| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Ahmed Mohamed | + 816 word(s) | 816 | 2020-09-09 11:07:51 | | | |
| 2 | Dean Liu | Meta information modification | 816 | 2020-09-10 10:20:56 | | |
Video Upload Options
Unlike arboviruses, which have a dual-host tropism by cycling between vertebrate hosts and arthropod vectors, ISVs replicate exclusively in arthropod populations, causing a persistent viral infection, as such they are mainly maintained in nature by vertical transmission route. ISVs are also refereed to as mosquito-specific viruses as they are generally identified and discovered in mosquitoes. They are nonetheless an important part of the mosquito microbiome. The first ISV identified is cell-fusing agent virus (CFAV), which was isolated from an Aedes aegypti (Ae. aegypti) cell culture.
1. Introduction
Since the discovery of CFAV, wild mosquito populations have been shown to act as a reservoir for a wide variety of ISVs, which suggests a significant heterogeneity among these viruses. These viruses are characterized by their competency to replicate in their vectors, while their replication are restricted in vertebrate cells.[1] Thanks to the advent of next-generation sequencing applications and advanced bioinformatics tools, metagenomic studies in this scope have identified a large number of ISVs harboring wild-caught mosquitoes over wide geographical areas (reviewed in [2]). Phylogenetic analyses based on sequence identities between ISVs and other mosquito-borne viruses suggest strong evidence that ISVs may be ancestral to arboviruses. Thereby, ISVs could serve as a model to study arbovirus evolution and their transition from single- to dual-host identity.
2. Classification of ISVs
ISVs have been classified within multiple different taxa, mostly in the family Flaviviridae and the order Bunyavirales (Table 1). The Togaviridae, Rhabdoviridae, and Mesoniviridae also contain a smaller number of ISVs, as well as other taxa.[3]
| Taxa | Genus | ISV | Host 1 | Reference |
|---|---|---|---|---|
| Flaviviridae | Flavivirus | Binjari virus | Aedes normanensis | [4] |
| Cell fusing agent virus | Aedes spp. | [5] | ||
| Kamiti River virus | Aedes macintoshi | [6] | ||
| Niénokoué virus | Culex spp. | [7] | ||
| Palm Creek virus | Coquillettidia xanthogaster | [8] | ||
| Parramatta River virus | Aedes vigilax | [9] | ||
| Bunyavirales | Phasivirus | Phasi Charoen-like phasivirus | Aedes aegypti | [10] |
| Orthoferavirus | Ferak orthoferavirus | Culex decens | [11] | |
| Goukovirus | Gouléako goukovirus | Anopheles spp. Culex spp. Uranotaenia spp. |
[12] | |
| Herbevirus | Herbert herbevirus | Culex nebulosus | [13] | |
| Orthojonvirus | Jonchet orthojonvirus | Culex spp. | [11] | |
| Birnaviridae | Entomobirnavirus | Espirito Santo virus | N/A | [14] |
| Mesoniviridae | Alphamesonivirus 1 | Cavally virus | Aedes spp. Anopheles spp. Culex spp. Uranotaenia spp. |
[15] |
| Nam Dinh virus | Culex spp. Aedes albopictus |
[16][17] | ||
| Dianke virus | Aedes spp. Anopheles spp. Culex spp. Mansonia spp. Uranotaenia spp. ceratopogonids |
[18] | ||
| Reoviridae | Dinovernavirus | Fako virus | Aedes spp. Eretmapodites spp. |
[19] |
| Rhabdoviridae | Almendravirus | Arboretum almendavirus | Ochlerotatusfulvus | [20] |
