
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Michael Davies | + 2966 word(s) | 2966 | 2021-12-29 03:42:50 | | | |
| 2 | Lindsay Dong | -4 word(s) | 2962 | 2022-02-24 05:09:27 | | |
Video Upload Options
Covalent crosslinks within or between proteins play a key role in determining the structure and function of proteins. Some of these are formed intentionally by either enzymatic or molecular reactions and are critical to normal physiological function. Others are generated as a consequence of exposure to oxidants (radicals, excited states or two-electron species) and other endogenous or external stimuli, or as a result of the actions of a number of enzymes (e.g., oxidases and peroxidases). Increasing evidence indicates that the accumulation of unwanted crosslinks, as is seen in ageing and multiple pathologies, has adverse effects on biological function.
1. Introduction
2. Enzymatic Protein Crosslinking
Multiple enzymes can mediate the crosslinking of proteins, with a few key examples briefly summarized below. Enzyme-generated crosslinks are critical to the formation of many three-dimensional structures as these provide strength and rigidity, if biologically required. Examples include crosslinks formed within the extracellular matrix (ECM) of most, if not all, tissues, such as those formed between matrix proteins, and particularly collagens by the copper-containing lysyl oxidase (LOX) and LOX-like (LOXL) enzymes [5]. LOX oxidizes specific lysine (Lys) and hydroxylysine residues to carbonyls that undergo subsequent reactions to crosslink collagens (e.g., types I and III) and elastin [5][6][7][8]. In contrast, the LOXL family of enzymes acts on collagen type IV and drives the assembly of basement membranes [5][9]. Other enzymes also contribute to collagen crosslinking in the ECM with peroxidasin, a member of the heme peroxidase superfamily, mediating the formation of highly specific methionine (Met) to Lys crosslinks within the NC1 domains on collagen via generation of the oxidant hypobromous acid (HOBr). This species reacts rapidly with the Met residue to form an intermediate that then reacts with a suitably positioned Lys residue [10][11] (see also below). This type of crosslinking has been reported across many species [12]. Other members of the peroxidase superfamilies (e.g., horseradish peroxidase, myeloperoxidase, laccase) can also generate crosslinks via enzyme-mediated oxidation of substrates to radicals which then undergo radical–radical coupling. A classic example is oxidative coupling of Tyr and a wide range of other phenols via phenoxyl radical generation [13][14][15].
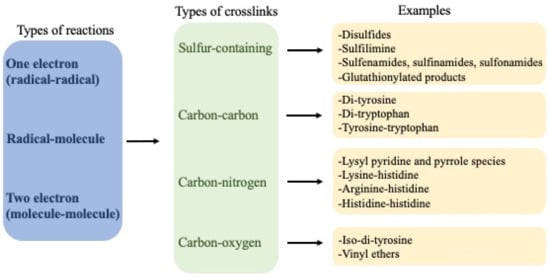
3. One-Electron (Radical–Radical) Reactions
Dimerization of two radicals to form a new covalent bond is typically a very fast process due to the low energy barriers for such reactions. Therefore, they are a major source of crosslinks in peptides and proteins when the radical flux is high and there are limited competing reactions. Most carbon-centered protein radicals (P•) formed from aliphatic side-chains by hydrogen–atom abstraction reactions react rapidly with O2 at diffusion-controlled rates (k ~ 109 M−1 s−1) to give peptide or protein peroxyl radicals (P-OO•) [16]. The rapidity of these reactions limits direct reactions of two P•, except in circumstances where the O2 concentration is low. This is of biological relevance, as hypoxia is a common phenomenon, with endogenous levels of O2 being typically in the range 3–70 μM [17]. However, lower concentrations are present in situations where demand is great (e.g., high metabolic rates) or perfusion is poor (e.g., in the core of many solid tumors), thereby limiting P-OO• formation and allowing (P-P) dimer formation [18]. For the limited number of P•, where reaction with O2 is slow or modest, as is the case for Cys-derived thiyl radicals (RS•, k < 107 M−1 s−1 [19]), tryptophan (Trp) indolyl radicals (Trp•, k < 4 × 106 M−1 s−1 [20][21]) and Tyr phenoxyl radicals (Tyr•, k < 103 M−1 s−1 [22]), formation of disulfides (cystine) from two RS•, di-tyrosine from two Tyr•, di-tryptophan from two Trp•, and crossed dimers between these (e.g., Tyr–Trp) can be generated.
