Gemini surfactants are dimeric structures, composed of two hydrophobic chains and two hydrophilic heads, linked by a spacer at or near the head groups. They present lower CMC, better efficiency to form micelles, and solubilization capacity comparedto their conventional (monomeric) counterparts. They can also reduce the surface tension of water and the oil–water interfacial tension from 10 to 100 times. This behaviour depends mainly on the nature of their components (heads, hydrophobic chains and spacer); thus, their synthesis is focused mainly on varying the type and length of these components.
- Gemini Surfactants
- Structure
- Applications
1. Structure
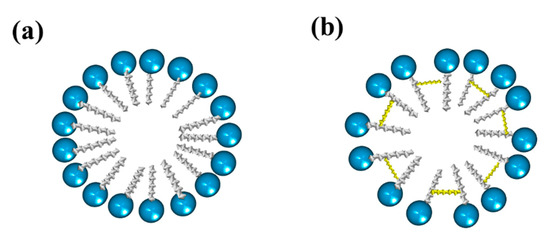
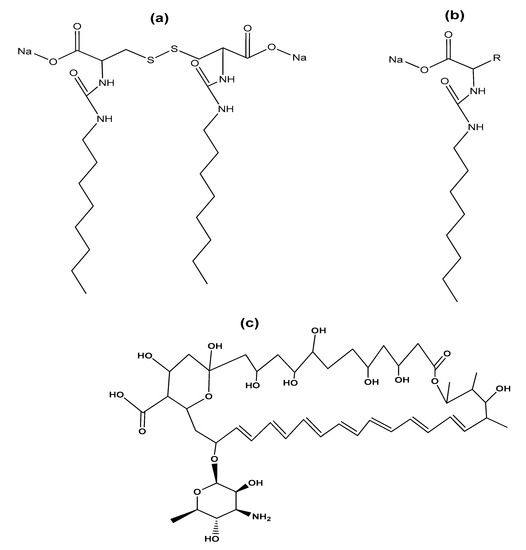
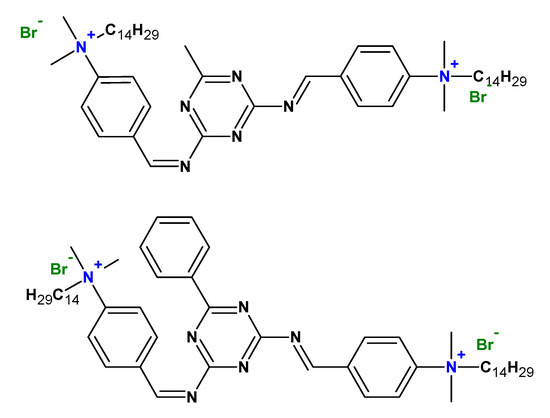
2. Type of Gemini Surfactants
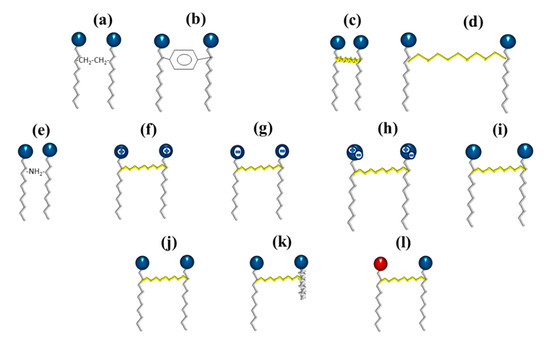
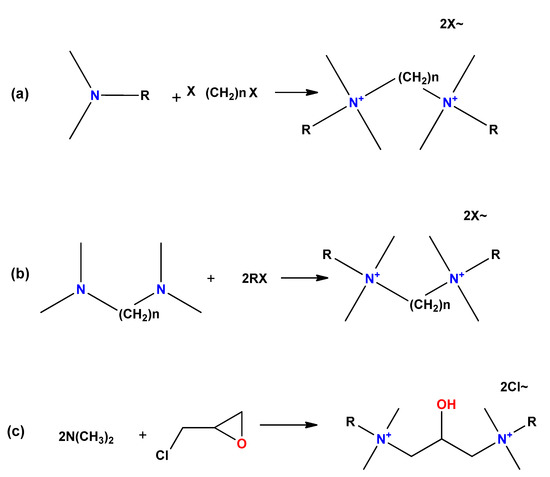
3. Synthesis Pathways
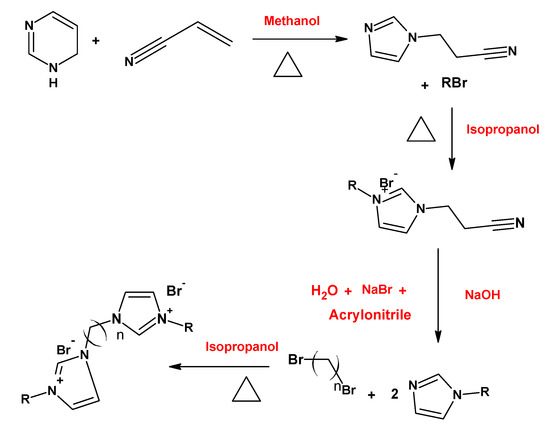
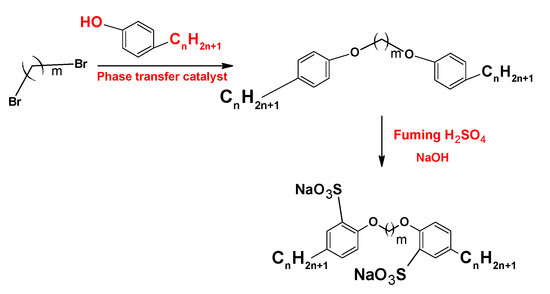