| Mousrhavirus | Moussa Mousrhavirus | Culex decens | [21] | |
| Togaviridae | Alphavirus | Agua Salud alphavirus | Culex declarator | [22] |
| Eilat virus | Anopheles coustani | [23] | ||
| Yada yada virus | N/A | [24] |
1 The host range of insect-specific viruses identified up to date. N/A the vector species of ISV are not determined yet.
3. ISV maintenance in nature
The vertical transmission routes (transovarial or transovum transmission) are considered the primary means by which ISVs are maintained and propagated in their vector populations.[26][27] They have also been shown to be venereally transmitted, yet to a lesser extent.[28] In addition, ISVs such as Eilat virus (EILV) and Negev virus could be experimentally transmitted to adult mosquitoes via a high-titer of an infectious blood meal.[29][30]
4. Host Restriction of ISVs
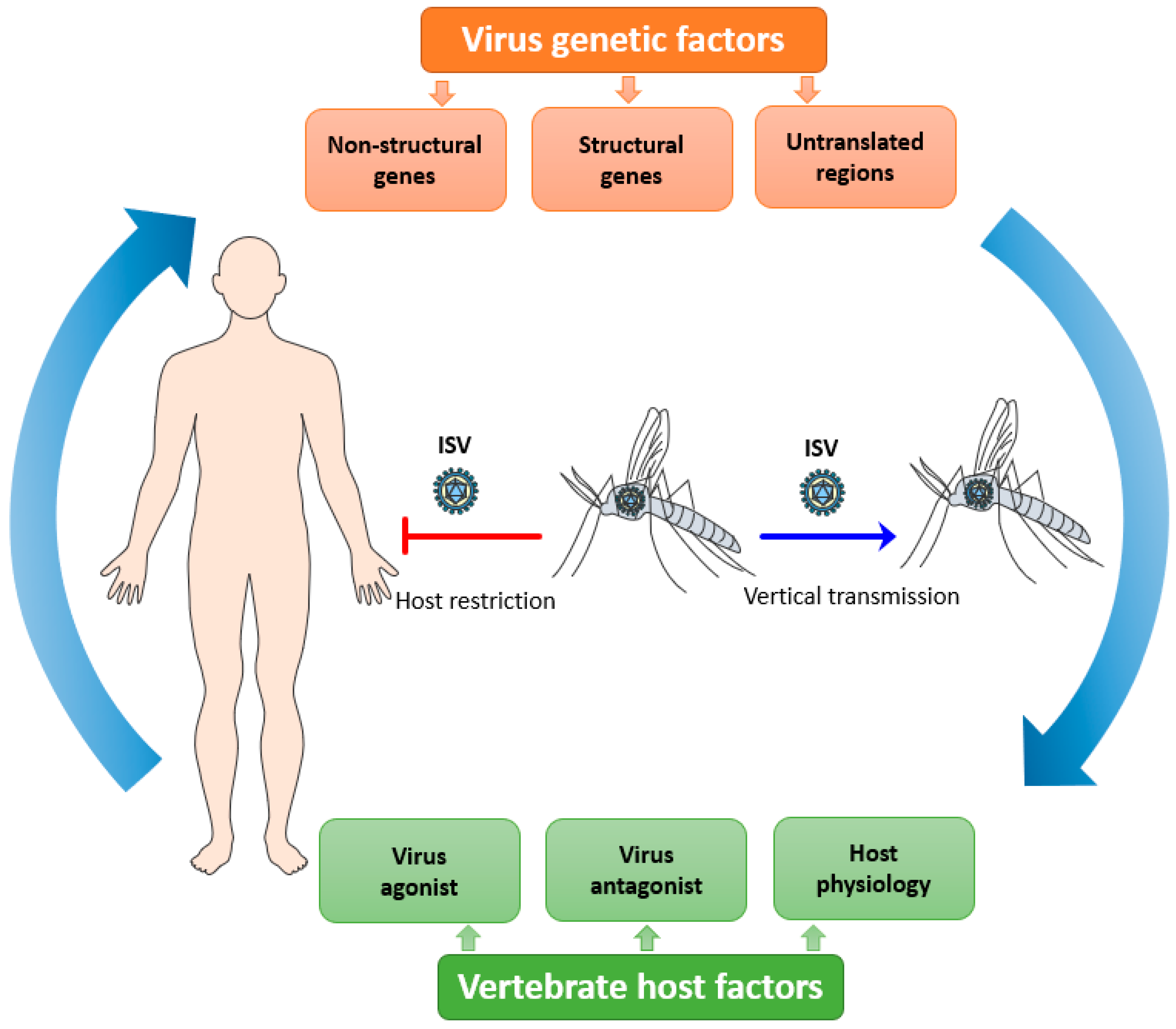
Viral tropism is fundamental to consider with regards to host restriction of ISVs. It is defined as the ability of a particular virus to productively infect and replicate in a specific cell type, tissue, or species.[25] Viral tropism is determined by host cell susceptibility and permissiveness. For an efficient viral replication cycle in the host cell, the virus should be able to interact with multiple host factors at each step of its replicative cycle and to antagonize the host immune response that hinders its replication. Consequently, ISVs must overcome several integrated bottlenecks present at different levels to potentially emerge as a new dual-host virus: their genetic determinants, the vertebrate host factors, and the host microenvironment needed for efficient replication (Figure 1) (reviewed in [1]).