Light, particularly of wavelengths >~280 nm, which are not absorbed by the ozone layer, can penetrate significantly into biological structures and be absorbed either directly by protein residues, particularly Trp, Tyr and cystine [23], or by other species with high extinction coefficients in the long wavelength UV or visible regions. Energy absorption by non-protein species can give rise to indirect protein oxidation via the formation of excited states (e.g., singlet oxygen, 1O2 and reactive triplets) and/or radicals [23]. Direct UV absorption by proteins can form RS• from homolysis of the –S–S– bond of cystine (with C-S cleavage being an alternative pathway), and Tyr and Trp radicals by photo-ionization of these side-chains. These species can then give rise to crosslinks.
4. Radical–Molecule Reactions
5. Two-Electron (Molecule–Molecule) Reactions
6. Types of Crosslinks Detected within and between Proteins and Peptides
| Crosslinked Residues | Protein(s) | Chemical Nature and/or Mechanism of Formation of the Crosslink | Method(s) | Refs |
|---|---|---|---|---|
| Tyr-Cys |
|
|
Mass spectrometry (a) X-ray crystallography (b, c) |
[27][28][29] |
| Trp-Cys | Human growth hormone (hGH) |
|
Mass spectrometry | [26] |
| Met-Hydroxy-lysine | Collagen IV | Formation of S=N bridge (sulfilimine bond) induced by peroxidasin/HOBr | Mass spectrometry | [11] |
| Lys-Cys | Transaldolase | Nitrogen–oxygen–sulfur (NOS) link/redox switch | X-ray crystallography | [30] |
| Cys-Ser |
|
|
Mass spectrometry (a) X-ray crystallography (b) |
[26][31] |
| Cys-Phe | hGH | Crosslink between thioaldehyde from Cys and dehydrophenylalanine generated from Phe | Mass spectrometry | [26] |
| Cys-DHA Cys-DHB |
Lens proteins (βB1, βB2, βA3, βA4 and γS crystallins) | Nucleophilic addition from Cys (GSH) to DHA or DHB | Mass spectrometry | [32] |
| Tyr-Gly | Insulin | Michael addition of primary amines (N-terminal Gly) to oxidized Tyr species | Mass spectrometry | [33] |
| Trp-Gly | Matrilysin (Matrix metalloproteinase 7) | Crosslink between 3-chloroindolenine (3-Cl-Trp) and the main-chain amide adjacent to a Gly | NMR spectroscopy | [34] |
| Tyr-His | Insulin | Michael addition from His to oxidized Tyr | Mass spectrometry | [33] |
| Tyr-Tyr (selected data) |
Isolated proteins including: α-lactalbumin, caseins, glucose 6-phosphate dehydrogenase, lysozyme, fibronectin, laminins, tropoelastin, cAMP receptor protein, α-synuclein, calmodulin, insulins, hemoglobin, human Δ25 centrin 2. Human lipoproteins Human plasma proteins, including those from people with chronic renal failure Human atherosclerotic lesions Erythrocytes exposed to H2O2 Brain proteins (amyloid-beta and α-synuclein) from Alzheimer’s subjects Lipofuscin from aged human brain Urine from people with diabetes Human lens proteins Bacterial spore coat proteins Parasite oocysts |
C–C and/or C–O crosslinks via radical–radical reactions | Western blotting UPLC/HPLC with various detection methods Mass spectrometry |
[33][35][36][37][38][39][40][41][42][43][44][45][46][47][48][49][50][51][52][53][54][55][56][57][58][59][60][61][62] |
| Trp-Trp |
|
C–C or C–N crosslinks via radical–radical reactions | Mass spectrometry | [35][42][63][64][65] |
| Tyr-Trp |
|
C–C (or C–O and C–N) crosslinks via radical–radical reactions | X-ray crystallography (a) Mass spectrometry (b–g) |
[35][38][41][42][65][66] |
| His-His |
|
Nucleophilic addition of His to oxidized His | Mass spectrometry (a,b) NMR (c) |
[67][68][69][70] |
| His-Arg | Ribonuclease A (RNAse) | Nucleophilic addition of Arg to oxidized His | Mass spectrometry | [71] |
| His-Lys | Immunoglobulin G1 | Nucleophilic addition of Lys to oxidized His | Mass spectrometry | [67][69] |
| His-Cys | Immunoglobulin G1 | Nucleophilic addition of Cys to oxidized His | Mass spectrometry | [69] |
| Tyr-Lys |
|
Michael addition of Lys to oxidized Tyr | Mass spectrometry | [33][71][72] |
6. Secondary Reactions of Crosslinks
7. Detection of Crosslinks, including Advantages and Disadvantages of Different Methods
7.1. Analysis of Changes in Molecular Mass by Electrophoresis and Size Exclusion Chromatography (SEC)
7.2. Analysis of Protein Crosslinks by Western (Immuno-) Blotting and ELISA Assays
7.3. Direct Detection by Spectrophotometric and Fluorometric Assays
7.4. HPLC/UPLC Methodologies
7.5. Detection and Characterization of Crosslinked Proteins Using Other Biophysical Approaches
7.6. Mass Spectrometry (MS)-Based Detection and Structural Characterization of Crosslinked Proteins
8. Crosslink Quantification
References
- Hogg, P.J. Disulfide bonds as switches for protein function. Trends Biochem. Sci. 2003, 28, 210–214.
