Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | René D. Peralta | + 2942 word(s) | 2942 | 2022-02-14 07:59:15 | | | |
| 2 | Jason Zhu | -3 word(s) | 2939 | 2022-02-21 03:03:24 | | | | |
| 3 | Lluvia Azhalea Guerrero | Meta information modification | 2939 | 2022-02-21 21:12:50 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Peralta, R.; Guerrero, L. Gemini Surfactants. Encyclopedia. Available online: https://encyclopedia.pub/entry/19647 (accessed on 04 March 2026).
Peralta R, Guerrero L. Gemini Surfactants. Encyclopedia. Available at: https://encyclopedia.pub/entry/19647. Accessed March 04, 2026.
Peralta, René, Lluvia Guerrero. "Gemini Surfactants" Encyclopedia, https://encyclopedia.pub/entry/19647 (accessed March 04, 2026).
Peralta, R., & Guerrero, L. (2022, February 19). Gemini Surfactants. In Encyclopedia. https://encyclopedia.pub/entry/19647
Peralta, René and Lluvia Guerrero. "Gemini Surfactants." Encyclopedia. Web. 19 February, 2022.
Copy Citation
Gemini surfactants are dimeric structures, composed of two hydrophobic chains and two hydrophilic heads, linked by a spacer at or near the head groups. They present lower CMC, better efficiency to form micelles, and solubilization capacity comparedto their conventional (monomeric) counterparts. They can also reduce the surface tension of water and the oil–water interfacial tension from 10 to 100 times. This behaviour depends mainly on the nature of their components (heads, hydrophobic chains and spacer); thus, their synthesis is focused mainly on varying the type and length of these components.
Gemini Surfactants
Structure
Applications
1. Structure
Gemini surfactants have attracted interest among the scientific community in various applications due to their very low CMC, greater solubilization power, as well as better wetting and foaming properties compared to single-chain surfactants [1]. Gemini surfactants have a polymorphic phase behaviour and a great variety of self-assembled structures forming aggregates that can be observed as micelles, bilayers, and vesicles, depending on the head groups, the size of the hydrophobic tails, and the nature of the spacer [2]. Figure 1 shows the structure of normal micelles obtained from conventional and gemini surfactants.
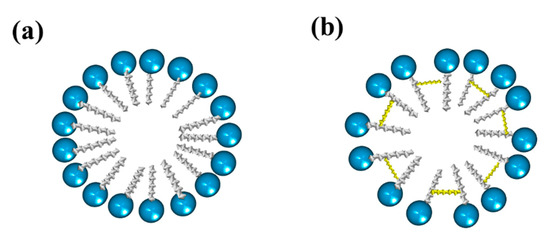
Figure 1. Representation of micelles formed from (a) conventional surfactants and (b) gemini surfactants.
2. Type of Gemini Surfactants
Gemini surfactants are classified by their physicochemical characteristics, groups present in the hydrophobic tails, and spacers. Regarding rigidity, the spacers in the chemical structure of a gemini surfactant can be classified into two subcategories, flexible (methylenes) and rigid (stilbene) (Figure 2a,b, respectively). Spacers also can be classified according to their length into short (Figure 2c) or long (Figure 2d). It is worth mentioning that the length of the spacer influences the geometry of the micelles. The presence of short spacers increases the repulsion between the head groups, resulting in micelles with a fiber-like structure, even at low concentrations of surfactant (Figure 3a). On the contrary, when the spacers are long, the micelles have elliptical geometries (Figure 3b). The transition from spherical micelles (Figure 3c) (4–8 carbon atoms in the spacer) to elliptical micelles occurs when repulsion between the groups of the polar heads decreases [3].
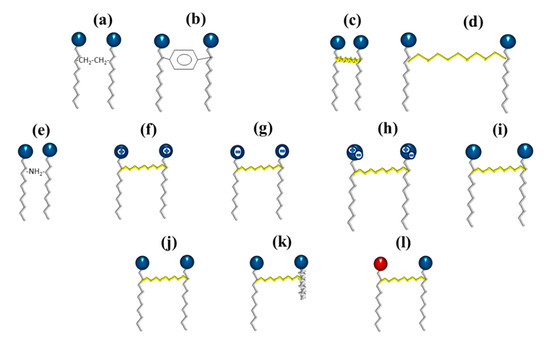
Figure 2. Schematic representation of the different types of gemini surfactants: (a) flexible spacer, (b) rigid spacer, (c) short chain spacer, (d) long-chain spacer, (e) polar spacer, (f) cationic, (g) anionic, (h) zwitterionic, (i) non-ionic, (j) two identical hydrophilic heads and hydrophobic chains, (k) two non-identical hydrophobic chains, and (l) two non-identical hydrophilic heads.
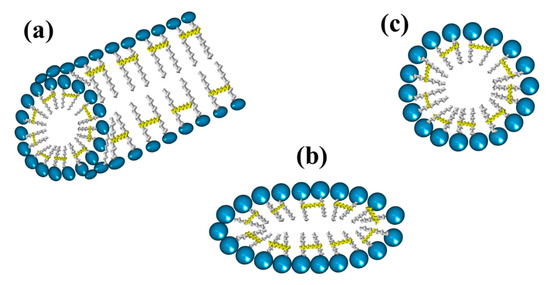
Figure 3. Geometries of micelles from gemini surfactants linked by the tail: (a) fiber-like, (b) elliptical, and (c) spherical.
On the other hand, the groups present in the spacer can be classified into polar (Figure 2e) or nonpolar (aliphatic and aromatic groups). Furthermore, the polar head can be positive, negative, zwitterionic, or non-ionic (Figure 2f–i). Finally, gemini dissymmetric (heterogeminis) surfactants contain two groups of non-identical polar heads (or identical) and different (or identical) lengths of alkyl tails, so they can also be classified into gemini surfactants of different head or hydrophobic tails and gemini surfactants of identical head and hydrophobic tails (Figure 2j–l) [4].
