| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Wolfgang Kreisel | + 816 word(s) | 816 | 2020-08-31 15:01:44 | | | |
| 2 | Peter Tang | + 74 word(s) | 890 | 2020-09-11 04:33:09 | | | | |
| 3 | Peter Tang | Meta information modification | 890 | 2020-09-13 05:20:10 | | | | |
| 4 | Peter Tang | Meta information modification | 890 | 2020-09-13 05:29:01 | | | | |
| 5 | Peter Tang | Meta information modification | 890 | 2020-09-13 09:19:01 | | |
Video Upload Options
Here, we review the role of phosphodiesterases in regulation of portal pressure in healthy liver and in liver cirrhosis and we present data, that inhibitors of phosphodiesterase-5 (PDE-5) might be a promising novel therapeutic approach in cirrhotic portal hypertension.
1. Introduction
Liver cirrhosis is a frequent condition with high impact on patients' life expectancy and health care systems [1][2][3]. Cirrhotic portal hypertension (PH) gradually develops with deteriorating liver function and can lead to life-threatening complications [4][5][6][7]. Other than an increase in intrahepatic flow resistance due to morphological remodeling of the organ, a functional dysregulation of the sinusoids, the smallest functional units of liver vasculature, plays a pivotal role [5][7][8]. The current view of pathophysiology of PH has led to the hypothesis of the "NO-paradox" which describes a reduced NO availability inside the liver and an elevated NO production in the peripheral systemic circulation, necessitating a tailored therapeutic approach [8][9][10].
The two cyclic nucleotides cyclic adenosine monophosphate (cAMP) and cyclic guanosine monophosphate (cGMP) are intracellular second messengers regulating important metabolic or regulatory pathways. They are formed by adenylate cyclases (ACs) or guanylate cyclases (GCs) and degraded by phosphodiesterases (PDEs). Thus, PDEs affect various metabolic processes, inflammatory mediator production and action or function of ion channels, muscle contraction, and myocardial contractility [11][12][13][14]. The best known target of pharmacological modulation is PDE-5. An increasing number of PDE-5 inhibitors with different pharmacological profiles have been developed. Known indications are erectile dysfunction, pulmonal arterial hypertension, high altitude edema, and lower urinary tract syndromes. Potential emerging applications include heart failure, stroke, neurodegenerative diseases, diabetic nephropathy, peripheral arterial disease, peripheral neuropathy, intestinal motility disorders, COVID-19 (adjunct therapy only), and—as we propose—cirrhotic portal hypertension (PH) [14][15][16].
Vascular tone is primarily regulated by the nitric oxide-cyclic guanosine monophosphate (NO-cGMP) pathway, wherein soluble guanylate cyclase (sGC) and phosphodiesterase-5 (PDE-5) are key enzymes [17]. Data about pathobiochemistry of the sinusoidal regulation in healthy and cirrhotic liver are reviewed.
2. Results and Biochemical Model
Recent data showed characteristic alterations in the expression of these regulatory enzymes or metabolite levels in liver cirrhosis [8][18][19][20][21]. Additionally, a disturbed zonation of the components of this pathway along the sinusoids was detected [19][21][22][23].
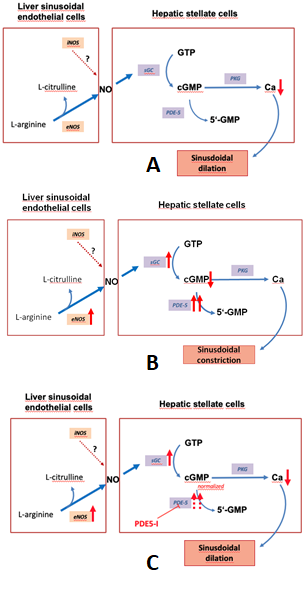
Figure 1 depicts the regulation of the NO-cGMP pathway in liver cirrhosis.
