
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Cyril Fersing | + 3631 word(s) | 3631 | 2022-02-24 10:19:21 | | | |
| 2 | Lindsay Dong | -1 word(s) | 3630 | 2022-02-25 03:35:18 | | | | |
| 3 | Lindsay Dong | Meta information modification | 3630 | 2022-02-28 08:02:05 | | |
Video Upload Options
The chelating agent AAZTA features a mesocyclic seven-membered diazepane ring, conferring some of the properties of both acyclic and macrocyclic chelating agents. Described in the early 2000s, AAZTA and its derivatives exhibited interesting properties once complexed with metals and radiometals, combining a fast kinetic of formation with a slow kinetic of dissociation. Importantly, the extremely short coordination reaction times allowed by AAZTA derivatives were particularly suitable for short half-life radioelements (i.e., 68Ga).
1. Introduction
2. Design, Synthesis, and Kinetic Properties
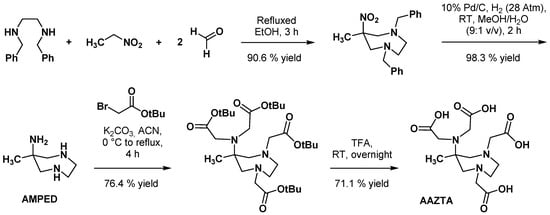
2.1. Original AAZTA Derivatives
2.2. AAZTA Modulations to Improve Coordination Behavior
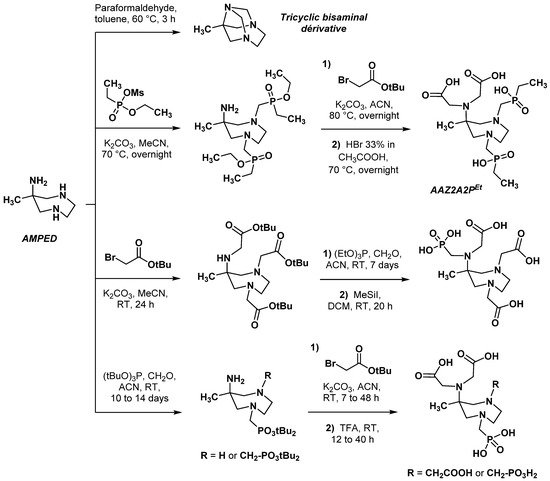
2.2.1. Modifications in the Number and Nature of Coordinating Side Arms
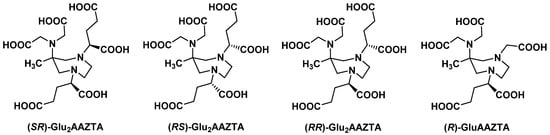