The surface activity of heterogeneous surfactants is highly dependent on the degree of asymmetry. For pyrene-based asymmetric gemini surfactants synthesized in five-step reactions, the Krafft temperature increases as the alkyl chain length increases. Similarly, the CMC values are much lower than their symmetric counterparts [5].
3. Synthesis Pathways
There are three main routes to synthesize symmetric gemini surfactants (Figure 4): (a) reaction of long chain tertiary amines with dihalogenated substrates as organic dibromides or dichlorides; (b) reaction of N,N,N′,N′-tetramethylpolymethylene diamines with alkyl halides; and (c) reaction of long chain tertiary amines with a haloalkylene oxide substrate.
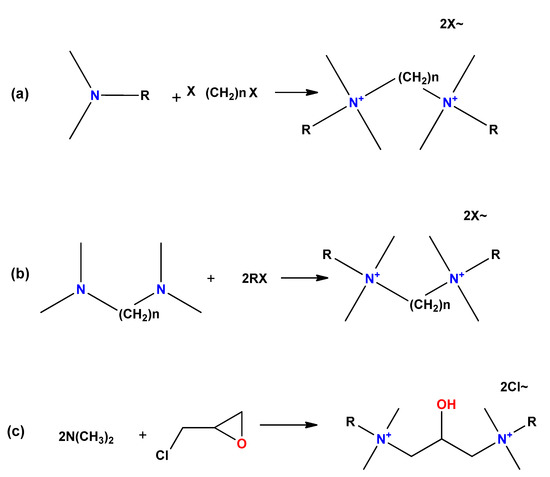
Figure 4. General routes to obtain gemini surfactants. (a) Reaction of long chain tertiary amines with dihalogenated substrates as organic dibromides or dichlorides; (b) reaction of N,N,N′,N′-tetramethylpolymethylene diamines with alkyl halides; and (c) reaction of long chain tertiary amines with a haloalkylene oxide substrate.
The yield of the synthesis of gemini surfactants depends mainly on the reactivity of the dihalogenoalkanes and the polarity and protic character of the solvent [6]. The best results have been achieved in aprotic and polar solvents. Some of these reactions can also be carried out without a solvent under mild conditions with very high yields [7]. Amino acid-based gemini surfactants are synthesized by condensation reactions at the amino group or the carboxyl group of the amino acid [8]. There are many studies on the synthesis and biological evaluation of gemini surfactants based on amino acids derived from arginine [9]. Some gemini surfactants have also been obtained from lysine, glycine, and cysteine [10][11]. Wang et al. synthesized a sugar-based gemini surfactant with a N, N′-acetylethylenediamine spacer (N,N′ (N-dodecyl-2-D-glucosaminyl acetyl) ethylenediamine and D-(+)-glucono-1,5-lactone as the starting material, in three steps. The CMC value (10−5 mol·L−1) determined by surface tension indicates a higher surface activity than the corresponding monomeric sugar-based surfactants [12]. With the aim of applying the surfactants in the oilfield, Hussain et al. [13] synthesized quaternary ammonium gemini surfactants with a different length of the spacer group (C8, C10, and C12), by solvent-free amidation of glycolic acid ethoxylate lauryl ether with 3-(dimethylamino)-1-propylamine. Similarly, Zhou et al. synthesized gemini surfactants in three steps using triethylene tetramine, fatty-acid methyl esters, ethyl chloride, N, N′-dimethyl ethylenediamine, and 3-chloro-2-hydroxypropane sulfonic acid sodium as the main raw materials to be applied in oilfields [14].
Thermodynamic and surface parameters are often evaluated for gemini surfactants. The effect of variations in the hydrophobic chain length of the gemini imidazolium surfactants on thermodynamic and surface parameters was studied by Ren et al. [15] (Figure 5). The results indicated that the micellization process could be both enthalpy and entropy driven, and that the increase in alkyl chain length causes the decreases in CMC and aggregation number.
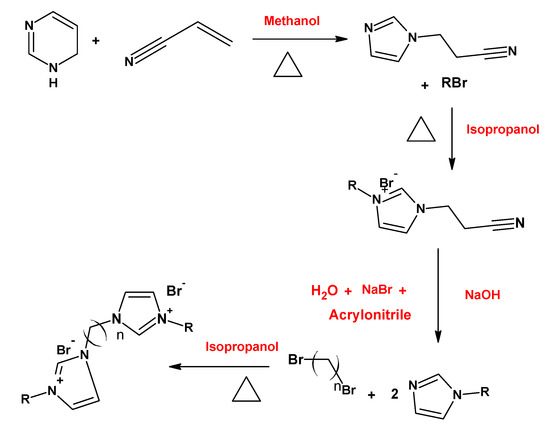
On the other hand, the synthesis and characterization of the anionic sulfonate gemini surfactants (Figure 6) with different hydrophobic chain length shows that this kind of surfactant presents a lower density, viscosity, and CMC than sodium dodecylbenzene sulfonate (SDBS), a monomeric surfactant with twelve carbon atoms in the hydrophobic chain [16].
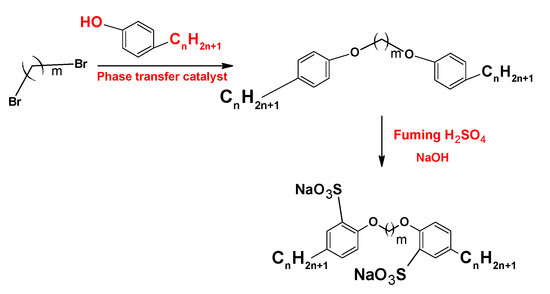
Figure 6. Synthesis of sulfonate gemini surfactants.
To improve the biodegradability of the cationic gemini surfactants, biodegradable moieties such as ester and amide groups have been used (Figure 7). It has been found that gemini surfactants are pH-responsive in alkaline conditions due to the ester group between the cationic head groups. The cationic gemini surfactants with an ester group in the spacer are more biodegradable than those with the ester bond in the tail [17].