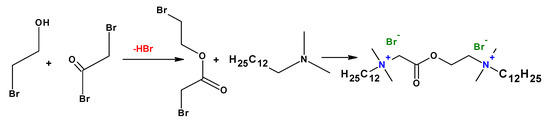
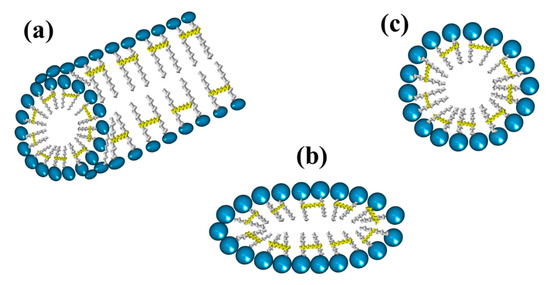
4. Micelles Formation
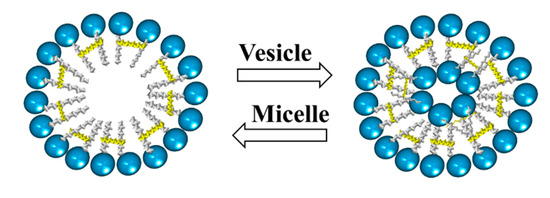
5. Applications
This entry is adapted from the peer-reviewed paper 10.3390/ijms23031798
References
- Damen, M.; Cristóbal-Lecina, E.; Sanmartí, G.C.; Van Dongen, S.F.M.; García, C.L.; Dolbnya, I.P.; Roeland, J.M.; Feiters, M.C. Structure–delivery relationships of lysine-based gemini surfactants and their lipoplexes. Soft Matter 2014, 10, 5702–5714.
- Bordes, R.; Holmberg, K. Amino acid-based surfactants-Do they deserve more attention? Adv. Colloid Interface Sci. 2015, 222, 79–91.
- Zhai, Z.; Yan, X.; Song, Z.; Shang, S.; Rao, X. Annular and threadlike wormlike micelles formed by a bio-based surfactant containing an extremely large hydrophobic group. Soft Matter 2018, 14, 499–507.
- Hossain, M.; Roy, A.; Malik, S.; Ghosh, A.; Saha, B. Review on chemically bonded geminis with cationic heads: Second-generation interfactants. Res. Chem. Intermed. 2016, 42, 1913–1928.