- Hägglund, P.; Mariotti, M.; Davies, M.J. Identification and characterization of protein cross-links induced by oxidative reactions. Expert Rev. Proteom. 2018, 18, 665–681.
- Adam, O.; Theobald, K.; Lavall, D.; Grube, M.; Kroemer, H.K.; Ameling, S.; Schafers, H.J.; Bohm, M.; Laufs, U. Increased lysyl oxidase expression and collagen cross-linking during atrial fibrillation. J. Mol. Cell Cardiol. 2011, 50, 678–685.
- Andringa, G.; Lam, K.Y.; Chegary, M.; Wang, X.; Chase, T.N.; Bennett, M.C. Tissue transglutaminase catalyzes the formation of alpha-synuclein crosslinks in Parkinson’s disease. FASEB J. 2004, 18, 932–934.
- Pehrsson, M.; Mortensen, J.H.; Manon-Jensen, T.; Bay-Jensen, A.C.; Karsdal, M.A.; Davies, M.J. Enzymatic cross-linking of collagens in organ fibrosis—Resolution and assessment. Expert Rev. Mol. Diagn. 2021, 21, 1049–1064.
- Kagan, H.M. Lysyl oxidase: Mechanism, regulation and relationship to liver fibrosis. Pathol. Res. Pract. 1994, 190, 910–919.
- López, B.; González, A.; Hermida, N.; Valencia, F.; de Teresa, E.; Díez, J. Role of lysyl oxidase in myocardial fibrosis: From basic science to clinical aspects. Am. J. Physiol. Heart Circ. Physiol. 2010, 299, H1–H9.
- Trackman, P.C. Lysyl Oxidase isoforms and potential therapeutic opportunities for fibrosis and cancer. Expert Opin. Ther. Targets 2016, 20, 935–945.
- Bignon, M.; Pichol-Thievend, C.; Hardouin, J.; Malbouyres, M.; Brechot, N.; Nasciutti, L.; Barret, A.; Teillon, J.; Guillon, E.; Etienne, E.; et al. Lysyl oxidase-like protein-2 regulates sprouting angiogenesis and type IV collagen assembly in the endothelial basement membrane. Blood 2011, 118, 3979–3989.
- McCall, A.S.; Cummings, C.F.; Bhave, G.; Vanacore, R.; Page-McCaw, A.; Hudson, B.G. Bromine is an essential trace element for assembly of collagen IV scaffolds in tissue development and architecture. Cell 2014, 157, 1380–1392.
- Bhave, G.; Cummings, C.F.; Vanacore, R.M.; Kumagai-Cresse, C.; Ero-Tolliver, I.A.; Rafi, M.; Kang, J.-S.; Pedchenko, V.; Fessler, L.I.; Fessler, J.H.; et al. Peroxidasin forms sulfilimine chemical bonds using hypohalous acids in tissue genesis. Nat. Chem. Biol. 2012, 8, 784–790.
- Peterfi, Z.; Geiszt, M. Peroxidasins: Novel players in tissue genesis. Trends Biochem. Sci. 2014, 39, 305–307.