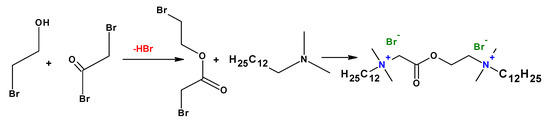
Figure 7. Synthesis of gemini surfactants containing an ester group.
4. Micelles Formation
Gemini surfactants can produce aggregates such as micelles, bilayers, vesicles, and other structures with different additives [18]. Several authors have carried out recent studies related to the formation of aggregates from gemini surfactants due to the benefits of these surfactants compared to those with a single hydrophobic chain.
A study of the interaction of the drug amitriptyline hydrochloride and the gemini surfactant ethane-1,2-diyl bis (N,N-dimethyl-N-tetradecylammonium acetoxy) (14-E2-14) in three aqueous media showed the high ability of gemini surfactants to form spherical micelles in aqueous systems [19].
Yang et al. studied the properties of different gemini surfactants synthesized with different sizes of hydrophobic chains [20]. During the analysis, they found that the size of the aggregates formed by the surfactants increased when the surfactant concentration was raised, reaching sizes from 200 to 400 nm. In the case of studies using the transmission electron microscopy (TEM) technique, surfactants with hydrophobic chains of 12, 16, and 18 carbon atoms formed spherical groups of hundreds of nanometers in solution with a tendency to form spherical aggregates.
In 2017, Feng et al. synthesized gemini alkyl glucoside surfactants to develop vesicles using (+)—catechin (C) and (−)—epigallocatechin (EGC) laureate, finding that the thermal stability of C or EGC was improved due to the encapsulation in more ordered structures. In addition, the incorporation of these drugs at low concentrations strengthened the bilayer formed [21].
In 2018, Gan et al. reported the formation of vesicles and micelles from gemini surfactants based on glucono-δ-lactone, which depended on the length of the hydrocarbon chain as well as the surfactant concentration [22].
In addition, there are studies on the influence of some parameters, such as the concentration, pH, temperature, and the presence of salts, on the morphology of aggregates formed by cationic gemini surfactants. These studies have shown a change from micelles to vesicles and vice versa by varying either the pH or temperature. Furthermore, the presence of salts may cause a transition from vesicles to micelles (Figure 8) [23].
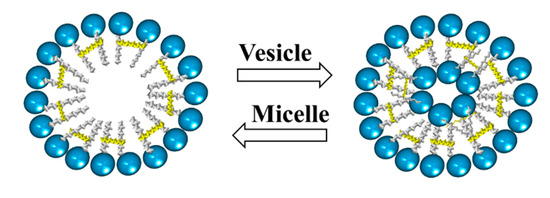
Figure 8. Scheme of transition from micelles to vesicles and vice versa.
More recently, Asadov et al. synthesized and characterized the cationic gemini surfactant N,N′-bis(alkyl)-N,N′-bis (2-hydroxypropyl) ethylene diammonium dibromide with chain lengths of 9, 12, and 14 carbon atoms [24]. They found that the aggregate diameters decreased when temperature was increased. In another work, Rajput et al. studied the effect of the addition of diclofenac sodium to gemini surfactant micellar aggregates, reporting a transition from micelles to vesicles as a result of an increase in the drug:gemini surfactant molar ratio. They claimed that the stability of vesicles at the human body temperature also makes them candidates for use in drug release [25].
5. Applications
Gemini surfactants have found application in medicine, physics, optics, and electronics. Polymerizable gemini anionic surfactants also have been synthesized to improve its interfacial properties [26]. These surfactants have been used as a template for the synthesis of nanoparticles. Tiwari et al. described the preparation and characterization of gold, silver, and gold-silver alloy nanoparticles using gemini surfactants as stabilizers of the nanoparticles around metal surfaces [27]. In addition, gemini surfactants have been used to obtain supramolecular solvents (SUPRAS), which are nanostructured liquids formed by aggregates of surfactants obtained through a self-assembly process. This type of solvent is assigned mainly to microextraction methods with applications in the cosmetic industry [28]. On the other hand, the formation of a spatial network of well-dispersed molecules is very important for biomedical and optoelectronic applications and these surfactants have been effective to form a three-dimensional network with supramolecular micellar hybridization [29]. Furthermore, these surfactants have been used as stabilizers in enhanced oil recovery [30]. For applications in this field, sulfonates gemini surfactants were shown to reduce the oil–water interfacial tension to ultralow values, around 10−3 mN/m, with surfactant concentrations less than 0.5 wt % [31]. Another important parameter for applications in oilfields, is thermal stability. In this sense, Hussain et al. studied the thermal degradation of three cationic poly(ethylene oxide) gemini surfactants containing flexible and rigid spacers. The thermal gravimetric analysis showed a degradation temperature higher than that observed in an oilfield (90 °C) [32].
In the polymer area, gemini surfactants play an important role in the synthesis of hybrid systems based on surfactant-polymer materials that have different applications. Hussain et al. investigated the properties of a surfactant-polymer hybrid material as candidate for carbonate reservoir at high temperatures [33]. They studied how the spacer length of the surfactant affects the rheological properties of the surfactant-polymer solutions. Furthermore, nanoemulsions stabilized by a gemini surfactant (14-6-14 GS) have been reported [34]. In the polymerization, Dreja and Thieke [35] reported the polymerization of styrene by free radicals at 25 °C in oil-in-water microemulsions stabilized by a series of cationic dimeric (gemini) surfactants and initiated by 60Co-γ-radiation. The resulting polymeric dispersions contained spherical latex particles (30–60 nm average diameter) and their size could be controlled by the monomer/surfactant ratio as well as by the surfactant spacer length. The polymer weight average molecular weight varied from 0.164 to 1.400 × 106 Da and depended on the spacer length and crosslinking. In a more recent study, Wang et al. [36] synthetized six quaternary ammonium salts from cardanol, a renewable resource, that can perform as gemini reactive surfactants. The surfactants, with a spacer consisting of a saturated aliphatic hydrocarbon chain, had a CMC of ≤0.2 mmol·L−1. A photo-active gemini surfactant with CMC = 0.05 mmol·L−1 was the stabilizer of a methyl methacrylate (MMA) emulsion, which was successfully polymerized using 2,2′-azobisisobutyronitrile as the initiator. Additionally, the gemini surfactant containing benzyl bromide was used as initiator and emulsifier during the atom transfer radical polymerization. The polymer obtained contained a cardanol-end unit and had an Mn = 45.1 kDa.