- Al Muslim, A.; Ayyash, D.; Gujral, S.S.; Mekhail, G.M.; Rao, P.P.N.; Wettig, S.D. Synthesis and characterization of asymmetrical gemini surfactants. Phys. Chem. Chem. Phys. 2017, 19, 1953–1962.
- Lu, T.; Huang, J. Synthesis and properties of novel gemini surfactant with short spacer. Chin. Sci. Bull. 2007, 52, 2618–2620.
- Brycki, B.; Drgas, M.; Bielawska, M.; Zdziennicka, A.; Jańczuk, B. Synthesis, spectroscopic studies, aggregation and surface behavior of hexamethylene-1,6-bis(N,N-dimethyl-N dodecylammonium bromide). J. Mol. Liq. 2016, 221, 108.
- Morán, M.C.; Pinazo, A.; Pérez, L.; Clapés, P.; Angelet, M.; García, M.T.; Vinardell, P.; Infante, R. “Green” amino acid-based surfactants. Green Chem. 2004, 6, 233–240.
- Clapés, P.; Infante, R. Amino acid-based surfactants: Enzymatic synthesis, properties and potential applications. Biocatal. Biotransform. 2002, 20, 215–233.
- Colomer, A.; Pinazo, A.; Manresa, M.A.; Vinardell, M.P.; Mitjans, M.; Infante, M.R.; Pérez, L. Cationic Surfactants Derived from Lysine: Effects of Their Structure and Charge Type on Antimicrobial and Hemolytic Activities. J. Med. Chem. 2011, 54, 989–1002.
- Faustino, C.; Serafim, C.; Ferreira, I.; Pinheiro, L.; Calado, A. Solubilization power of an amino acid-based gemini surfactant towards the hydrophobic drug amphotericin B. Colloids Surfaces A Physicochem. Eng. Asp. 2015, 480, 426–432.
- Wang, R.; WanYan, R.; Yang, S.; Wang, D.; Yin, Z. Synthesis and aggregation of novel sugar-based gemini surfactant with a N, N’-acetylethylenediamine spacer in aqueous solution. J. Surfactants Deterg. 2020, 23, 697–703.
- Hussain, S.M.S.; Kamal, M.S.; Murtaza, M. Synthesis of Novel Ethoxylated Quaternary Ammonium Gemini Surfactants for Enhanced Oil Recovery Application. Energies 2019, 12, 1731.
- Zhou, M.; Zhou, L.; Guo, X. Synthesis of Sulfobetaine-Type Zwitterionic Gemini Surfactants (EAPMAC) and Their Oilfield Application Properties. J. Surfactants Deterg. 2019, 22, 23–32.
- Ren, C.; Wang, F.; Zhang, Z.; Nie, H.; Li, N.; Cui, M. Synthesis, surface activity and aggregation behavior of Gemini imidazolium surfactants 1,3-bis(3-alkylimidazolium-1-yl) propane bromide. Colloids Surfaces A Physicochem. Eng. Asp. 2015, 467, 1–8.
- Hordyjewicz-Baran, Z.; Woch, J.; Kuliszewska, E.; Zimoch, J.; Libera, M.; Dworak, A.; Trzebicka, B. Aggregation behavior of anionic sulfonate gemini surfactants with dodecylphenyl tails. Colloids Surfaces A Physicochem. Eng. Asp. 2015, 484, 336–344.
- Tehrani-Bagha, A.R.; Holmberg, K.; Ginkel, C.G.; Kean, M. Cationic gemini surfactants with cleavable spacer: Chemical hydrolysis, biodegradation, and toxicity. J. Colloid Interf. Sci. 2015, 449, 72–79.
- Kumar, D.; Abdul, M.R. Catalytic influence of 16-s-16 gemini surfactants on the rate constant of histidine and ninhydrin. Roy. Soc. Open Sci. 2020, 7, 191648.
- Abdul, R.M. Investigation of micellar and interfacial phenomenon of amitriptyline hydrochloride with cationic ester-bonded gemini surfactant mixture in different solvent media. PLoS ONE 2020, 15, e0241300.