2.2.2. Backbone Rigidification
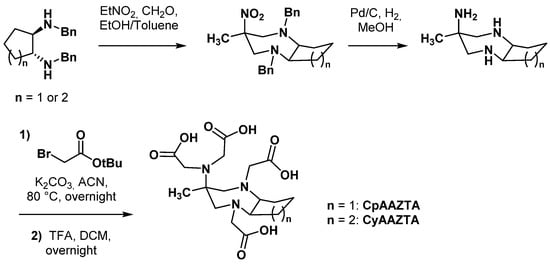
2.3. AAZTA Modulations to Obtain Bifunctional Chelating Agents (BCA)
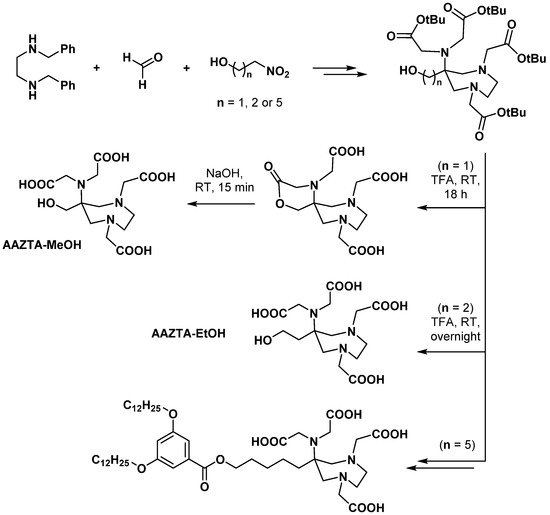
2.4. AAZ3A Derivatives
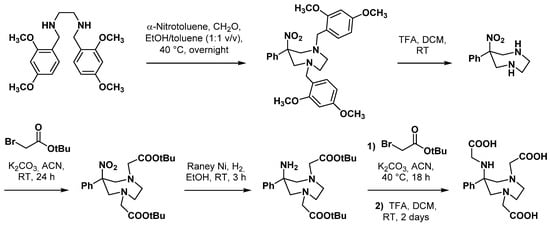
3. Applications in Nuclear Medicine
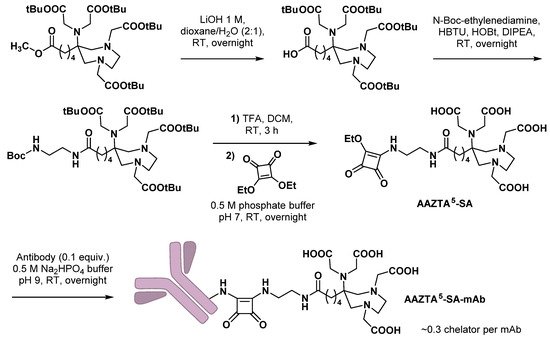
3.1. Monoclonal Antibodies
3.2. Small Molecules
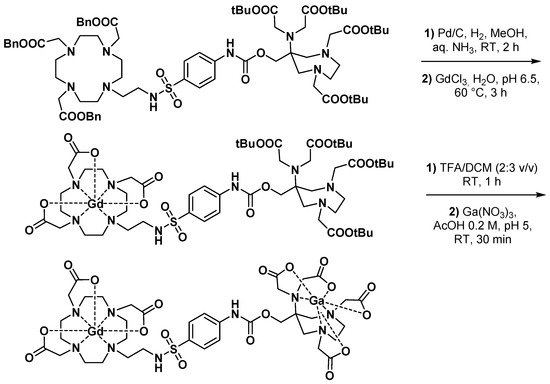
3.2.1. Dual MRI/PET Imaging Agents
3.2.2. Bisphosphonate Derivatives
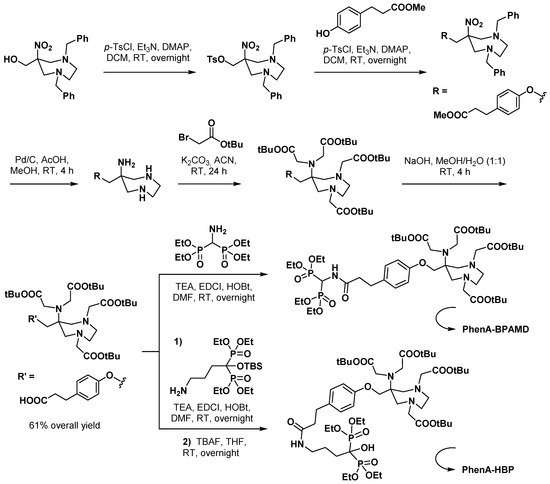
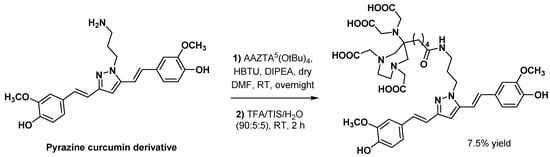
3.2.3. Curcumin Derivatives
3.2.4. Prostate-Specific Antigen Ligands
Sinnes et al. explored the influence of the chelator part on the in vitro characteristics of a PSMA vector molecule by replacing DOTA with AAZTA5 in PSMA-617 [36]. The resulting AAZTA5-PSMA-617 (Figure 2) was successfully radiolabeled with 68Ga, 44Sc, and 177Lu at RT within 5 min, reaching >99% RCY for a chelator-to-radiometal molar ratio = 10:1. Both [68Ga]Ga- and [44Sc]Sc-AAZTA5-PSMA-617 were extremely stable in human serum, PBS, and EDTA/DTPA in PBS (>90% RCP over 2 h for the 68Ga derivative and >95% RCP over 8 h for the 44Sc derivative).