Regarding the use of gemini surfactants in the biomedical area, the research of Cardoso et al. studied the effectiveness of complexes of serine-derived gemini surfactants and DNA in mitochondrial expression [37]. For their part, Faustino et al. reported the synthesis of gemini anionic surfactants from L-cysteine, D-cysteine, DL-cysteine, and their monomeric counterparts (Figure 9a,b), as well as the study of their properties in solution at physiological pH. Gemini surfactants showed low CMC values and higher efficiency than their monomeric counterparts. Furthermore, surfactants were found to interact with bile acids, membrane phospholipids, oligosaccharides, and bovine serum albumin protein [11]. Furthermore, it has been reported that the solubilization of the drug amphotericin B (AmB) in micelles formed with an anionic gemini surfactant (derived from the amino acid cysteine) prevents self-aggregation of the drug, which makes it less toxic during administration (Figure 9c). In addition, the use of gemini surfactants avoids organic solvents, often used in the preparation of other drug carriers such as polymeric micelles, liposomes, and nanoparticles [38].
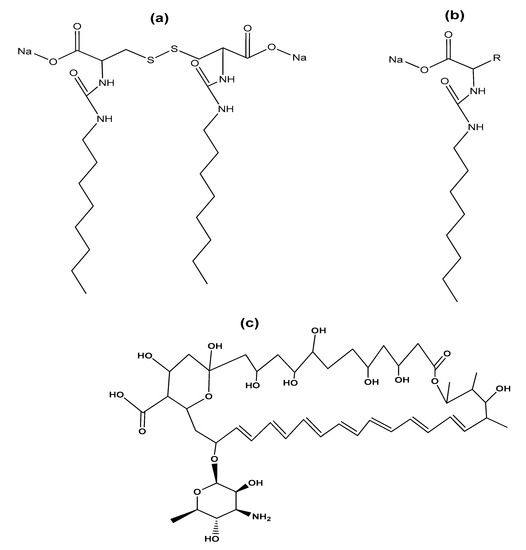
Figure 9. (a) Anionic gemini surfactant derived from cysteine and (b) its monomeric counterpart; (c) chemical structure of antifungal polyene antibiotic amphotericin B.
Specifically, in drug delivery, Cruz et al. used cationic gemini surfactants to deliver RNA for gioblastoma treatment [39], while Michel et al. developed a cationic gemini surfactant modified with β-cyclodextrin to improve the biological and physicochemical behavior of the drug mephalan. [40]. There are some reports on the use of amino acid-derived gemini surfactants for drug delivery; in this regard, lysine-derived surfactants have been used to form niosomes as delivery systems for the parenteral administration of the anticancer drug methotrexate [41]. Srivastava et al. developed gemini surfactant vesicles for encapsulation and release of the anticancer drug doxorubicin. They found that vesicles reduce the toxicity and showed better therapeutic effects at high drug concentrations [42]. Recently, Choi et al. synthesized disulfide-bridged gemini surfactants and their micellar properties were analyzed in the release of drugs for reactive oxygen species. The self-assembled surfactants as stable micellar aggregates were subjected to a reductive environment that caused destabilization of the micelles, suggesting that this response of the micelles could be used in the release of anticancer drugs [43].
On the other hand, one of the properties of gemini surfactants that allows their uses in medicine is their antimicrobial activity, for example, against Gram-positive bacteria such as Bacillis subtilis and Staphylococcus aureus [44]. This property makes them good capping agents for metal nanoparticles synthesis with unique and strengthened biocidal properties [45].
Cationic gemini surfactants have also found application as corrosion inhibitors (Figure 10) [46] and in the area of environmental protection, for example, in soil remediation to remove hydrophobic organic pollutants, heavy metals, and radionuclides from the soil [47].
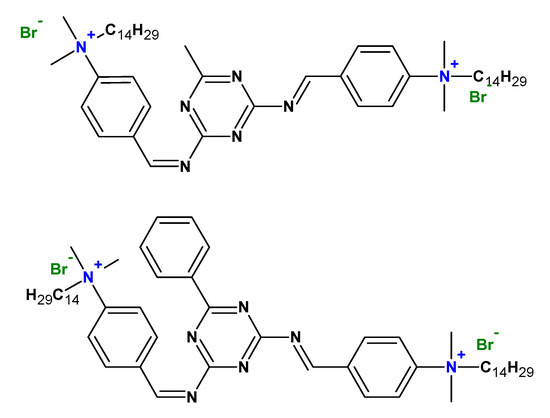
Figure 10. Chemical structures of some gemini surfactants used as corrosion inhibitors.
Otherwise, the study of the interactions between proteins and surfactants is very important due to the numerous technical applications in the fields of pharmaceuticals, cosmetics, paints, coatings, etc. [48][49][50][51]. Surfactants can cause the protein conformational changes via electrostatic and hydrophobic interactions, leading to the protein folding or unfolding depending on the concentrations of surfactants and proteins [52][53][54]
Recently, gemini surfactants were shown to be more efficient to interact with proteins by comparing them with single-chain surfactants [55][56][57][58]. Zhou et al. studied the effect of the structure of cationic surfactants on the conformation of bovine serum albumin (BSA) with a series of imidazolium gemini surfactants. The results showed that the gemini surfactant with either a shorter spacer or longer chain has a larger effect on BSA unfolding, and that the interactions of BSA with imidazolium gemini surfactants are stronger than those for single quaternary ammonium surfactants [59]. For their part, Branco et al. studied the interaction between a cationic amino acid-based gemini surfactant derived from cysteine and BSA under physiological conditions [60].
