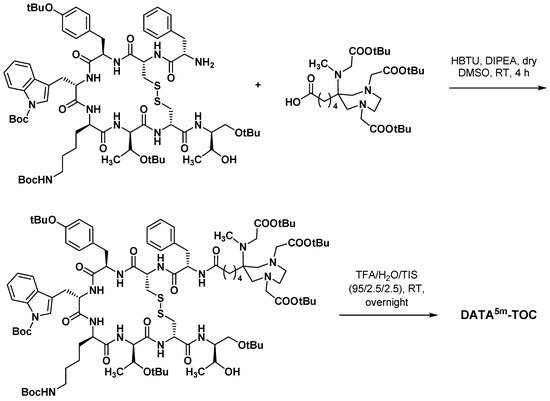
The chelating agent AAZTA features a mesocyclic seven-membered diazepane ring, conferring some of the properties of both acyclic and macrocyclic chelating agents. Described in the early 2000s, AAZTA and its derivatives exhibited interesting properties once complexed with metals and radiometals, combining a fast kinetic of formation with a slow kinetic of dissociation. Importantly, the extremely short coordination reaction times allowed by AAZTA derivatives were particularly suitable for short half-life radioelements (i.e., 68Ga).
- AAZTA
- bifunctional chelator
- radiopharmaceuticals
- theranostics
- nuclear medicine
1. Introduction
2. Design, Synthesis, and Kinetic Properties
2.1. Original AAZTA Derivatives
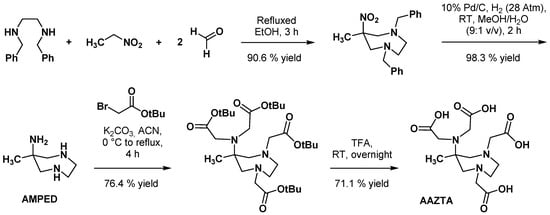
2.2. AAZTA Modulations to Improve Coordination Behavior
2.2.1. Modifications in the Number and Nature of Coordinating Side Arms
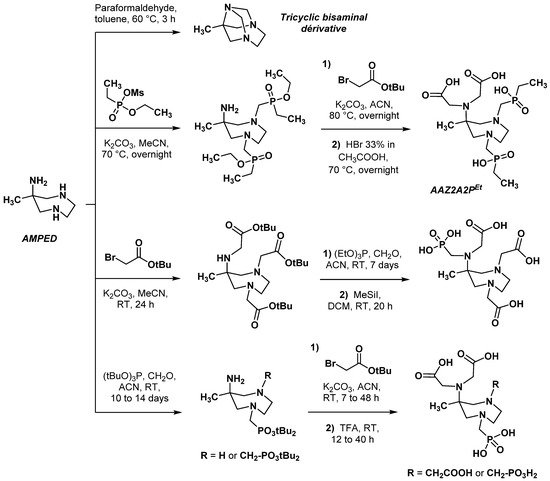
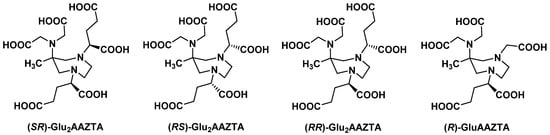
2.2.2. Backbone Rigidification
2.3. AAZTA Modulations to Obtain Bifunctional Chelating Agents (BCA)
2.4. AAZ3A Derivatives
3. Applications in Nuclear Medicine
3.1. Monoclonal Antibodies
Bifunctional chelating agents compatible with mild radiolabeling reaction conditions are particularly needed for monoclonal antibodies (mAbs) functionalization, as mAbs are heat- and pH-sensitive macromolecules. In this view, Klasen et al. combined an AAZTA5 scaffold with a squaramide ester motive and coupled this BCA to a model antibody (bevacizumab) [30][60]. This ligation technique, based on the pH-dependent reactivity of squaric acid diethyl ester, is increasingly used in the design of new radiopharmaceuticals [31][61]. Starting from protected AAZTA5, methyl ester could be selectively hydrolyzed with lithium hydroxide and the resulting carboxylic acid further coupled with N-Boc-ethylenediamine. After deprotection of both tert-butyl-ester- and Boc-protecting groups, the resulting compound was immediately coupled with squaric acid (SA) diethyl ester. Finally, bioconjugation with bevacizumab was conducted in mild conditions (Scheme 610).