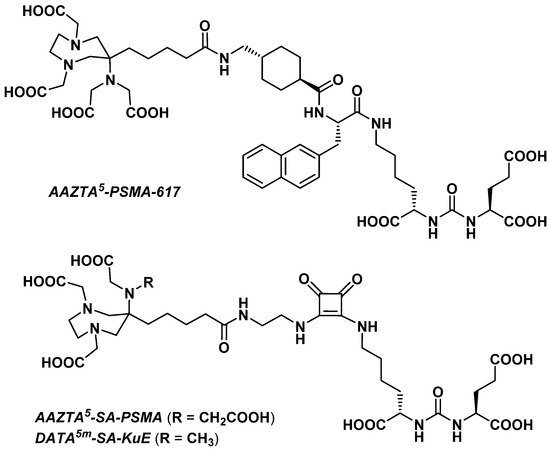
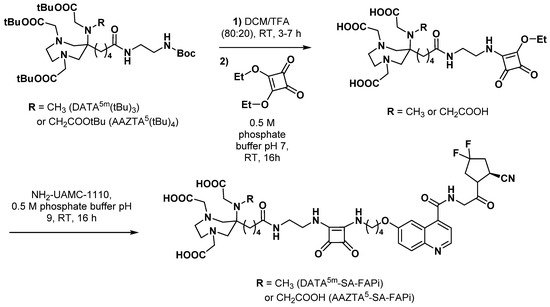
3.2.5. Fibroblast Activation Protein Inhibitors
3.3. Peptides
3.3.1. RGD Peptides
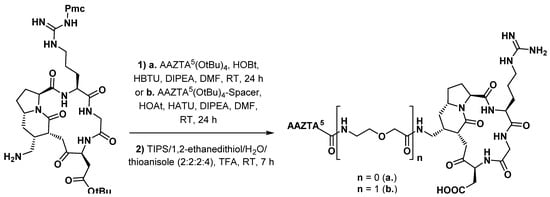
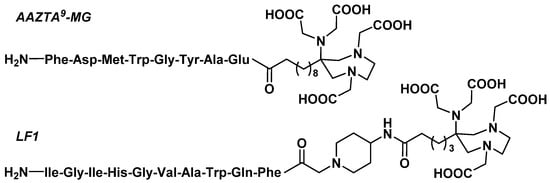
3.3.2. Gastrin Analogues
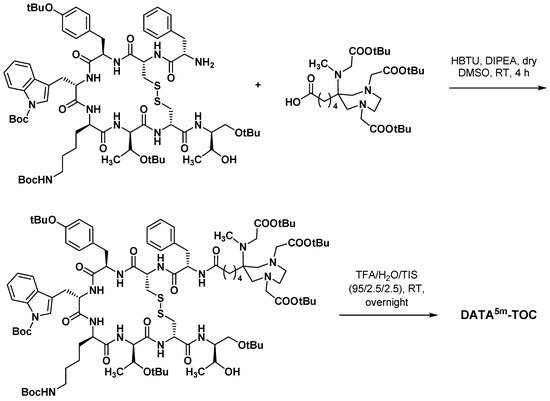
3.3.3. Octreotide Analogues
4. Conclusions
References
- Kelkar, S.S.; Reineke, T.M. Theranostics: Combining imaging and therapy. Bioconjug. Chem. 2011, 22, 1879–1903.
- Cai, Y.; Chen, X.; Si, J.; Mou, X.; Dong, X. All-in-one nanomedicine: Multifunctional single-component nanoparticles for cancer theranostics. Small 2021, 17, 2103072.
- Kharbikar, B.N.; Zhong, J.X.; Cuylear, D.L.; Perez, C.A.; Desai, T.A. Theranostic biomaterials for tissue engineering. Curr. Opin. Biomed. Eng. 2021, 19, 100299.
- Langbein, T.; Weber, W.A.; Eiber, M. Future of theranostics: An outlook on precision oncology in Nuclear Medicine. J. Nucl. Med. 2019, 60, 13S–19S.
- Hertz, S. Radioactive iodine in the study of thyroid physiology: VII. The use of radioactive iodine therapy in hyperthyroidism. JAMA 1946, 131, 81–86.
- Seidlin, S.M. Radioactive iodine therapy: Effect on functioning metastases of adenocarcinoma of the thyroid. JAMA 1946, 132, 838–847.