Figure 1. The nitric oxide-cyclic guanosine monophosphate (NO-cGMP) pathway, a regulator of sinusoidal tone (adapted from Schaffner).(A):Regulation of sinusoidal tone in healthy livers: the activation of the NO-cGMP pathway takes place once NO is generated by eNOS in sinusoidal endothelial cells and diffuses into the neighboring hepatic stellate cells, where it binds to the enzyme sGC. The following activation of sGC, in turn, catalyzes the conversation of GTP to cGMP. cGMP, an intracellular second messenger, triggers distinct downstream ignalling effects, which eventually exert vasodilation. As a negative feedback mechanism, rising cGMP concentrations initiate the activation of the enzyme PDE-5 which mediates cGMP inactivation. (B): Disturbed regulation of sinusoidal tone in liver cirrhosis: altered expression of key enzymes in the NO-cGMP pathway lead to reduced cGMP concentrations and thus sinusoidal constriction. (C): Effects of PDE-5 inhibitors in liver cirrhosis: application of PDE-5 inhibitors lead to a normalization of cGMP concentrations and thus sinusoidal dilation. (↑ increased expression; ↑↑ markedly increased expression; ↓ decreased concentration; ⊥ inhibition).
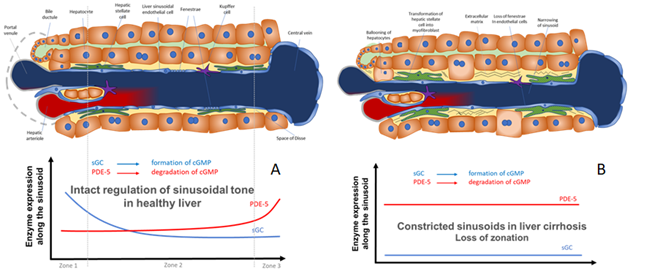
Figure 2. Model for the regulation of sinusoidal tone by the key enzymes soluble guanylate (sGC) and PDE-5 in healthy and diseased liver. The role of zonation of sGC and PDE-5 in healthy and cirrhotic liver. (A) In healthy livers an opposing zonation of sGC and PDE-5 may lead to a high cGMP production in the peripheral parts of the hepatic lobule, in which cGMP may exert its physiological function inside the sinusoids. However, excess cGMP might be degraded by high PDE-5 presence (zone 3) before entering the extrahepatic vasculature. (B) In cirrhotic livers the loss of physiological hepatic zonation, marked PDE-5 overexpression, and resulting increase in cGMP degradation might lead to sinusoidal constriction and elevation of portal pressure.
In Figure 2 we show a biochemical model how the opposing zonation of the enzymes sGC and PDE-5 and the loss of correct zonation in liver cirrhosis may regulate the sinusoidal diameter, thus regulating portal pressure.
Experimental and clinical data showed, that inhibitors of PDE-5 induced a reduction of portal pressure that may predict a beneficial effect in terms of reduced rate of esophageal variceal bleeding or rebleeding and other sequelae of liver cirrhosis [19][20][24][25][26][27][28][29]. The reviewed data suggest that deranged cGMP availability better explains the contrasting findings of intrahepatic vasoconstriction and peripheral systemic vasodilation than the mere focus on NO. Thus, we suggest considering the term "cGMP-paradox" to describe the circulatory findings in liver cirrhosis: eNOS and sGC are overexpressed in cirrhosis. However, this effect is overridden by a very marked overexpression of PDE-5. This results in low levels of cGMP inside the cirrhotic liver leading to sinusoidal constriction. Inhibition of PDE-5 normalizes cGMP levels and lowers portal pressure [19]. In peripheral arteries both eNOS and sGC are upregulated, however PDE-5 is downregulated [30][31][32]. This results in high peripheral cGMP and systemic vasodilation.
3. Conclusions
Since altered activities and/or zonation of sGC and PDE-5 may play a pivotal role in this process, these enzymes should be investigated more systematically as potential targets in medical therapy of PH. Moreover, since there are first hints showing antifibrotic effects induced by sGC and PDE-5, these might represent interesting targets for the medical therapy of liver fibrosis/cirrhosis.