Figure 1. Putative overview of the host tropism of insect-specific viruses (ISVs). ISVs are maintained in mosquito populations by a vertical route of transmission. The infection and replication of ISVs are restricted in vertebrate hosts due to the complex interplay between multiple viral, host, and microenvironmental factors.[1]
5. Potential applications of ISVs
5.1. Novel biocontrol agents
Since ISV discovery in natural mosquito populations as circulating viruses more attention has been given to their role in modulating pathogenic arbovirus transmission. Recently, different studies have focused on ISV interactions with dual-host arboviruses by investigating the phenomenon of superinfection exclusion, reviewed in [2][31][32]. Accordingly, ISVs can modulate the replication or dissemination of dual-host viruses in their competent vectors.[33]
5.2. Vaccine and diagnostic platforms
Thanks to ISV host-restriction in vertebrate cells, ISVs have been manipulated as vaccine and diagnostic platforms. Additionally, the similarity between ISVs and medically-important arboviruses in gene structure and order have paved the way to generate chimeras that could harbor structural proteins of these arboviruses. Consequently, the dual-host structural proteins could maintain the native structure and conformation required for vaccine and diagnostic platforms. In this regard, Eilat virus and Binjari virus have been utilized as recombinant platforms for vaccine and diagnostic development.[34][35][36].
References
- Ahmed Me Elrefaey; Rana Abdelnabi; Ana Lucia Rosales Rosas; Lanjiao Wang; Sanjay Basu; Leen Delang; Understanding the Mechanisms Underlying Host Restriction of Insect-Specific Viruses. Viruses 2020, 12, 964, 10.3390/v12090964.
- Eric Agboli; Mayke Leggewie; Mine Altinli; Esther Schnettler; Mosquito-Specific Viruses—Transmission and Interaction. Viruses 2019, 11, 873, 10.3390/v11090873.
- Charles H. Calisher; Stephen Higgs; The Discovery of Arthropod-Specific Viruses in Hematophagous Arthropods: An Open Door to Understanding the Mechanisms of Arbovirus and Arthropod Evolution?. Annual Review of Entomology 2018, 63, 87-103, 10.1146/annurev-ento-020117-043033.
- Jessica J. Harrison; Jody Hobson-Peters; Agathe M. G. Colmant; Joanna Koh; Natalee D. Newton; David Warrilow; Helle Bielefeldt-Ohmann; Thisun B. H. Piyasena; Caitlin A. O’Brien; Laura J. Vet; et al.Devina ParamithaJames R. PotterSteven S. DavisCheryl A. JohansenYin Xiang SetohAlexander A. KhromykhRoy A. Hall Antigenic Characterization of New Lineage II Insect-Specific Flaviviruses in Australian Mosquitoes and Identification of Host Restriction Factors. mSphere 2020, 5, 1, 10.1128/msphere.00095-20.
- Victor Stollar; Virginia L. Thomas; An agent in the Aedes aegypti cell line (Peleg) which causes fusion of Aedes albopictus cells. Virology 1975, 64, 367-377, 10.1016/0042-6822(75)90113-0.
- M. B. Crabtree; R. C. Sang; V. Stollar; L. M. Dunster; B. R. Miller; Genetic and phenotypic characterization of the newly described insect flavivirus, Kamiti River virus. Archives of Virology 2003, 148, 1095-1118, 10.1007/s00705-003-0019-7.