- Strosberg, J.R.; Caplin, M.E.; Kunz, P.L.; Ruszniewski, P.B.; Bodei, L.; Hendifar, A.; Mittra, E.; Wolin, E.M.; Yao, J.C.; Pavel, M.E.; et al. 177Lu-Dotatate plus long-acting octreotide versus high-dose long-acting octreotide in patients with midgut neuroendocrine tumours (NETTER-1): Final overall survival and long-term safety results from an open-label, randomised, controlled, phase 3 trial. Lancet Oncol. 2021, 22, 1752–1763.
- Khreish, F.; Ghazal, Z.; Marlowe, R.J.; Rosar, F.; Sabet, A.; Maus, S.; Linxweiler, J.; Bartholomä, M.; Ezziddin, S. 177Lu-PSMA-617 radioligand therapy of metastatic castration-resistant prostate cancer: Initial 254-patient results from a prospective registry (REALITY Study). Eur. J. Nucl. Med. Mol. Imaging 2021, in press.
- Sartor, O.; de Bono, J.; Chi, K.N.; Fizazi, K.; Herrmann, K.; Rahbar, K.; Tagawa, S.T.; Nordquist, L.T.; Vaishampayan, N.; El-Haddad, G.; et al. Lutetium-177–PSMA-617 for metastatic castration-resistant prostate cancer. N. Engl. J. Med. 2021, 385, 1091–1103.
- Mikolajczak, R.; Huclier-Markai, S.; Alliot, C.; Haddad, F.; Szikra, D.; Forgacs, V.; Garnuszek, P. Production of scandium radionuclides for theranostic applications: Towards standardization of quality requirements. EJNMMI Radiopharm. Chem. 2021, 6, 19.
- Price, E.W.; Orvig, C. Matching chelators to radiometals for radiopharmaceuticals. Chem. Soc. Rev. 2014, 43, 260–290.
- Aime, S.; Calabi, L.; Cavallotti, C.; Gianolio, E.; Giovenzana, G.B.; Losi, P.; Maiocchi, A.; Palmisano, G.; Sisti, M. : A new structural entry for an improved generation of MRI contrast agents. Inorg. Chem. 2004, 43, 7588–7590.
- Baranyai, Z.; Uggeri, F.; Giovenzana, G.B.; Bényei, A.; Brücher, E.; Aime, S. Equilibrium and kinetic properties of the lanthanoids(III) and various divalent metal complexes of the heptadentate ligand AAZTA. Chem. Eur. J. 2009, 15, 1696–1705.
- Mathias, C.J.; Sun, Y.; Welch, M.J.; Connett, J.M.; Philpott, G.W.; Martell, A.E. N,N′-bis(2-hydroxybenzyl)-1-(4-bromoacetamidobenzyl)-1,2-ethylene-diamine-N,N′-diacetic acid: A new bifunctional chelate for radio-labeling antibodies. Bioconjug. Chem. 1990, 1, 204–211.
- Eder, M.; Wängler, B.; Knackmuss, S.; LeGall, F.; Little, M.; Haberkorn, M.; Mier, W.; Eisenhut, M. Tetrafluorophenolate of HBED-CC: A versatile conjugation agent for 68Ga-labeled small recombinant antibodies. Eur. J. Nucl. Med. Mol. Imaging 2008, 35, 1878–1886.
- Hennrich, U.; Eder, M. Ga-PSMA-11: The first FDA-approved 68Ga-radiopharmaceutical for PET imaging of prostate cancer. Pharmaceuticals 2021, 14, 713.
- Ermelindo, A.; Gambino, G.; Tei, L. Synthesis of a mixed carboxylate–phosphinate AAZTA-like ligand and relaxometric characterization of its GdIII complex. Tetrahedron Lett. 2013, 54, 6378–6380.
- Elemento, E.M.; Parker, D.; Aime, S.; Gianolio, E.; Lattuada, L. Variation of water exchange dynamics with ligand structure and stereochemistry in lanthanide complexes based on 1,4-diazepine derivatives. Org. Biomol. Chem. 2009, 7, 1120–1131.