Luo et al. focused on the investigations of the interactions between single-chain or gemini quaternary ammonium surfactants with hemoglobin. They observed that the interactions between the surfactants and hemoglobin were mainly caused by both electrostatic and hydrophobic interactions, and the hydrophobic chain length and linking group length of the surfactants had a significant influence on tuning the conformations of hemoglobin [61]. For their part, Amiri et al. reported the interactions of gemini surfactants with ribonuclease Sa, and the results indicated that the tune of protein conformations is changed with the structure of surfactants and proteins [62]. More recently, Aslam et al. reported the preparation of pyridinium-based gemini surfactants and the study of interaction with BSA. They found a strong interaction between the gemini surfactants and protein due to the decrease of the CMC of surfactant as the BSA concentration was increased [63].
Micellar catalysis is a process that consists of the accumulation of a catalyst in the internal part of a micelle [64]. The micellar catalysis was shown to improve the reaction rate between the oil–water interphase and selectivity of the target molecules in organic reactions, such as electrophilic and nucleophilic substitution, hydrolysis, etc. [65][66].
Micellar catalysis using gemini surfactants was shown to have high catalytic efficiency and accelerate processes reducing the generation of secondary reactions [67][68]. Bunton et al. proposed for the first time the use of gemini surfactants in micellar catalysis [69]. The gemini surfactant synthesized by this group showed better catalytic efficiency than CTAB in nucleophilic substitutions reactions. Since then, more studies have been reported [70][71][72][73]. Micellar catalysis using gemini surfactants has been applied in reactions of ester hydrolysis [74], chloromethylation [75], and nucleophilic and electrophilic substitutions [68]. Furthermore, the catalytic properties of these surfactants have favoured the development of aqueous micellar catalytic processes, where the substitution of organic solvents for water is achieved, contributing to the development of more sustainable and environmentally friendly processes [76].
References
- Damen, M.; Cristóbal-Lecina, E.; Sanmartí, G.C.; Van Dongen, S.F.M.; García, C.L.; Dolbnya, I.P.; Roeland, J.M.; Feiters, M.C. Structure–delivery relationships of lysine-based gemini surfactants and their lipoplexes. Soft Matter 2014, 10, 5702–5714.
- Bordes, R.; Holmberg, K. Amino acid-based surfactants-Do they deserve more attention? Adv. Colloid Interface Sci. 2015, 222, 79–91.
- Zhai, Z.; Yan, X.; Song, Z.; Shang, S.; Rao, X. Annular and threadlike wormlike micelles formed by a bio-based surfactant containing an extremely large hydrophobic group. Soft Matter 2018, 14, 499–507.
- Hossain, M.; Roy, A.; Malik, S.; Ghosh, A.; Saha, B. Review on chemically bonded geminis with cationic heads: Second-generation interfactants. Res. Chem. Intermed. 2016, 42, 1913–1928.
- Al Muslim, A.; Ayyash, D.; Gujral, S.S.; Mekhail, G.M.; Rao, P.P.N.; Wettig, S.D. Synthesis and characterization of asymmetrical gemini surfactants. Phys. Chem. Chem. Phys. 2017, 19, 1953–1962.
- Lu, T.; Huang, J. Synthesis and properties of novel gemini surfactant with short spacer. Chin. Sci. Bull. 2007, 52, 2618–2620.
- Brycki, B.; Drgas, M.; Bielawska, M.; Zdziennicka, A.; Jańczuk, B. Synthesis, spectroscopic studies, aggregation and surface behavior of hexamethylene-1,6-bis(N,N-dimethyl-N dodecylammonium bromide). J. Mol. Liq. 2016, 221, 108.
- Morán, M.C.; Pinazo, A.; Pérez, L.; Clapés, P.; Angelet, M.; García, M.T.; Vinardell, P.; Infante, R. “Green” amino acid-based surfactants. Green Chem. 2004, 6, 233–240.
- Clapés, P.; Infante, R. Amino acid-based surfactants: Enzymatic synthesis, properties and potential applications. Biocatal. Biotransform. 2002, 20, 215–233.
- Colomer, A.; Pinazo, A.; Manresa, M.A.; Vinardell, M.P.; Mitjans, M.; Infante, M.R.; Pérez, L. Cationic Surfactants Derived from Lysine: Effects of Their Structure and Charge Type on Antimicrobial and Hemolytic Activities. J. Med. Chem. 2011, 54, 989–1002.
- Faustino, C.; Serafim, C.; Ferreira, I.; Pinheiro, L.; Calado, A. Solubilization power of an amino acid-based gemini surfactant towards the hydrophobic drug amphotericin B. Colloids Surfaces A Physicochem. Eng. Asp. 2015, 480, 426–432.
- Wang, R.; WanYan, R.; Yang, S.; Wang, D.; Yin, Z. Synthesis and aggregation of novel sugar-based gemini surfactant with a N, N’-acetylethylenediamine spacer in aqueous solution. J. Surfactants Deterg. 2020, 23, 697–703.
- Hussain, S.M.S.; Kamal, M.S.; Murtaza, M. Synthesis of Novel Ethoxylated Quaternary Ammonium Gemini Surfactants for Enhanced Oil Recovery Application. Energies 2019, 12, 1731.
- Zhou, M.; Zhou, L.; Guo, X. Synthesis of Sulfobetaine-Type Zwitterionic Gemini Surfactants (EAPMAC) and Their Oilfield Application Properties. J. Surfactants Deterg. 2019, 22, 23–32.