- Yang, W.; Cao, Y.; Ju, H.; Wang, Y.; Jiang, Y.; Geng, T. Amide Gemini surfactants linked by rigid spacer group 1,4-dibromo-2-butene: Surface properties, aggregate and application properties. J. Mol. Liq. 2021, 326, 115339.
- Feng, J.; Lin, C.; Wang, H.; Liu, S. Gemini dodecyl O-glucoside-based vesicles as nanocarriers for catechin laurate. J. Funct. Foods 2017, 32, 256–265.
- Gan, C.; Li, H.; Cai, K. Novel Sugar-Based Gemini Surfactants and Their Surface Properties. J. Surfactants Deterg. 2018, 21, 859–866.
- Parikh, K.; Singh, S.; Kumar, S. Self assembly in an aqueous gemini surfactant containing sugar based (isosorbide) spacer. Arab. J. Chem. 2020, 13, 1848–1857.
- Asadov, Z.H.; Ahmadova, G.A.; Rahimov, R.A.; Hashimzade, S.-Z.F.; Ismailov, E.H.; Asadova, N.Z.; Suleymanova, S.A.; Zubkov, F.I.; Mammadov, A.M.; Agamaliyeva, D.B. Micellization and Adsorption Properties of New Cationic Gemini Surfactants Having Hydroxyisopropyl Group. J. Chem. Eng. Data 2019, 64, 952–962.
- Rajput, S.M.; Kumar, S.; Aswal, V.K.; El Seoud, O.A.; Malek, N.I.; Kailasa, S.K. Drug-Induced Micelle-to-Vesicle Transition of a Cationic Gemini Surfactant: Potential Applications in Drug Delivery. ChemPhysChem 2018, 19, 865–872.
- Sakai, K.; Wada, M.; Matsuda, W.; Tsuchiya, K.; Takamatsu, Y.; Tsubone, K.; Endo, T.; Torigoe, K.; Sakai, H.; Abe, M. Polymerizable anionic gemini surfactants: Physicochemical properties in aqueous solution and polymerization behavior. J. Oleo Sci. 2009, 58, 403–413.
- Tiwari, A.K.; Gangopadhyay, S.; Chang, C.-H.; Pande, S.; Saha, S.K. Study on metal nanoparticles synthesis and orientation of gemini surfactant molecules used as stabilizer. J. Colloid Interface Sci. 2015, 445, 76–83.
- Feizi, N.; Yamini, Y.; Moradi, M.; Karimi, M.; Salamat, Q.; Amanzadeh, H. A new generation of nano-structured supramolecular solvents based on propanol/gemini surfactant for liquid phase microextraction. Anal. Chim. Acta 2017, 953, 1–9.
- Fu, C.; He, D.; Yu, Y.; Wu, S.; Dong, C.; Wang, H. Fluorescent sensitization of gemini surfactant micellar-hybridized supramolecular hydrogels. J. Lumin. 2017, 181, 8–13.
- Pal, N.; Hoteit, H.; Mandal, A. Structural aspects, mechanisms and emerging prospects of Gemini surfactant-basedalternative Enhanced Oil Recovery technology: A review. J. Mol. Liq. 2021, 339, 116811.
- Mpelwa, M.; Tang, S.; Jin, L.; Hu, R. New sulfonate Gemini surfactants: Synthesis and evaluation for enhanced oil recovery applications. J. Dispers. Sci. Technol. 2020, 41, 2091–2099.
- Hussain, S.M.S.; Kamal, M.S.; Solling, T.; Murtaza, M.; Fogang, L.T. Surface and thermal properties of synthesized cationic poly(ethylene oxide) gemini surfactants: The role of the spacer. RSC Adv. 2019, 9, 30154–30163.
- Hussain, S.S.; Kamal, M.S. Effect of large spacer on surface activity, thermal, and rheological properties of novel amido-amine cationic gemini surfactants. J. Mol. Liq. 2017, 242, 1131–1137.