- Jacob, J.S.; Cistola, D.P.; Hsu, F.F.; Muzaffar, S.; Mueller, D.M.; Hazen, S.L.; Heinecke, J.W. Human phagocytes employ the myeloperoxidase-hydrogen peroxide system to synthesize dityrosine, trityrosine, pulcherosine, and isodityrosine by a tyrosyl radical-dependent pathway. J. Biol. Chem. 1996, 271, 19950–19956.
- Selinheimo, E.; Autio, K.; Kruus, K.; Buchert, J. Elucidating the mechanism of laccase and tyrosinase in wheat bread making. J. Agric. Food Chem. 2007, 55, 6357–6365.
- Dunford, H.B. Peroxidases. In Advances in Inorganic Biochemistry; Elsevier: Amsterdam, The Netherlands, 1982; pp. 41–68.
- Davies, M.J. Protein oxidation and peroxidation. Biochem. J. 2016, 473, 805–825.
- Carreau, A.; El Hafny-Rahbi, B.; Matejuk, A.; Grillon, C.; Kieda, C. Why is the partial oxygen pressure of human tissues a crucial parameter? Small molecules and hypoxia. J. Cell Mol. Med. 2011, 15, 1239–1253.
- Dizdaroglu, M.; Simic, M.G. Isolation and characterization of radiation-induced aliphatic peptide dimers. Int. J. Radiat. Biol. 1983, 44, 231–239.
- Schoneich, C. Thiyl radicals and induction of protein degradation. Free Radic. Res. 2016, 50, 143–149.
- Fang, X.; Jin, F.; Jin, H.; von Sonntag, C. Reaction of the superoxide radical with the N-centred radical derived from N-acetyltryptophan methyl ester. J. Chem. Soc. Perkin. Trans. 1998, 259–263.
- Candeias, L.P.; Wardman, P.; Mason, R.P. The reaction of oxygen with radicals from oxidation of tryptophan and indole-3-acetic acid. Biophys. J. 1997, 67, 229–237.
- Hunter, E.P.; Desrosiers, M.F.; Simic, M.G. The effect of oxygen, antioxidants, and superoxide radical on tyrosine phenoxyl radical dimerization. Free Radic. Biol. Med. 1989, 6, 581–585.
- Pattison, D.I.; Rahmanto, A.S.; Davies, M.J. Photo-oxidation of proteins. PhotoChem. PhotoBiol. Sci. 2012, 11, 38–53.
- Siodlak, D. α,β-Dehydroamino acids in naturally occurring peptides. Amino Acids 2015, 47, 1–17.
- Friedman, M. Chemistry, biochemistry, nutrition, and microbiology of lysinoalanine, lanthionine, and histidinoalanine in food and other proteins. J. Agric. Food Chem. 1999, 47, 1295–1319.
- Steinmann, D.; Mozziconacci, O.; Bommana, R.; Stobaugh, J.F.; Wang, Y.J.; Schoneich, C. Photodegradation pathways of protein disulfides: Human growth hormone. Pharm. Res. 2017, 34, 2756–2778.
- Nagy, P.; Lechte, T.P.; Das, A.B.; Winterbourn, C.C. Conjugation of glutathione to oxidized tyrosine residues in peptides and proteins. J. Biol. Chem. 2012, 287, 26068–26076.
- Rokhsana, D.; Howells, A.E.; Dooley, D.M.; Szilagyi, R.K. Role of the Tyr-Cys cross-link to the active site properties of galactose oxidase. Inorg. Chem. 2012, 51, 3513–3524.
- Davies, C.G.; Fellner, M.; Tchesnokov, E.P.; Wilbanks, S.M.; Jameson, G.N. The Cys-Tyr cross-link of cysteine dioxygenase changes the optimal pH of the reaction without a structural change. Biochemistry 2014, 53, 7961–7968.
- Wensien, M.; von Pappenheim, F.R.; Funk, L.M.; Kloskowski, P.; Curth, U.; Diederichsen, U.; Uranga, J.; Ye, J.; Fang, P.; Pan, K.T.; et al. A lysine-cysteine redox switch with an NOS bridge regulates enzyme function. Nature 2021, 593, 460–464.
- Van Montfort, R.L.; Congreve, M.; Tisi, D.; Carr, R.; Jhoti, H. Oxidation state of the active-site cysteine in protein tyrosine phosphatase 1B. Nature 2003, 423, 773–777.
- Wang, Z.; Lyons, B.; Truscott, R.J.; Schey, K.L. Human protein aging: Modification and crosslinking through dehydroalanine and dehydrobutyrine intermediates. Aging Cell 2014, 13, 226–234.