- Ren, C.; Wang, F.; Zhang, Z.; Nie, H.; Li, N.; Cui, M. Synthesis, surface activity and aggregation behavior of Gemini imidazolium surfactants 1,3-bis(3-alkylimidazolium-1-yl) propane bromide. Colloids Surfaces A Physicochem. Eng. Asp. 2015, 467, 1–8.
- Hordyjewicz-Baran, Z.; Woch, J.; Kuliszewska, E.; Zimoch, J.; Libera, M.; Dworak, A.; Trzebicka, B. Aggregation behavior of anionic sulfonate gemini surfactants with dodecylphenyl tails. Colloids Surfaces A Physicochem. Eng. Asp. 2015, 484, 336–344.
- Tehrani-Bagha, A.R.; Holmberg, K.; Ginkel, C.G.; Kean, M. Cationic gemini surfactants with cleavable spacer: Chemical hydrolysis, biodegradation, and toxicity. J. Colloid Interf. Sci. 2015, 449, 72–79.
- Kumar, D.; Abdul, M.R. Catalytic influence of 16-s-16 gemini surfactants on the rate constant of histidine and ninhydrin. Roy. Soc. Open Sci. 2020, 7, 191648.
- Abdul, R.M. Investigation of micellar and interfacial phenomenon of amitriptyline hydrochloride with cationic ester-bonded gemini surfactant mixture in different solvent media. PLoS ONE 2020, 15, e0241300.
- Yang, W.; Cao, Y.; Ju, H.; Wang, Y.; Jiang, Y.; Geng, T. Amide Gemini surfactants linked by rigid spacer group 1,4-dibromo-2-butene: Surface properties, aggregate and application properties. J. Mol. Liq. 2021, 326, 115339.
- Feng, J.; Lin, C.; Wang, H.; Liu, S. Gemini dodecyl O-glucoside-based vesicles as nanocarriers for catechin laurate. J. Funct. Foods 2017, 32, 256–265.
- Gan, C.; Li, H.; Cai, K. Novel Sugar-Based Gemini Surfactants and Their Surface Properties. J. Surfactants Deterg. 2018, 21, 859–866.
- Parikh, K.; Singh, S.; Kumar, S. Self assembly in an aqueous gemini surfactant containing sugar based (isosorbide) spacer. Arab. J. Chem. 2020, 13, 1848–1857.
- Asadov, Z.H.; Ahmadova, G.A.; Rahimov, R.A.; Hashimzade, S.-Z.F.; Ismailov, E.H.; Asadova, N.Z.; Suleymanova, S.A.; Zubkov, F.I.; Mammadov, A.M.; Agamaliyeva, D.B. Micellization and Adsorption Properties of New Cationic Gemini Surfactants Having Hydroxyisopropyl Group. J. Chem. Eng. Data 2019, 64, 952–962.
- Rajput, S.M.; Kumar, S.; Aswal, V.K.; El Seoud, O.A.; Malek, N.I.; Kailasa, S.K. Drug-Induced Micelle-to-Vesicle Transition of a Cationic Gemini Surfactant: Potential Applications in Drug Delivery. ChemPhysChem 2018, 19, 865–872.
- Sakai, K.; Wada, M.; Matsuda, W.; Tsuchiya, K.; Takamatsu, Y.; Tsubone, K.; Endo, T.; Torigoe, K.; Sakai, H.; Abe, M. Polymerizable anionic gemini surfactants: Physicochemical properties in aqueous solution and polymerization behavior. J. Oleo Sci. 2009, 58, 403–413.
- Tiwari, A.K.; Gangopadhyay, S.; Chang, C.-H.; Pande, S.; Saha, S.K. Study on metal nanoparticles synthesis and orientation of gemini surfactant molecules used as stabilizer. J. Colloid Interface Sci. 2015, 445, 76–83.
- Feizi, N.; Yamini, Y.; Moradi, M.; Karimi, M.; Salamat, Q.; Amanzadeh, H. A new generation of nano-structured supramolecular solvents based on propanol/gemini surfactant for liquid phase microextraction. Anal. Chim. Acta 2017, 953, 1–9.
- Fu, C.; He, D.; Yu, Y.; Wu, S.; Dong, C.; Wang, H. Fluorescent sensitization of gemini surfactant micellar-hybridized supramolecular hydrogels. J. Lumin. 2017, 181, 8–13.
- Pal, N.; Hoteit, H.; Mandal, A. Structural aspects, mechanisms and emerging prospects of Gemini surfactant-basedalternative Enhanced Oil Recovery technology: A review. J. Mol. Liq. 2021, 339, 116811.
- Mpelwa, M.; Tang, S.; Jin, L.; Hu, R. New sulfonate Gemini surfactants: Synthesis and evaluation for enhanced oil recovery applications. J. Dispers. Sci. Technol. 2020, 41, 2091–2099.
- Hussain, S.M.S.; Kamal, M.S.; Solling, T.; Murtaza, M.; Fogang, L.T. Surface and thermal properties of synthesized cationic poly(ethylene oxide) gemini surfactants: The role of the spacer. RSC Adv. 2019, 9, 30154–30163.
- Hussain, S.S.; Kamal, M.S. Effect of large spacer on surface activity, thermal, and rheological properties of novel amido-amine cationic gemini surfactants. J. Mol. Liq. 2017, 242, 1131–1137.
- Pal, N.; Kumar, N.; Saw, R.K.; Mandal, A. Gemini surfactant/polymer/silica stabilized oil-in-water nanoemulsions: Design and physicochemical characterization for enhanced oil recovery. J. Pet. Sci. Eng. 2019, 183, 106464.
- Dreja, M.; Thieke, B. Polymerization of styrene in ternary microemulsion using cationic gemini surfactants. Langmuir 1998, 14, 800–807.