- Guanci, C.; Pinalli, R.; Aime, S.; Gianolio, E.; Lattuada, L.; Giovenzana, G.B. Synthesis of phosphonic analogues of AAZTA and relaxometric evaluation of the corresponding Gd(III) complexes as potential MRI contrast agents. Tetrahedron Lett. 2015, 56, 1994–1997.
- Camera, L.; Kinuya, S.; Garmestani, K.; Wu, C.; Brechbiel, M.W.; Pai, L.H.; McMurry, T.J.; Gansow, O.A.; Pastan, I.; Paik, C.H. Evaluation of the serum stability and in vivo biodistribution of CHX-DTPA and other ligands for yttrium labeling of monoclonal antibodies. J. Nucl. Med. 1994, 35, 882–889.
- Stimmel, J.B.; Kull, F.C. Samarium-153 and lutetium-177 chelation properties of selected macrocyclic and acyclic ligands. Nucl. Med. Biol. 1998, 25, 117–125.
- Vágner, A.; D’Alessandria, C.; Gambino, G.; Schwaiger, M.; Aime, S.; Maiocchi, A.; Tóth, I.; Baranyai, Z.; Tei, L. A Rigidified AAZTA-like ligand as efficient chelator for 68Ga radiopharmaceuticals. ChemistrySelect 2016, 1, 163–171.
- Martinelli, J.; Martorana, E.; Tei, L. Synthesis of a rigidified bicyclic AAZTA-like ligand and relaxometric characterization of its GdIII complex. Tetrahedron Lett. 2020, 61, 152573.
- Rosa, L.D.; Romanelli, A.; D’Andrea, L.D. Introduction to chemical ligation reactions. In Chemical Ligation; D’Andrea, L.D., Romanelli, A., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2017; pp. 1–87. ISBN 978-1-119-04411-6.
- Spang, P.; Herrmann, C.; Roesch, F. Bifunctional gallium-68 chelators: Past, present, and future. Semin. Nucl. Med. 2016, 46, 373–394.
- Sengar, R.S.; Nigam, A.; Geib, S.J.; Wiener, E.C. Syntheses and crystal structures of gadolinium and europium complexes of AAZTA analogues. Polyhedron 2009, 28, 1525–1531.
- Gianolio, E.; Giovenzana, G.B.; Ciampa, A.; Lanzardo, S.; Imperio, D.; Aime, S. A novel method of cellular labeling: Anchoring MR-imaging reporter particles on the outer cell surface. ChemMedChem 2008, 3, 60–62.
- Bandoli, G.; Dolmella, A.; Tisato, F.; Porchia, M.; Refosco, F. Mononuclear six-coordinated Ga(III) complexes: A comprehensive survey. Coord. Chem. 2009, 253, 56–77.
- Parker, D.; Waldron, B.P. Conformational analysis and synthetic approaches to polydentate perhydro-diazepine ligands for the complexation of gallium(III). Org. Biomol. Chem. 2013, 11, 2827–2838.
- Klasen, B.; Moon, E.S.; Rösch, F. AAZTA5-Squaramide ester competing with DOTA-, DTPA- and CHX-A″-DTPA-analogues: Promising tool for 177Lu-labeling of monoclonal antibodies under mild conditions. Nucl. Med. Biol. 2021, 96, 80–93.
- Grus, T.; Lahnif, H.; Klasen, B.; Moon, E.-S.; Greifenstein, L.; Roesch, F. Squaric acid-based radiopharmaceuticals for tumor imaging and therapy. Bioconjug. Chem. 2021, 32, 1223–1231.
- Vologdin, N.; Rolla, G.A.; Botta, M.; Tei, L. Orthogonal synthesis of a heterodimeric ligand for the development of the GdIII–GaIII ditopic complex as a potential pH-sensitive MRI/PET probe. Org. Biomol. Chem. 2013, 11, 1683–1690.
- Lowe, M.P.; Parker, D.; Reany, O.; Aime, S.; Botta, M.; Castellano, G.; Gianolio, E.; Pagliarin, R. pH-Dependent modulation of relaxivity and luminescence in macrocyclic gadolinium and europium complexes based on reversible intramolecular sulfonamide ligation. J. Am. Chem. Soc. 2001, 123, 7601–7609.