- Torosantucci, R.; Mozziconacci, O.; Sharov, V.; Schöneich, C.; Jiskoot, W. Chemical modifications in aggregates of recombinant human insulin induced by metal-catalyzed oxidation: Covalent cross-linking via michael addition to tyrosine oxidation products. Pharm. Res. 2012, 29, 2276–2293.
- Fu, X.; Kao, J.L.; Bergt, C.; Kassim, S.Y.; Huq, N.P.; d’Avignon, A.; Parks, W.C.; Mecham, R.P.; Heinecke, J.W. Oxidative cross-linking of tryptophan to glycine restrains matrix metalloproteinase activity: Specific structural motifs control protein oxidation. J. Biol. Chem. 2004, 279, 6209–6212.
- Zhao, Z.; Engholm-Keller, K.; Poojary, M.M.; Boelt, S.G.; Rogowska-Wrzesinska, A.; Skibsted, L.H.; Davies, M.J.; Lund, M.N. Generation of aggregates of alpha-lactalbumin by UV-B light exposure. J. Agric. Food Chem. 2020, 68, 6701–6714.
- Fuentes-Lemus, E.; Silva, E.; Leinisch, F.; Dorta, E.; Lorentzen, L.G.; Davies, M.J.; López-Alarcón, C. alpha- and beta-casein aggregation induced by riboflavin-sensitized photo-oxidation occurs via di-tyrosine cross-links and is oxygen concentration dependent. Food Chem. 2018, 256, 119–128.
- Colombo, G.; Clerici, M.; Altomare, A.; Rusconi, F.; Giustarini, D.; Portinaro, N.; Garavaglia, M.L.; Rossi, R.; Dalle-Donne, I.; Milzani, A. Thiol oxidation and di-tyrosine formation in human plasma proteins induced by inflammatory concentrations of hypochlorous acid. J. Proteom. 2017, 152, 22–32.
- Leinisch, F.; Mariotti, M.; Rykaer, M.; López-Alarcón, C.; Hägglund, P.; Davies, M.J. Peroxyl radical- and photo-oxidation of glucose 6-phosphate dehydrogenase generates cross-links and functional changes via oxidation of tyrosine and tryptophan residues. Free Radic. Biol. Med. 2017, 112, 240–252.
- Fuentes-Lemus, E.; Mariotti, M.; Hägglund, P.; Leinisch, F.; Fierro, A.; Silva, E.; Davies, M.J.; López-Alarcón, C. Oxidation of lysozyme induced by peroxyl radicals involves amino acid modifications, loss of activity, and formation of specific crosslinks. Free Radic. Biol. Med. 2021, 167, 258–270.
- Fuentes-Lemus, E.; Mariotti, M.; Hägglund, P.; Leinisch, F.; Fierro, A.; Silva, E.; López-Alarcón, C.; Davies, M.J. Binding of rose bengal to lysozyme modulates photooxidation and cross-linking reactions involving tyrosine and tryptophan. Free Radic. Biol. Med. 2019, 143, 375–386.
- Fuentes-Lemus, E.; Mariotti, M.; Reyes, J.; Leinisch, F.; Hägglund, P.; Silva, E.; Davies, M.J.; López-Alarcón, C. Photo-oxidation of lysozyme triggered by riboflavin is O2-dependent, occurs via mixed type 1 and type 2 pathways, and results in inactivation, site-specific damage and intra- and inter-molecular crosslinks. Free Radic. Biol. Med. 2020, 152, 61–73.
- Mariotti, M.; Rogowska-Wrzesinska, A.; Hägglund, P.; Davies, M.J. Cross-linking and modification of fibronectin by peroxynitrous acid: Mapping and quantification of damage provides a new model for domain interactions. J. Biol. Chem. 2021, 296, 100360.
- Degendorfer, G.; Chuang, C.Y.; Hammer, A.; Malle, E.; Davies, M.J. Peroxynitrous acid induces structural and functional modifications to basement membranes and its key component, laminin. Free Radic. Biol. Med. 2015, 89, 721–733.
- Leinisch, F.; Mariotti, M.; Andersen, S.H.; Lindemose, S.; Hägglund, P.; Mollegaard, N.E.; Davies, M.J. UV oxidation of cyclic AMP receptor protein, a global bacterial gene regulator, decreases DNA binding and cleaves DNA at specific sites. Sci. Rep. 2020, 10, 3106.
