and zooxanthellae in Montastraea faveolata collected from the Florida reef tract. Limnol. Oceanogr. 2005, 50, 1049–1058.
- Alamaru, A.; Loya, Y.; Brokovich, E.; Yam, R.; Shemesh, A. Carbon and nitrogen utilization in two species of Red Sea corals along a depth gradient: Insights from stable isotope analysis of total organic material and lipids. Geochim. Cosmochim. Acta 2009, 73, 5333–5342.
- Reynaud, S.; Martinez, P.; Houlbrèque, F.; Billy, I.; Allemand, D.; Ferrier-Pagès, C. Effect of light and feeding on the nitrogen isotopic composition of a zooxanthellate coral: Role of nitrogen recycling. Mar. Ecol. Prog. Ser. 2009, 392, 103–110.