- Rebecca Halbach; Sandra Junglen; Ronald P. Van Rij; Mosquito-specific and mosquito-borne viruses: evolution, infection, and host defense. Current Opinion in Insect Science 2017, 22, 16-27, 10.1016/j.cois.2017.05.004.
- Jody Hobson-Peters; Alice Wei Yee Yam; Jennifer Lu; Yin Xiang Setoh; Fiona May; Nina Kurucz; Susan Walsh; Natalie A. Prow; Steven S. Davis; Richard Weir; et al.Lorna MelvilleNeville HuntRichard I. WebbBradley J. BlitvichPeter WhelanRoy A. Hall A New Insect-Specific Flavivirus from Northern Australia Suppresses Replication of West Nile Virus and Murray Valley Encephalitis Virus in Co-infected Mosquito Cells. PLOS ONE 2013, 8, e56534, 10.1371/journal.pone.0056534.
- Breeanna J. McLean; Jody Hobson-Peters; Cameron E. Webb; Daniel Watterson; Natalie A. Prow; Hong Duyen Nguyen; Sonja Hall-Mendelin; David Warrilow; Cheryl A. Johansen; Cassie C. Jansen; et al.Andrew F. Van Den HurkNigel W. BeebeEsther SchnettlerRoss T. BarnardRoy A. Hall A novel insect-specific flavivirus replicates only in Aedes-derived cells and persists at high prevalence in wild Aedes vigilax populations in Sydney, Australia. Virology 2015, 486, 272-283, 10.1016/j.virol.2015.07.021.
- Xiaomin Zhang; Suibin Huang; Tao Jin; Peng Lin; Yalan Huang; Chunli Wu; Bo Peng; Lan Wei; Hin Chu; Miao Wang; et al.Zhirong JiaShaohua ZhangJianbin XieJinquan ChengChengsong WanRenli Zhang Discovery and high prevalence of Phasi Charoen-like virus in field-captured Aedes aegypti in South China. Virology 2018, 523, 35-40, 10.1016/j.virol.2018.07.021.
- Marco Marklewitz; Florian Zirkel; Andreas Kurth; Christian Drosten; Sandra Junglen; Evolutionary and phenotypic analysis of live virus isolates suggests arthropod origin of a pathogenic RNA virus family. Proceedings of the National Academy of Sciences 2015, 112, 7536-7541, 10.1073/pnas.1502036112.
- Marco Marklewitz; S. Handrick; W. Grasse; A. Kurth; Alexander N. Lukashev; C. Drosten; H. Ellerbrok; F. H. Leendertz; G. Pauli; Sandra Junglen; et al. Gouleako Virus Isolated from West African Mosquitoes Constitutes a Proposed Novel Genus in the Family Bunyaviridae. Journal of Virology 2011, 85, 9227-9234, 10.1128/jvi.00230-11.
- Marco Marklewitz; Florian Zirkel; Innocent B. Rwego; Hanna Heidemann; Pascal Trippner; Andreas Kurth; René Kallies; Thomas Briese; W. Ian Lipkin; Christian Drosten; et al.Thomas R. GillespieSandra Junglen Discovery of a Unique Novel Clade of Mosquito-Associated Bunyaviruses. Journal of Virology 2013, 87, 12850-12865, 10.1128/jvi.01862-13.
- Ricardo Vancini; Angel Paredes; Mariana Ribeiro; Kevin Blackburn; Davis Ferreira; Joseph P. Kononchik; Raquel Hernandez; Dennis T. Brown; Espirito Santo Virus: a New Birnavirus That Replicates in Insect Cells. Journal of Virology 2011, 86, 2390-2399, 10.1128/jvi.06614-11.
- Florian Zirkel; Andreas Kurth; Phenix-Lan Quan; Thomas Briese; Heinz Ellerbrok; Georg Pauli; Fabian H. Leendertz; W. Ian Lipkin; John Ziebuhr; Christian Drosten; et al.Sandra Junglen An Insect Nidovirus Emerging from a Primary Tropical Rainforest. mBio 2011, 2, 1, 10.1128/mbio.00077-11.