- Ursem, R.; Swarge, B.; Abhyankar, W.R.; Buncherd, H.; de Koning, L.J.; Setlow, P.; Brul, S.; Kramer, G. Identification of native cross-links in Bacillus subtilis spore coat proteins. J. Proteome Res. 2021, 20, 1809–1816.
- Leeuwenburgh, C.; Rasmussen, J.E.; Hsu, F.F.; Mueller, D.M.; Pennathur, S.; Heinecke, J.W. Mass spectrometric quantification of markers for protein oxidation by tyrosyl radical, copper, and hydroxyl radical in low density lipoprotein isolated from human atherosclerotic plaques. J. Biol. Chem. 1997, 272, 3520–3526.
- Giulivi, C.; Davies, K.J. Dityrosine and tyrosine oxidation products are endogenous markers for the selective proteolysis of oxidatively modified red blood cell hemoglobin by (the 19 S) proteasome. J. Biol. Chem. 1993, 268, 8752–8759.
- Truscott, R.J.W.; Friedrich, M.G. The etiology of human age-related cataract. Proteins don’t last forever. Biochim. Biophys. Acta 2016, 1860, 192–198.
- Serpell, L.C.; Williams, T.L.; Stewart-Parker, M.; Ford, L.; Skaria, E.; Cole, M.; Bucher, W.G.r.; Morris, K.L.; Sada, A.A.; Thorpe, J.R. A central role for dityrosine crosslinking of Amyloid-β in Alzheimer’s disease. Acta Neuropath Commun. 2013, 1, 83.
- Tiwari, M.K.; Leinisch, F.; Sahin, C.; Møller, I.M.; Otzen, D.E.; Davies, M.J.; Bjerrum, M.J. Early events in copper-ion catalyzed oxidation of α-synuclein. Free Radic. Biol. Med. 2018, 121, 38–50.
- Pennathur, S.; Jackson-Lewis, V.; Przedborski, S.; Heinecke, J.W. Mass spectrometric quantification of 3-nitrotyrosine, ortho-tyrosine, and o,o′-dityrosine in brain tissue of 1-methyl-4-phenyl-1,2,3,6- tetrahydropyridine-treated mice, a model of oxidative stress in Parkinson’s disease. J. Biol. Chem. 1999, 274, 34621–34628.
- Kato, Y.; Maruyama, W.; Naoi, M.; Hashizume, Y.; Osawa, T. Immunohistochemical detection of dityrosine in lipofuscin pigments in the aged human brain. FEBS Lett. 1998, 439, 231–234.
- Kato, Y.; Dozaki, N.; Nakamura, T.; Kitamoto, N.; Yoshida, A.; Naito, M.; Kitamura, M.; Osawa, T. Quantification of modified tyrosines in healthy and diabetic human urine using liquid chromatography/tandem mass spectrometry. J. Clin. Biochem. Nutr. 2009, 44, 67–78.
- Wu, G.R.; Cheserek, M.; Shi, Y.H.; Shen, L.Y.; Yu, J.; Le, G.W. Elevated plasma dityrosine in patients with hyperlipidemia compared to healthy individuals. Ann. Nutr. Metab. 2014, 66, 44–50.
- Ziouzenkova, O.; Asatryan, L.; Akmal, M.; Tetta, C.; Wratten, M.L.; Loseto-Wich, G.; Jurgens, G.; Heinecke, J.; Sevanian, A. Oxidative cross-linking of ApoB100 and hemoglobin results in low density lipoprotein modification in blood. Relevance to atherogenesis caused by hemodialysis. J. Biol. Chem. 1999, 274, 18916–18924.
- Francis, G.A.; Mendez, A.J.; Bierman, E.L.; Heinecke, J.W. Oxidative tyrosylation of high density lipoprotein by peroxidase enhances cholesterol removal from cultured fibroblasts and macrophage foam cells. Proc. Natl. Acad. Sci. USA 1993, 90, 6631–6635.
- Malencik, D.A.; Anderson, S.R. Dityrosine formation in calmodulin: Cross-linking and polymerization catalyzed by Arthromyces peroxidase. Biochemistry 1996, 35, 4375–4386.
- Aeschbach, R.; Amado, R.; Neukom, H. Formation of dityrosine cross-links in proteins by oxidation of tyrosine residues. Biochim. Biophys. Acta 1976, 439, 292–301.