- Pal, N.; Kumar, N.; Saw, R.K.; Mandal, A. Gemini surfactant/polymer/silica stabilized oil-in-water nanoemulsions: Design and physicochemical characterization for enhanced oil recovery. J. Pet. Sci. Eng. 2019, 183, 106464.
- Dreja, M.; Thieke, B. Polymerization of styrene in ternary microemulsion using cationic gemini surfactants. Langmuir 1998, 14, 800–807.
- Wang, R.; Luo, Y.; Cheng, C.J.; Huang, Q.H.; Huang, H.S.; Qin, S.H.; Tu, Y.M. Syntheses of cardanol-based cationic surfactants and their use in emulsion polymerisation. Chem. Pap. 2016, 70, 1218–1227.
- Cardoso, A.M.; Morais, C.M.; Cruz, A.R.; Cardoso, A.L.; Silva, S.G.; Vale, M.L.; Marques, E.; Pedroso de Lima, M.C.; Jurado, A. Gemini surfactants mediate efficient mitochondrial gene delivery and expression. Mol. Pharm. 2015, 12, 716–730.
- Serafim, C.; Ferreira, I.; Rijo, P.; Pinheiro, L.; Faustino, C.; Calado, A.; Garcia-Rio, L. Lipoamino acid-based micelles as promising delivery vehicles for monomeric amphotericin B. Int. J. Pharm. 2016, 497, 23–35.
- Cruz, R.A.; Morais, C.M.; Cardoso, A.M.; Silva, S.G.; Luisa do Vale, M.; Marques, E.F.; Pedroso de Lima, M.C.; Jurado, A.S. Enhancing glioblastoma cell sensitivity to Chemotherapeutics: A strategy involving survin gen silencing mediated gemini surfactants-based complexes. Eur. J. Pharm. Biopharm. 2016, 104, 7–18.
- Michel, D.; Mohammed-Saeid, W.; Getson, H.; Roy, C.; Poorghorban, M.; Chitanda, J.M.; Verrall, R.; Badea, I. Evaluation of β-cyclodextrin-modified gemini surfactant-based delivery systems in melanoma models. Int. J. Nanomed. 2016, 11, 6703–6712.
- Muzzalupo, R.; Pérez, L.; Pinazo, A.; Tavano, L. Pharmaceutical versatility of cationic niosomes derived from amino acid-based surfactants: Skin penetration behavior and controlled drug release. Int. J. Pharm. 2017, 529, 245–252.
- Srivastava, A.; Liu, C.; Lv, J.; Deb, D.K.; Qiao, W. Enhanced intercellular release of anticancer drug by using nano-sized catanionic vesicles of doxorubicin hydrochloride and gemini surfactants. J. Mol. Liq. 2018, 259, 398–410.
- Choi, Y.I.; Choi, E.-S.; Mun, K.H.; Lee, S.G.; Lee, S.J.; Jeong, S.W.; Lee, S.W.; Kim, H.-C. Dual-responsive Gemini Micelles for Efficient Delivery of Anticancer Therapeutics. Polymers 2019, 11, 604.
- Gawali, I.; Usmani, G. Synthesis, surface active properties and applications of cationic gemini surfactants from triethylenetetramine. J. Disper. Sci. Technol. 2020, 41, 450–460.
- Chiappisi, L.; Keiderling, U.; Ulloa, C.E.G.; Gómez, R.; Valiente, M.; Gradzielski, M. Aggregation behavior of surfactants with cationic and anionic dendronic head groups. J. Colloid Interface Sci. 2019, 534, 430–439.
- Singh, R.K.; Kukrety, A.; Saxena, R.C.; Thakre, G.D.; Atray, N.; Ray, S.S. Novel Triazine Schiff Base-Based Cationic Gemini Surfactants: Synthesis and Their Evaluation as Antiwear, Antifriction, and Anticorrosive Additives in Polyol. Ind. Eng. Chem. Res. 2016, 55, 2520–2526.
- Mao, X.; Jiang, R.; Xiao, W.; Yu, J. Use of surfactants for the remediation of contaminated soils: A review. J. Hazard. Mater. 2015, 285, 419–435.