- 1371/journal.ppat.1002215
- 1186/1743-422X-11-97
- Moussa Moïse Diagne; Alioune Gaye; Marie Henriette Dior Ndione; Martin Faye; Gamou Fall; Idrissa Dieng; Steven G. Widen; Thomas G. Wood; Vsevolod Popov; Hilda Guzman; et al.Yamar BâScott C. WeaverMawlouth DialloRobert TeshOusmane FayeNikos VasilakisAmadou A. Sall Dianke virus: A new mesonivirus species isolated from mosquitoes in Eastern Senegal.. Virus Research 2019, 275, 197802, 10.1016/j.virusres.2019.197802.
- Albert J. Auguste; Jason T. Kaelber; Eric B. Fokam; Hilda Guzman; Christine V. F. Carrington; Jesse H. Erasmus; Basile Kamgang; Vsevolod L. Popov; Joanita Jakana; Xiangan Liu; et al.Thomas G. WoodSteven G. WidenNikos VasilakisRobert B. TeshWah ChiuScott C Weaver A Newly Isolated Reovirus Has the Simplest Genomic and Structural Organization of Any Reovirus. Journal of Virology 2014, 89, 676-687, 10.1128/jvi.02264-14.
- Nikos Vasilakis; Fanny Castro-Llanos; Steven G. Widen; Patricia V. Aguilar; Hilda Guzman; Carolina Guevara; Roberto Fernandez; Albert J. Auguste; Thomas G. Wood; Vsevolod Popov; et al.Kirk MundalElodie GhedinTadeusz J. KochelEdward C. HolmesPeter J. WalkerRobert B. Tesh Arboretum and Puerto Almendras viruses: two novel rhabdoviruses isolated from mosquitoes in Peru. Journal of General Virology 2014, 95, 787-792, 10.1099/vir.0.058685-0.
- Phenix-Lan Quan; S. Junglen; Alla Tashmukhamedova; Sean Conlan; Stephen K. Hutchison; Andreas Kurth; Heinz Ellerbrok; Michael Egholm; Thomas Briese; Fabian H. Leendertz; et al.W. Ian Lipkin Moussa virus: A new member of the Rhabdoviridae family isolated from Culex decens mosquitoes in Côte d’Ivoire. Virus Research 2010, 147, 17-24, 10.1016/j.virusres.2009.09.013.
- Kyra Hermanns; Marco Marklewitz; Florian Zirkel; Gijs J. Overheul; Rachel A. Page; Jose R. Loaiza; Christian Drosten; Ronald P. Van Rij; Sandra Junglen; Agua Salud alphavirus defines a novel lineage of insect-specific alphaviruses discovered in the New World. Journal of General Virology 2020, 101, 96-104, 10.1099/jgv.0.001344.
- Farooq Nasar; Gustavo Palacios; Rodion V. Gorchakov; Hilda Guzman; Amelia P. Travassos Da Rosa; Nazir Savji; Vsevolod L. Popov; Michael B. Sherman; W. Ian Lipkin; Robert B. Tesh; et al.Scott C Weaver Eilat virus, a unique alphavirus with host range restricted to insects by RNA replication. Proceedings of the National Academy of Sciences 2012, 109, 14622-14627, 10.1073/pnas.1204787109.
- Jana Batovska; Jan P. Buchmann; Edward C. Holmes; Stacey E. Lynch; Coding-Complete Genome Sequence of Yada Yada Virus, a Novel Alphavirus Detected in Australian Mosquitoes. Microbiology Resource Announcements 2020, 9, 1, 10.1128/mra.01476-19.
- Grant McFadden; Mohamed R. Mohamed; Masmudur M. Rahman; Eric Bartee; Cytokine determinants of viral tropism. Nature Reviews Immunology 2009, 9, 645-655, 10.1038/nri2623.
- Maria Angelica Contreras-Gutierrez; Hilda Guzman; Saravanan Thangamani; Nikos Vasilakis; Robert B. Tesh; Experimental Infection with and Maintenance of Cell Fusing Agent Virus (Flavivirus) in Aedes aegypti.. The American Journal of Tropical Medicine and Hygiene 2017, 97, 299-304, 10.4269/ajtmh.16-0987.