References
- Tsochatzis EA, Bosch J, Burroughs AK. Liver cirrhosis. Lancet 2014, 383,1749–1761.
- De Franchis, R.; Primignani, M. Why do varices bleed? Gastroenterol. Clin. North Am. 1992, 21, 85–101.
- de Franchis R, Baveno V Faculty. Revising consensus in portal hypertension: report of the Baveno V consensus workshop on methodology of diagnosis and therapy in portal hypertension. J Hepatol 2010;53:762–8 [PMID: 20638742 DOI: 10.1016/j.jhep.2010.06.004].
- Kalra A, Yetiskul E, Wehrle CJ, Tuma F. Physiology, Liver [Internet]. In: StatPearls. Treasure Island (FL): StatPearls Publishing; 2020 [cited 2020 Aug 17]. Available from: http://www.ncbi.nlm.nih.gov/books/NBK535438/.
- Bosch, J. Vascular Deterioration in Cirrhosis. J. Clin. Gastroenterol. 2007, 41, S247–S253, doi:10.1097/mcg.0b013e3181572357.
- Turco, L.; Garcia-Tsao, G. Portal Hypertension: Pathogenesis and Diagnosis. Clin. Liver Dis. 2019, 23, 573–587, doi:10.1016/j.cld.2019.07.007.
- Gracia-Sancho, J.; Maeso-Díaz, R.; Bosch, J. Pathophysiology and a Rational Basis of Therapy. Dig. Dis. 2015, 33, 508–514, doi:10.1159/000374099.
- Iwakiri, Y. Pathophysiology of portal hypertension. Clin. Liver Dis. 2014, 18, 281–91, doi:10.1016/j.cld.2013.12.001.
- Shah, V.H.; Lyford, G.; Gores, G.; Farrugia, G. Nitric oxide in gastrointestinal health and disease. Gastroenterol. 2004, 126, 903–913, doi:10.1053/j.gastro.2003.11.046.
- Langer, D.A.; Shah, V.H. Nitric oxide and portal hypertension: Interface of vasoreactivity and angiogenesis. J. Hepatol. 2006, 44, 209–216, doi:10.1016/j.jhep.2005.10.004.
- Corbin, J.D.; Francis, S.H. Cyclic GMP Phosphodiesterase-5: Target of Sildenafil. J. Boil. Chem. 1999, 274, 13729–13732, doi:10.1074/jbc.274.20.13729.
- Lincoln, T.M. Cyclic GMP and Phosphodiesterase 5 Inhibitor Therapies: What’s on the Horizon?: Fig. 1. Mol. Pharmacol. 2004, 66, 11–13, doi:10.1124/mol.104.001388.
- Manallack DT, Hughes RA, Thompson PE. The next generation of phosphodiesterase inhibitors: structural clues to ligand and substrate selectivity of phosphodiesterases. J Med Chem 2005;48:3449–62 [PMID: 15887951 DOI: 10.1021/jm040217u].
- Ribaudo, G.; Pagano, M.A.; Bova, S.; Zagotto, G. New Therapeutic Applications of Phosphodiesterase 5 Inhibitors (PDE5-Is). Curr. Med. Chem. 2016, 23, 1239–1249, doi:10.2174/0929867323666160428110059.
- Francis, S.H.; Busch, J.L.; Corbin, J.D. cGMP-Dependent Protein Kinases and cGMP Phosphodiesterases in Nitric Oxide and cGMP Action. Pharmacol. Rev. 2010, 62, 525–563, doi:10.1124/pr.110.002907.
- Jeon, Y.H.; Heo, Y.-S.; Kim, C.M.; Hyun, Y.-L.; Lee, T.G.; Ro, S.; Cho, J.M. Phosphodiesterase: overview of protein structures, potential therapeutic applications and recent progress in drug development. Cell. Mol. Life Sci. 2005, 62, 1198–1220, doi:10.1007/s00018-005-4533-5.