- Gospodarczyk, W.; Szutkowski, K.; Kozak, M. Interaction of Bovine Serum Albumin (BSA) with Novel Gemini Surfactants Studied by Synchrotron Radiation Scattering (SR-SAXS), Circular Dichroism (CD), and Nuclear Magnetic Resonance (NMR). J. Phys. Chem. B 2014, 118, 8652–8661.
- Akram, M.; Bhat, I.A.; Din, K.-U. Binding of a novel 12-E2-12 gemini surfactant to xanthine oxidase: Analysis involving tensiometric, spectroscopic, microscopic and molecular docking approach. J. Lumin. 2016, 170, 56–63.
- Bhat, I.A.; Roy, B. Synthesis and biophysical analysis of a novel gemini surfactant with lysozyme: Industrial perspective. J. Ind. Eng. Chem. 2018, 63, 348–358.
- Akram, M.; Bhat, I.A.; Anwar, S.; Ahmad, A.; Din, K.-U. Biophysical perspective of the binding of ester-functionalized gemini surfactants with catalase. Int. J. Biol. Macromol. 2016, 88, 614–623.
- Andersen, K.K.; Otzen, D.E. Denaturation of alpha-lactalbumin and myoglobin by the anionic biosurfactant rhamnolipid. Biochim. Biophys. Acta 2014, 1844, 2338–2345.
- Mehan, S.; Aswal, V.K.; Kohlbrecher, J. Cationic versus Anionic Surfactant in Tuning the Structure and Interaction of Nanoparticle, Protein, and Surfactant Complexes. Langmuir 2014, 30, 9941–9950.
- Kumar, D.; Rub, M.A.; Akram, M.; Din, K.-U. Effect of gemini (alkanediyl-α,ω-bis(dimethylcetylammonium bromide)) (16-s-16, s=4, 5, 6) surfactants on the interaction of ninhydrin with chromium-glycylphenylalanine. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 132, 288–294.
- Ge, Y.-S.; Tai, S.-X.; Xu, Z.-Q.; Lai, L.; Tian, F.-F.; Li, D.-W.; Jiang, F.-L.; Liu, Y.; Gao, Z.-N. Synthesis of Three Novel Anionic Gemini Surfactants and Comparative Studies of Their Assemble Behavior in the Presence of Bovine Serum Albumin. Langmuir 2012, 28, 5913–5920.
- Mir, M.A.; Khan, J.M.; Khan, R.H.; Rather, G.M.; Dar, A.A. Effect of spacer length of alkanediyl-α,ω-bis(dimethylcetylammonium bromide) gemini homologues on the interfacial and physicochemical properties of BSA. Colloids Surfaces B Biointerfaces 2010, 77, 54–59.
- Wang, Y.; Jiang, X.; Zhou, L.; Yang, L.; Xia, G.; Chen, Z.; Duan, M. Synthesis and binding with BSA of a new gemini surfactant. Colloids Surfaces A Physicochem. Eng. Asp. 2013, 436, 1159–1169.
- Faustino, C.M.C.; Calado, A.; Garcia-Rio, L. Gemini Surfactant−Protein Interactions: Effect of pH, Temperature, and Surfactant Stereochemistry. Biomacromolecules 2009, 10, 2508–2514.
- Zhou, T.; Ao, M.; Xu, G.; Liu, T.; Zhang, J. Interactions of bovine serum albumin with cationic imidazolium and quaternary ammonium gemini surfactants: Effects of surfactant architecture. J. Colloid Interface Sci. 2013, 389, 175–181.
- Branco, M.A.; Pinheiro, L.; Faustino, C. Amino acid-based cationic gemini surfactant–protein interactions. Colloids Surfaces A Physicochem. Eng. Asp. 2015, 480, 105–112.
- Luo, X.; Gao, J.; Cao, M.; Xiang, C.; Zhang, Y.; Sun, T.; Xie, H.; Lei, Q.; Fang, W. Tuning the conformations of hemoglobin via interactions with single-chain and Gemini quaternary ammonium surfactants. Chem. Phys. Lett. 2019, 728, 115–123.