- Joel J. L. Lutomiah; Charles Mwandawiro; Japhet Magambo; Rosemary Sang; Infection and Vertical Transmission of Kamiti River Virus in Laboratory Bred Aedes aegypti Mosquitoes. Journal of Insect Science 2007, 7, 1-7, 10.1673/031.007.5501.
- Bethany G. Bolling; Francisco J. Olea-Popelka; Lars Eisen; Chester G. Moore; Carol D. Blair; Transmission dynamics of an insect-specific flavivirus in a naturally infected Culex pipiens laboratory colony and effects of co-infection on vector competence for West Nile virus. Virology 2012, 427, 90-97, 10.1016/j.virol.2012.02.016.
- Farooq Nasar; Andrew D. Haddow; Robert B. Tesh; Scott C Weaver; Eilat virus displays a narrow mosquito vector range. Parasites & Vectors 2014, 7, 595, 10.1186/s13071-014-0595-2.
- Nikos Vasilakis; Naomi L. Forrester; Gustavo Palacios; Farooq Nasar; Nazir Savji; Shannan L. Rossi; Hilda Guzman; Thomas G. Wood; Vsevolod Popov; Rodion Gorchakov; et al.Ana Vázquez GonzálezAndrew D. HaddowUglas M. WattsAmelia P. A. Travassos Da RosaScott C WeaverW. Ian LipkinRobert B. Tesh Negevirus: a Proposed New Taxon of Insect-Specific Viruses with Wide Geographic Distribution. Journal of Virology 2012, 87, 2475-2488, 10.1128/jvi.00776-12.
- Pontus Öhlund; Hanna Lundén; Anne-Lie Blomström; Insect-specific virus evolution and potential effects on vector competence. Virus Genes 2019, 55, 127-137, 10.1007/s11262-018-01629-9.
- Edward I Patterson; Jandouwe Villinger; Joseph N Muthoni; Lucien Dobel-Ober; Grant L Hughes; Exploiting insect-specific viruses as a novel strategy to control vector-borne disease. Current Opinion in Insect Science 2020, 39, 50-56, 10.1016/j.cois.2020.02.005.
- Artem Baidaliuk; Elliott F. Miot; Sebastian Lequime; Isabelle Moltini-Conclois; Fanny Delaigue; Stéphanie Dabo; Laura B. Dickson; Fabien Aubry; Sarah H. Merkling; Van-Mai Cao-Lormeau; et al.Louis Lambrechts Cell-Fusing Agent Virus Reduces Arbovirus Dissemination in Aedes aegypti Mosquitoes In Vivo. Journal of Virology 2019, 93, e00705-19, 10.1128/jvi.00705-19.
- Jesse H. Erasmus; Albert J. Auguste; Jason T. Kaelber; Huanle Luo; Shannan L. Rossi; Karla Fenton; Grace Leal; Dal Young Kim; Wah Chiu; Tian Wang; et al.Ilya FrolovFarooq NasarScott C Weaver A chikungunya fever vaccine utilizing an insect-specific virus platform. Nature Medicine 2016, 23, 192-199, 10.1038/nm.4253.
- Jesse H. Erasmus; James Needham; Syamal Raychaudhuri; Michael S. Diamond; David W. C. Beasley; Stan Morkowski; Henrik Salje; Ildefonso Fernandez Salas; Dal Young Kim; Ilya Frolov; et al.Farooq NasarScott C Weaver Utilization of an Eilat Virus-Based Chimera for Serological Detection of Chikungunya Infection. PLOS Neglected Tropical Diseases 2015, 9, e0004119, 10.1371/journal.pntd.0004119.
- Jesse H. Erasmus; Robert L. Seymour; Jason T. Kaelber; Dal Young Kim; Grace Leal; Michael B. Sherman; Ilya Frolov; Wah Chiu; Scott C Weaver; Farooq Nasar; et al. Novel insect-specific Eilat virus-based chimeric vaccine candidates provide durable, mono- and multi-valent, single dose protection against lethal alphavirus challenge. Journal of Virology 2017, 92, JVI.01274-17, 10.1128/jvi.01274-17.