- Das, A.B.; Nauser, T.; Koppenol, W.H.; Kettle, A.J.; Winterbourn, C.C.; Nagy, P. Rapid reaction of superoxide with insulin-tyrosyl radicals to generate a hydroperoxide with subsequent glutathione addition. Free Radic. Biol. Med. 2014, 70, 86–95.
- Gatin, A.; Billault, I.; Duchambon, P.; Van der Rest, G.; Sicard-Roselli, C. Oxidative radicals (HO. or N3.) induce several di-tyrosine bridge isomers at the protein scale. Free Radic. Biol. Med. 2021, 162, 461–470.
- Degendorfer, G.; Chuang, C.Y.; Kawasaki, H.; Hammer, A.; Malle, E.; Yamakura, F.; Davies, M.J. Peroxynitrite-mediated oxidation of plasma fibronectin. Free Radic. Biol. Med. 2016, 97, 602–615.
- Mai, K.; Smith, N.C.; Feng, Z.P.; Katrib, M.; Šlapeta, J.; Šlapetova, I.; Wallach, M.G.; Luxford, C.; Davies, M.J.; Zhang, X.; et al. Peroxidase catalysed cross-linking of an intrinsically unstructured protein via dityrosine bonds in the oocyst wall of the apicomplexan parasite, Eimeria maxima. Int. J. Parasitol. 2011, 41, 1157–1164.
- Medinas, D.B.; Gozzo, F.C.; Santos, L.F.A.; Iglesias, A.H.; Augusto, O. A ditryptophan cross-link is responsible for the covalent dimerization of human superoxide dismutase 1 during its bicarbonate-dependent peroxidase activity. Free Radic. Biol. Med. 2010, 49, 1046–1053.
- Paviani, V.; Queiroz, R.F.; Marques, E.F.; Di Mascio, P.; Augusto, O. Production of lysozyme and lysozyme-superoxide dismutase dimers bound by a ditryptophan cross-link in carbonate radical-treated lysozyme. Free Radic. Biol. Med. 2015, 89, 72–82.
- Paviani, V.; Junqueira de Melo, P.; Avakin, A.; Di Mascio, P.; Ronsein, G.E.; Augusto, O. Human cataractous lenses contain cross-links produced by crystallin-derived tryptophanyl and tyrosyl radicals. Free Radic. Biol. Med. 2020, 160, 356–367.
- Bhaskar, B.; Immoos, C.E.; Shimizu, H.; Sulc, F.; Farmer, P.J.; Poulos, T.L. A novel heme and peroxide-dependent tryptophan-tyrosine cross-link in a mutant of cytochrome c peroxidase. J. Mol. Biol. 2003, 328, 157–166.
- Liu, M.; Zhang, Z.; Cheetham, J.; Ren, D.; Zhou, Z.S. Discovery and characterization of a photo-oxidative histidine-histidine cross-link in IgG1 antibody utilizing 18O-labeling and mass spectrometry. Anal. Chem. 2014, 86, 4940–4948.
- Powell, T.; Knight, M.J.; O’Hara, J.; Burkitt, W. Discovery of a photoinduced histidine-histidine cross-link in an IgG4 antibody. J. Am. Soc. Mass Spectrom. 2020, 31, 1233–1240.
- Xu, C.F.; Chen, Y.; Yi, L.; Brantley, T.; Stanley, B.; Sosic, Z.; Zang, L. Discovery and characterization of histidine oxidation initiated cross-links in an IgG1 monoclonal antibody. Anal. Chem. 2017, 89, 7915–7923.
- Agon, V.V.; Bubb, W.A.; Wright, A.; Hawkins, C.L.; Davies, M.J. Sensitizer-mediated photooxidation of histidine residues: Evidence for the formation of reactive side-chain peroxides. Free Radic Biol. Med. 2006, 40, 698–710.
- Leinisch, F.; Mariotti, M.; Hägglund, P.; Davies, M.J. Structural and functional changes in RNAse A originating from tyrosine and histidine cross-linking and oxidation. Free Radic. Biol. Med. 2018, 126, 73–86.
- Torosantucci, R.; Sharov, V.S.; Van Beers, M.; Brinks, V.; Schöneich, C.; Jiskoot, W. Identification of oxidation sites and covalent cross-links in metal catalyzed oxidized interferon beta-1a: Potential implications for protein aggregation and immunogenicity. Mol. Pharm. 2013, 10, 2311–2322.