- Wang, R.; Luo, Y.; Cheng, C.J.; Huang, Q.H.; Huang, H.S.; Qin, S.H.; Tu, Y.M. Syntheses of cardanol-based cationic surfactants and their use in emulsion polymerisation. Chem. Pap. 2016, 70, 1218–1227.
- Cardoso, A.M.; Morais, C.M.; Cruz, A.R.; Cardoso, A.L.; Silva, S.G.; Vale, M.L.; Marques, E.; Pedroso de Lima, M.C.; Jurado, A. Gemini surfactants mediate efficient mitochondrial gene delivery and expression. Mol. Pharm. 2015, 12, 716–730.
- Serafim, C.; Ferreira, I.; Rijo, P.; Pinheiro, L.; Faustino, C.; Calado, A.; Garcia-Rio, L. Lipoamino acid-based micelles as promising delivery vehicles for monomeric amphotericin B. Int. J. Pharm. 2016, 497, 23–35.
- Cruz, R.A.; Morais, C.M.; Cardoso, A.M.; Silva, S.G.; Luisa do Vale, M.; Marques, E.F.; Pedroso de Lima, M.C.; Jurado, A.S. Enhancing glioblastoma cell sensitivity to Chemotherapeutics: A strategy involving survin gen silencing mediated gemini surfactants-based complexes. Eur. J. Pharm. Biopharm. 2016, 104, 7–18.
- Michel, D.; Mohammed-Saeid, W.; Getson, H.; Roy, C.; Poorghorban, M.; Chitanda, J.M.; Verrall, R.; Badea, I. Evaluation of β-cyclodextrin-modified gemini surfactant-based delivery systems in melanoma models. Int. J. Nanomed. 2016, 11, 6703–6712.
- Muzzalupo, R.; Pérez, L.; Pinazo, A.; Tavano, L. Pharmaceutical versatility of cationic niosomes derived from amino acid-based surfactants: Skin penetration behavior and controlled drug release. Int. J. Pharm. 2017, 529, 245–252.
- Srivastava, A.; Liu, C.; Lv, J.; Deb, D.K.; Qiao, W. Enhanced intercellular release of anticancer drug by using nano-sized catanionic vesicles of doxorubicin hydrochloride and gemini surfactants. J. Mol. Liq. 2018, 259, 398–410.
- Choi, Y.I.; Choi, E.-S.; Mun, K.H.; Lee, S.G.; Lee, S.J.; Jeong, S.W.; Lee, S.W.; Kim, H.-C. Dual-responsive Gemini Micelles for Efficient Delivery of Anticancer Therapeutics. Polymers 2019, 11, 604.
- Gawali, I.; Usmani, G. Synthesis, surface active properties and applications of cationic gemini surfactants from triethylenetetramine. J. Disper. Sci. Technol. 2020, 41, 450–460.
- Chiappisi, L.; Keiderling, U.; Ulloa, C.E.G.; Gómez, R.; Valiente, M.; Gradzielski, M. Aggregation behavior of surfactants with cationic and anionic dendronic head groups. J. Colloid Interface Sci. 2019, 534, 430–439.
- Singh, R.K.; Kukrety, A.; Saxena, R.C.; Thakre, G.D.; Atray, N.; Ray, S.S. Novel Triazine Schiff Base-Based Cationic Gemini Surfactants: Synthesis and Their Evaluation as Antiwear, Antifriction, and Anticorrosive Additives in Polyol. Ind. Eng. Chem. Res. 2016, 55, 2520–2526.
- Mao, X.; Jiang, R.; Xiao, W.; Yu, J. Use of surfactants for the remediation of contaminated soils: A review. J. Hazard. Mater. 2015, 285, 419–435.
- Gospodarczyk, W.; Szutkowski, K.; Kozak, M. Interaction of Bovine Serum Albumin (BSA) with Novel Gemini Surfactants Studied by Synchrotron Radiation Scattering (SR-SAXS), Circular Dichroism (CD), and Nuclear Magnetic Resonance (NMR). J. Phys. Chem. B 2014, 118, 8652–8661.
- Akram, M.; Bhat, I.A.; Din, K.-U. Binding of a novel 12-E2-12 gemini surfactant to xanthine oxidase: Analysis involving tensiometric, spectroscopic, microscopic and molecular docking approach. J. Lumin. 2016, 170, 56–63.
- Bhat, I.A.; Roy, B. Synthesis and biophysical analysis of a novel gemini surfactant with lysozyme: Industrial perspective. J. Ind. Eng. Chem. 2018, 63, 348–358.
- Akram, M.; Bhat, I.A.; Anwar, S.; Ahmad, A.; Din, K.-U. Biophysical perspective of the binding of ester-functionalized gemini surfactants with catalase. Int. J. Biol. Macromol. 2016, 88, 614–623.
- Andersen, K.K.; Otzen, D.E. Denaturation of alpha-lactalbumin and myoglobin by the anionic biosurfactant rhamnolipid. Biochim. Biophys. Acta 2014, 1844, 2338–2345.
- Mehan, S.; Aswal, V.K.; Kohlbrecher, J. Cationic versus Anionic Surfactant in Tuning the Structure and Interaction of Nanoparticle, Protein, and Surfactant Complexes. Langmuir 2014, 30, 9941–9950.
- Kumar, D.; Rub, M.A.; Akram, M.; Din, K.-U. Effect of gemini (alkanediyl-α,ω-bis(dimethylcetylammonium bromide)) (16-s-16, s=4, 5, 6) surfactants on the interaction of ninhydrin with chromium-glycylphenylalanine. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 132, 288–294.
- Ge, Y.-S.; Tai, S.-X.; Xu, Z.-Q.; Lai, L.; Tian, F.-F.; Li, D.-W.; Jiang, F.-L.; Liu, Y.; Gao, Z.-N. Synthesis of Three Novel Anionic Gemini Surfactants and Comparative Studies of Their Assemble Behavior in the Presence of Bovine Serum Albumin. Langmuir 2012, 28, 5913–5920.