- Wu, Z.; Zha, Z.; Choi, S.R.; Plössl, K.; Zhu, L.; Kung, H.F. New 68Ga-phenA bisphosphonates as potential bone imaging agents. Nucl. Med. Biol. 2016, 43, 360–371.
- Orteca, G.; Sinnes, J.-P.; Rubagotti, S.; Iori, M.; Capponi, P.C.; Piel, M.; Rösch, F.; Ferrari, E.; Asti, M. Gallium-68 and scandium-44 labelled radiotracers based on curcumin structure linked to bifunctional chelators: Synthesis and characterization of potential PET radiotracers. J. Inorg. Biochem. 2020, 204, 110954.
- Sinnes, J.-P.; Bauder-Wüst, U.; Schäfer, M.; Moon, E.S.; Kopka, K.; Rösch, F. 68Ga, 44Sc and 177Lu-labeled AAZTA5-PSMA-617: Synthesis, radiolabeling, stability and cell binding compared to DOTA-PSMA-617 analogues. EJNMMI Radiopharm. Chem. 2020, 5, 28.
- Ghiani, S.; Hawala, I.; Szikra, D.; Trencsényi, G.; Baranyai, Z.; Nagy, G.; Vágner, A.; Stefania, R.; Pandey, S.; Maiocchi, A. Synthesis, radiolabeling, and pre-clinical evaluation of Sc-AAZTA conjugate PSMA inhibitor, a new tracer for high-efficiency imaging of prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 2351–2362.
- Le Roux, J.; Kleynhans, J.; Rubow, S. The use of HEPES-buffer in the production of gallium-68 radiopharmaceuticals—Time to reconsider strict Pharmacopoeial limits? EJNMMI Radiopharm. Chem. 2021, 6, 15.
- Puré, E.; Blomberg, R. Pro-tumorigenic roles of fibroblast activation protein in cancer: Back to the basics. Oncogene 2018, 37, 4343–4357.
- Jansen, K.; Heirbaut, L.; Verkerk, R.; Cheng, J.D.; Joossens, J.; Cos, P.; Maes, L.; Lambeir, A.-M.; De Meester, I.; Augustyns, K.; et al. Extended structure–activity relationship and pharmacokinetic investigation of (4-quinolinoyl)glycyl-2-cyanopyrrolidine inhibitors of fibroblast activation protein (FAP). J. Med. Chem. 2014, 57, 3053–3074.
- Lindner, T.; Loktev, A.; Giesel, F.; Kratochwil, C.; Altmann, A.; Haberkorn, U. Targeting of activated fibroblasts for imaging and therapy. EJNMMI Radiopharm. Chem. 2019, 4, 16.
- Lindner, T.; Giesel, F.L.; Kratochwil, C.; Serfling, S.E. Radioligands targeting fibroblast activation protein (FAP). Cancers 2021, 13, 5744.
- Moon, E.S.; Elvas, F.; Vliegen, G.; De Lombaerde, S.; Vangestel, C.; De Bruycker, S.; Bracke, A.; Eppard, E.; Greifenstein, L.; Klasen, B.; et al. Targeting fibroblast activation protein (FAP): Next generation PET radiotracers using squaramide coupled bifunctional DOTA and DATA5m chelators. EJNMMI Radiopharm. Chem. 2020, 5, 19.
- Moon, E.S.; Van Rymenant, Y.; Battan, S.; De Loose, J.; Bracke, A.; Van der Veken, P.; De Meester, I.; Rösch, F. In vitro evaluation of the squaramide-conjugated fibroblast activation protein inhibitor-based agents AAZTA5.SA.FAPi and DOTA.SA.FAPi. Molecules 2021, 26, 3482.
- Krenning, E.P.; Breeman, W.A.P.; Kooij, P.P.M.; Lameris, J.S.; Bakker, W.H.; Koper, J.W.; Ausema, L.; Reubi, J.C.; Lamberts, S.W.J. Localisation of endocrine-related tumours with radioiodinated analogue of somatostatin. Lancet 1989, 333, 242–244.
- Bakker, W.H.; Albert, R.; Bruns, C.; Breeman, W.A.P.; Hofland, L.J.; Marbach, P.; Pless, J.; Pralet, D.; Stolz, B.; Koper, J.W.; et al. -Octreotide, a potential radiopharmaceutical for imaging of somatostatin receptor-positive tumors: Synthesis, radiolabeling and In vitro validation. Life Sci. 1991, 49, 1583–1591.