- Breusing, N.; Grune, T. Biomarkers of protein oxidation from a chemical, biological and medical point of view. Exp. Gerontol. 2010, 45, 733–737.
- Lopezllorca, L.V.; Fry, S.C. Dityrosine, trityrosine and tetratyrosine, potential cross-links in structural proteins of plant-parasitic nematodes. Nematologica 1989, 35, 165–179.
- Dhayal, S.K.; Sforza, S.; Wierenga, P.A.; Gruppen, H. Peroxidase induced oligo-tyrosine cross-links during polymerization of alpha-lactalbumin. Biochim Biophys. Acta Proteins Proteom. 2015, 1854, 1898–1905.
- Silva, E.; Barrias, P.; Fuentes-Lemus, E.; Tirapegui, C.; Aspee, A.; Carroll, L.; Davies, M.J.; López-Alarcón, C. Riboflavin-induced Type 1 photo-oxidation of tryptophan using a high intensity 365nm light emitting diode. Free Radic. Biol. Med. 2019, 131, 133–143.
- Reid, L.O.; Vignoni, M.; Martins-Froment, N.; Thomas, A.H.; Dantola, M.L. Photochemistry of tyrosine dimer: When an oxidative lesion of proteins is able to photoinduce further damage. PhotoChem. PhotoBiol. Sci. 2019, 18, 1732–1741.
- Huang, Y.R.; Hua, Y.F.; Qiu, A.Y. Soybean protein aggregation induced by lipoxygenase catalyzed linoleic acid oxidation. Food Res. Int. 2006, 39, 240–249.
- Cui, X.H.; Xiong, Y.L.L.; Kong, B.H.; Zhao, X.H.; Liu, N. Hydroxyl radical-stressed whey protein isolate: Chemical and structural properties. Food Bioprocess. Technol. 2012, 5, 2454–2461.
- Hawkins, C.L.; Morgan, P.E.; Davies, M.J. Quantification of protein modification by oxidants. Free Radic. Biol. Med. 2009, 46, 965–988.
- Figueroa, J.D.; Zarate, A.M.; Fuentes-Lemus, E.; Davies, M.J.; López-Alarcón, C. Formation and characterization of crosslinks, including Tyr-Trp species, on one electron oxidation of free Tyr and Trp residues by carbonate radical anion. RSC Adv. 2020, 10, 25786–25800.
- Gamon, L.F.; Guo, C.; He, J.; Hägglund, P.; Hawkins, C.L.; Davies, M.J. Absolute quantitative analysis of intact and oxidized amino acids by LC-MS without prior derivatization. Redox Biol. 2020, 36, 101586.
- Desmons, A.; Thioulouse, E.; Hautem, J.Y.; Saintier, A.; Baudin, B.; Lamaziere, A.; Netter, C.; Moussa, F. Direct liquid chromatography tandem mass spectrometry analysis of amino acids in human plasma. J. Chromatogr. A 2020, 1622, 461135.
- Verzini, S.; Shah, M.; Theillet, F.X.; Belsom, A.; Bieschke, J.; Wanker, E.E.; Rappsilber, J.; Binolfi, A.; Selenko, P. Megadalton-sized dityrosine aggregates of alpha-synuclein retain high degrees of structural disorder and internal dynamics. J. Mol. Biol. 2020, 432, 166689.
- Thorn, D.C.; Grosas, A.B.; Mabbitt, P.D.; Ray, N.J.; Jackson, C.J.; Carver, J.A. The structure and stability of the disulfide-linked gammaS-crystallin dimer provide insight into oxidation products associated with lens cataract formation. J. Mol. Biol. 2019, 431, 483–497.
- Evrard, C.; Capron, A.; Marchand, C.; Clippe, A.; Wattiez, R.; Soumillion, P.; Knoops, B.; Declercq, J.P. Crystal structure of a dimeric oxidized form of human peroxiredoxin 5. J. Mol. Biol. 2004, 337, 1079–1090.
- Smeets, A.; Evrard, C.; Landtmeters, M.; Marchand, C.; Knoops, B.; Declercq, J.P. Crystal structures of oxidized and reduced forms of human mitochondrial thioredoxin 2. Protein Sci. 2005, 14, 2610–2621.