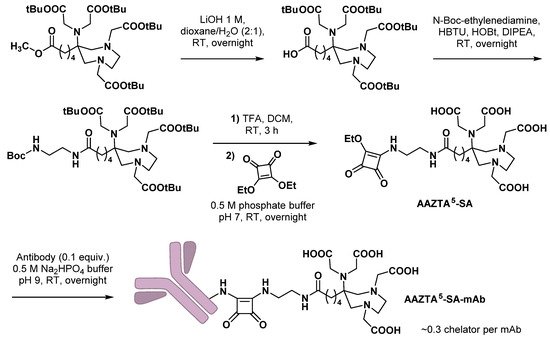
3.2. Small Molecules
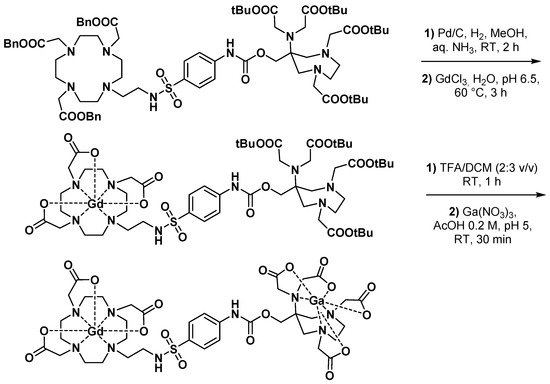
3.2.1. Dual MRI/PET Imaging Agents
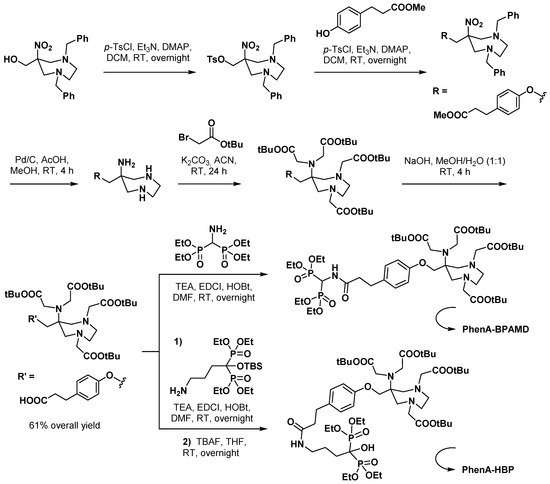
3.2.2. Bisphosphonate Derivatives
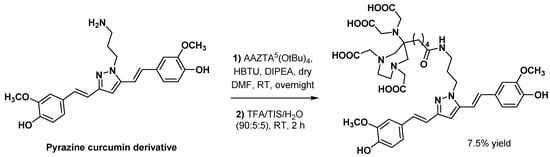
3.2.3. Curcumin Derivatives
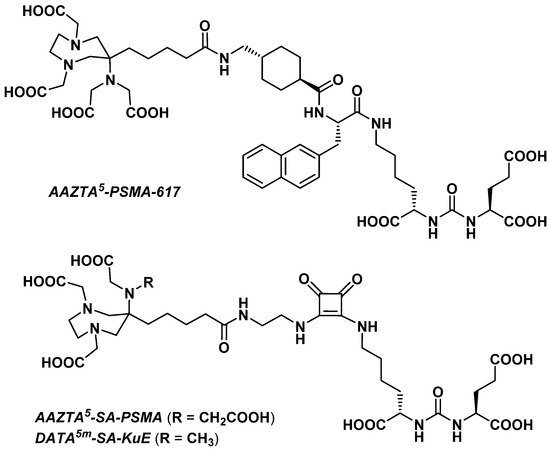
3.2.4. Prostate-Specific Antigen Ligands
Sinnes et al. explored the influence of the chelator part on the in vitro characteristics of a PSMA vector molecule by replacing DOTA with AAZTA5 in PSMA-617 [36][72]. The resulting AAZTA5-PSMA-617 (Figure 24) was successfully radiolabeled with 68Ga, 44Sc, and 177Lu at RT within 5 min, reaching >99% RCY for a chelator-to-radiometal molar ratio = 10:1. Both [68Ga]Ga- and [44Sc]Sc-AAZTA5-PSMA-617 were extremely stable in human serum, PBS, and EDTA/DTPA in PBS (>90% RCP over 2 h for the 68Ga derivative and >95% RCP over 8 h for the 44Sc derivative).
3.2.5. Fibroblast Activation Protein Inhibitors
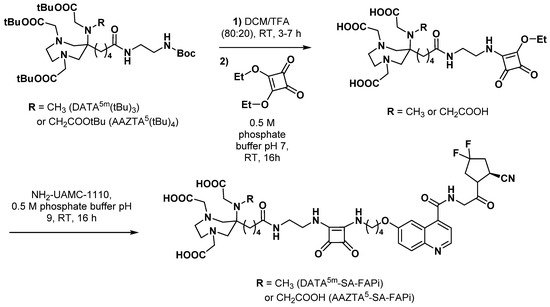
3.3. Peptides
3.3.1. RGD Peptides
Functionalization of a peptide with AAZTA5 was first described by Manzoni et al. [50][90], who prepared about ten RGD peptidomimetic conjugates bearing different chelating groups. Modified integrin αVβ3 ligand DB58, incorporating an azide group, was chosen for functionalization. After hydrogenation with Pd/C to get the corresponding amine, it was coupled to either AAZTA5(OtBu)4 or its spacer-containing analogue, using standard amide bond formation procedures (Scheme 115).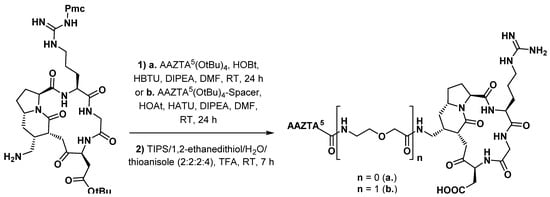
3.3.2. Gastrin Analogues
3.3.3. Octreotide Analogues