- Otte, A.; Jermann, E.; Behe, M.; Goetze, M.; Bucher, H.C.; Roser, H.W.; Heppeler, A.; Mueller-Brand, J.; Maecke, H.R. DOTATOC: A powerful new tool for receptor-mediated radionuclide therapy. Eur. J. Nucl. Med. 1997, 24, 792–795.
- Reubi, J.C.; Schär, J.C.; Waser, B.; Wenger, S.; Heppeler, A.; Schmitt, J.S.; Mäcke, H.R. Affinity profiles for human somatostatin receptor subtypes SST1-SST5 of somatostatin radiotracers selected for scintigraphic and radiotherapeutic use. Eur. J. Nucl. Med. 2000, 27, 273–282.
- Wild, D.; Schmitt, J.S.; Ginj, M.; Mäcke, H.R.; Bernard, B.F.; Krenning, E.; de Jong, M.; Wenger, S.; Reubi, J.-C. DOTA-NOC, a high-affinity ligand of somatostatin receptor subtypes 2, 3 and 5 for labelling with various radiometals. Eur. J. Nucl. Med. Mol. Imaging 2003, 30, 1338–1347.
- Manzoni, L.; Belvisi, L.; Arosio, D.; Bartolomeo, M.P.; Bianchi, A.; Brioschi, C.; Buonsanti, F.; Cabella, C.; Casagrande, C.; Civera, M.; et al. Synthesis of Gd and 68Ga complexes in conjugation with a conformationally optimized RGD sequence as potential MRI and PET tumor-imaging probes. ChemMedChem 2012, 7, 1084–1093.
- Reubi, J.C. Targeting CCK receptors in human cancers. Curr. Top. Med. Chem. 2007, 7, 1239–1242.
- Reubi, J.C.; Waser, B. Concomitant expression of several peptide receptors in neuroendocrine tumours: Molecular basis for in vivo multireceptor tumour targeting. Eur. J. Nucl. Med. Mol. Imaging 2003, 30, 781–793.
- Klingler, M.; Hörmann, A.A.; Guggenberg, E.V. Cholecystokinin-2 receptor targeting with radiolabeled peptides: Current status and future directions. Curr. Med. Chem. 2020, 27, 7112–7132.
- Pfister, J.; Summer, D.; Rangger, C.; Petrik, M.; von Guggenberg, E.; Minazzi, P.; Giovenzana, G.B.; Aloj, L.; Decristoforo, C. Influence of a novel, versatile bifunctional chelator on theranostic properties of a minigastrin analogue. EJNMMI Res. 2015, 5, 74.
- Eychenne, R.; Bouvry, C.; Bourgeois, M.; Loyer, P.; Benoist, E.; Lepareur, N. Overview of radiolabeled somatostatin analogs for cancer imaging and therapy. Molecules 2020, 25, 4012.
- Bombardieri, E.; Ambrosini, V.; Aktolun, C.; Baum, R.P.; Bishof-Delaloye, A.; Del Vecchio, S.; Maffioli, L.; Mortelmans, L.; Oyen, W.; Pepe, G.; et al. 111In-Pentetreotide scintigraphy: Procedure guidelines for tumour imaging. Eur. J. Nucl. Med. Mol. Imaging 2010, 37, 1441–1448.
- Sinnes, J.-P.; Nagel, J.; Rösch, F. AAZTA5/AAZTA5-TOC: Synthesis and radiochemical evaluation with 68Ga, 44Sc and 177Lu. EJNMMI Radiopharm. Chem. 2019, 4, 18.
- Seemann, J.; Waldron, B.; Parker, D.; Roesch, F. DATATOC: A novel conjugate for kit-type 68Ga labelling of TOC at ambient temperature. EJNMMI Radiopharm. Chem. 2016, 1, 4.
- Giovenzana, G.B.; Palmisano, G.; Sisti, M.; Cavallotti, C.; Aime, S.; Calabi, L. Multidentate Aza Ligands Able to Complex Metal Ions and the Use Thereof in Diagnostics and Therapy. International Patent Application No. WO2003008390A1, 30 January 2003.