- Mir, M.A.; Khan, J.M.; Khan, R.H.; Rather, G.M.; Dar, A.A. Effect of spacer length of alkanediyl-α,ω-bis(dimethylcetylammonium bromide) gemini homologues on the interfacial and physicochemical properties of BSA. Colloids Surfaces B Biointerfaces 2010, 77, 54–59.
- Wang, Y.; Jiang, X.; Zhou, L.; Yang, L.; Xia, G.; Chen, Z.; Duan, M. Synthesis and binding with BSA of a new gemini surfactant. Colloids Surfaces A Physicochem. Eng. Asp. 2013, 436, 1159–1169.
- Faustino, C.M.C.; Calado, A.; Garcia-Rio, L. Gemini Surfactant−Protein Interactions: Effect of pH, Temperature, and Surfactant Stereochemistry. Biomacromolecules 2009, 10, 2508–2514.
- Zhou, T.; Ao, M.; Xu, G.; Liu, T.; Zhang, J. Interactions of bovine serum albumin with cationic imidazolium and quaternary ammonium gemini surfactants: Effects of surfactant architecture. J. Colloid Interface Sci. 2013, 389, 175–181.
- Branco, M.A.; Pinheiro, L.; Faustino, C. Amino acid-based cationic gemini surfactant–protein interactions. Colloids Surfaces A Physicochem. Eng. Asp. 2015, 480, 105–112.
- Luo, X.; Gao, J.; Cao, M.; Xiang, C.; Zhang, Y.; Sun, T.; Xie, H.; Lei, Q.; Fang, W. Tuning the conformations of hemoglobin via interactions with single-chain and Gemini quaternary ammonium surfactants. Chem. Phys. Lett. 2019, 728, 115–123.
- Amiri, R.; Bordbar, A.-K.; Laurents, D.V. Gemini Surfactants Affect the Structure, Stability, and Activity of Ribonuclease Sa. J. Phys. Chem. B 2014, 118, 10633–10642.
- Aslam, J.; Lone, I.H.; Ansari, F.; Aslam, A.; Aslam, R.; Akram, M. Molecular binding interaction of pyridinium based gemini surfactants with bovine serum albumin: Insights from physicochemical, multispectroscopic, and computational analysis. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 250, 119350.
- Mohammed, H.; Al-Hazmi, S.M.; Alhagri, I.A.; Alhakimi, A.N.; Dahadha, A.; Al-Dhoun, M.; Batineh, Y. Micellar catalysis of chemical reactions by mixed surfactant systems and gemini surfactants. Asian J. Chem. 2021, 33, 1471–1480.
- Balakrishnan, V.K.; Buncel, E.; Vanloon, G.W. Micellar Catalyzed Degradation of Fenitrothion, an Organophosphorus Pesticide, in Solution and Soils. Environ. Sci. Technol. 2005, 39, 5824–5830.
- Xu, D.-Q.; Pan, Z.-W. Phase-transfer catalysis of a new cationic gemini surfactant with ester groups for nucleophilic substitution reaction. Chin. Chem. Lett. 2014, 25, 1169–1173.
- Dileep, K.; Malik, A.R. Study of the interaction between ninhydrin and chromium(III)-amino acid in an aqueous-micellar system: Influence of gemini surfactant micelles. J. Mol. Liq. 2020, 301, 112373.
- Xu, D.; Wang, H.; Pan, Z.; Zhang, T. The kinetics and effect of a new gemini surfactant on the efficiency of micellar catalysis for the hydrolysis reaction of 4-nitrophenyl acetate. J. Mol. Liq. 2018, 250, 223–228.
- Bunton, C.A.; Robinson, L.B.; Schaak, J.; Stam, M.F. Catalysis of nucleophilic substitutions by micelles of dicationic detergents. J. Org. Chem. 1971, 36, 2346–2350.
- Mirgorodskaya, A.B.; Yackevich, E.I.; Lukashenko, S.S.; Zakharova, L.Y.; Konovalov, A.I. Solubilization and catalytic behavior of micellar system based on gemini surfactant with hydroxyalkylated head group. J. Mol. Liq. 2012, 169, 106–109.
- Jiang, W.; Xu, B.; Lin, Q.; Li, J.; Fu, H.; Zeng, X.; Chen, H. Cleavage of phosphate diesters mediated by Zn(II) complex in Gemini surfactant micelles. J. Colloid Interface Sci. 2007, 311, 530–536.
- Qiu, L.-G.; Jiang, X.; Gu, L.-N.; Hu, G. Gemini metallomicellar catalysis: Hydrolysis of p-nitrophenyl picolinate catalyzed by Cu(II) and Ni(II) complexes of macrocyclic ligands in gemini surfactant micelles. J. Mol. Catal. A Chem. 2007, 277, 15–20.
- Qiu, L.-G.; Xie, A.-J.; Shen, Y.-H. Micellar effects of a triazole-based cationic gemini surfactant on the rate of a nucleophilic aromatic substitution reaction. Colloid Polym. Sci. 2005, 283, 1343–1348.
- Qiu, L.-G.; Xie, A.-J.; Shen, Y.-H. Understanding the adsorption of cationic gemini surfactants on steel surface in hydrochloric acid. Mater. Chem. Phys. 2004, 87, 237–240.
- Liu, Q.F.; Lu, M.; Wei, W. Chloromethylation of 2-chloroethylbenzene catalyzed bymicellar catalysis. Acta Chim. Sin. 2009, 39, 440–446.
- Shen, T.; Zhou, S.; Ruan, J.; Chen, X.; Liu, X.; Ge, X.; Qian, C. Recent advances on micellar catalysis in water. Adv. Colloid Interface Sci. 2021, 287, 102299.
More
Information
Subjects:
Biochemical Research Methods
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
11.3K
Entry Collection:
Organic Synthesis
Revisions:
3 times
(View History)
Update Date:
21 Feb 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