- Amiri, R.; Bordbar, A.-K.; Laurents, D.V. Gemini Surfactants Affect the Structure, Stability, and Activity of Ribonuclease Sa. J. Phys. Chem. B 2014, 118, 10633–10642.
- Aslam, J.; Lone, I.H.; Ansari, F.; Aslam, A.; Aslam, R.; Akram, M. Molecular binding interaction of pyridinium based gemini surfactants with bovine serum albumin: Insights from physicochemical, multispectroscopic, and computational analysis. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 250, 119350.
- Mohammed, H.; Al-Hazmi, S.M.; Alhagri, I.A.; Alhakimi, A.N.; Dahadha, A.; Al-Dhoun, M.; Batineh, Y. Micellar catalysis of chemical reactions by mixed surfactant systems and gemini surfactants. Asian J. Chem. 2021, 33, 1471–1480.
- Balakrishnan, V.K.; Buncel, E.; Vanloon, G.W. Micellar Catalyzed Degradation of Fenitrothion, an Organophosphorus Pesticide, in Solution and Soils. Environ. Sci. Technol. 2005, 39, 5824–5830.
- Xu, D.-Q.; Pan, Z.-W. Phase-transfer catalysis of a new cationic gemini surfactant with ester groups for nucleophilic substitution reaction. Chin. Chem. Lett. 2014, 25, 1169–1173.
- Dileep, K.; Malik, A.R. Study of the interaction between ninhydrin and chromium(III)-amino acid in an aqueous-micellar system: Influence of gemini surfactant micelles. J. Mol. Liq. 2020, 301, 112373.
- Xu, D.; Wang, H.; Pan, Z.; Zhang, T. The kinetics and effect of a new gemini surfactant on the efficiency of micellar catalysis for the hydrolysis reaction of 4-nitrophenyl acetate. J. Mol. Liq. 2018, 250, 223–228.
- Bunton, C.A.; Robinson, L.B.; Schaak, J.; Stam, M.F. Catalysis of nucleophilic substitutions by micelles of dicationic detergents. J. Org. Chem. 1971, 36, 2346–2350.
- Mirgorodskaya, A.B.; Yackevich, E.I.; Lukashenko, S.S.; Zakharova, L.Y.; Konovalov, A.I. Solubilization and catalytic behavior of micellar system based on gemini surfactant with hydroxyalkylated head group. J. Mol. Liq. 2012, 169, 106–109.
- Jiang, W.; Xu, B.; Lin, Q.; Li, J.; Fu, H.; Zeng, X.; Chen, H. Cleavage of phosphate diesters mediated by Zn(II) complex in Gemini surfactant micelles. J. Colloid Interface Sci. 2007, 311, 530–536.
- Qiu, L.-G.; Jiang, X.; Gu, L.-N.; Hu, G. Gemini metallomicellar catalysis: Hydrolysis of p-nitrophenyl picolinate catalyzed by Cu(II) and Ni(II) complexes of macrocyclic ligands in gemini surfactant micelles. J. Mol. Catal. A Chem. 2007, 277, 15–20.
- Qiu, L.-G.; Xie, A.-J.; Shen, Y.-H. Micellar effects of a triazole-based cationic gemini surfactant on the rate of a nucleophilic aromatic substitution reaction. Colloid Polym. Sci. 2005, 283, 1343–1348.
- Qiu, L.-G.; Xie, A.-J.; Shen, Y.-H. Understanding the adsorption of cationic gemini surfactants on steel surface in hydrochloric acid. Mater. Chem. Phys. 2004, 87, 237–240.
- Liu, Q.F.; Lu, M.; Wei, W. Chloromethylation of 2-chloroethylbenzene catalyzed bymicellar catalysis. Acta Chim. Sin. 2009, 39, 440–446.
- Shen, T.; Zhou, S.; Ruan, J.; Chen, X.; Liu, X.; Ge, X.; Qian, C. Recent advances on micellar catalysis in water. Adv. Colloid Interface Sci. 2021, 287, 102299.