- Münzel T, Feil R, Mülsch A, Lohmann SM, Hofmann F, Walter U. Physiology and Pathophysiology of Vascular Signaling Controlled by Cyclic Guanosine 3′,5′-Cyclic Monophosphate–Dependent Protein Kinase. Circulation 2003;108:2172–83 [DOI: 10.1161/01.CIR.0000094403.78467.C3].
- Tsuchida, T.; Friedman, S.L. Mechanisms of hepatic stellate cell activation. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 397–411, doi:10.1038/nrgastro.2017.38.
- Schaffner, D.; Lazaro, A.; Deibert, P.; Hasselblatt, P.; Stoll, P.; Fauth, L.; Baumstark, M.W.; Merfort, I.; Schmitt-Graeff, A.; Kreisel, W. Analysis of the nitric oxide-cyclic guanosine monophosphate pathway in experimental liver cirrhosis suggests phosphodiesterase-5 as potential target to treat portal hypertension. World J. Gastroenterol. 2018, 24, 4356–4368, doi:10.3748/wjg.v24.i38.4356.
- Schwabl, P.; Brusilovskaya, K.; Supper, P.; Bauer, D.; Königshofer, P.; Riedl, F.; Hayden, H.; Fuchs, C.D.; Stift, J.; Oberhuber, G.; et al. The soluble guanylate cyclase stimulator riociguat reduces fibrogenesis and portal pressure in cirrhotic rats. Sci. Rep. 2018, 8, 9372, doi:10.1038/s41598-018-27656-y.
- Davies, N.A.; Hodges, S.J.; Pitsillides, A.A.; Mookerjee, R.; Jalan, R.; Mehdizadeh, S. Hepatic guanylate cyclase activity is decreased in a model of cirrhosis: A quantitative cytochemistry study. FEBS Lett. 2006, 580, 2123–2128, doi:10.1016/j.febslet.2006.02.080.
- Hall, K.C.; Bernier, S.G.; Jacobson, S.; Liu, G.; Zhang, P.Y.; Sarno, R.; Catanzano, V.; Currie, M.G.; Masferrer, J.L. sGC stimulator praliciguat suppresses stellate cell fibrotic transformation and inhibits fibrosis and inflammation in models of NASH. Proc. Natl. Acad. Sci. 2019, 116, 11057–11062, doi:10.1073/pnas.1821045116.
- Su, T.; Yang, Y.; Lai, S.; Jeong, J.; Jung, Y.; McConnell, M.; Utsumi, T.; Iwakiri, Y. Single-cell transcriptomics reveals zone-specific alterations of liver sinusoidal endothelial cells in cirrhosis 2020.
- Deibert, P.; Schumacher, Y.-O.; Ruecker, G.; Opitz, O.G.; Blum, H.E.; Rossle, M.; Kreisel, W. Effect of vardenafil, an inhibitor of phosphodiesterase-5, on portal haemodynamics in normal and cirrhotic liver - results of a pilot study. Aliment. Pharmacol. Ther. 2006, 23, 121–128, doi:10.1111/j.1365-2036.2006.02735.x.
- Halverscheid, L.; Deibert, P.; Schmidt, R.; Blum, H.E.; Dunkern, T.; Pannen, B.H.; Kreisel, W. Phosphodiesterase-5 inhibitors have distinct effects on the hemodynamics of the liver. BMC Gastroenterol. 2009, 9, 69, doi:10.1186/1471-230x-9-69.
- Choi, S.-M.; Shin, J.-H.; Kim, J.-M.; Lee, C.-H.; Kang, K.-K.; Ahn, B.-O.; Yoo, M. Effect of Udenafil on Portal Venous Pressure and Hepatic Fibrosis in Rats. Arzneimittelforschung 2011, 59, 641–646, doi:10.1055/s-0031-1296453.
- Kreisel, W.; Deibert, P.; Kupcinskas, L.; Sumskiene, J.; Appenrodt, B.; Roth, S.; Neagu, M.; Rössle, M.; Zipprich, A.; Caca, K.; et al. The phosphodiesterase-5-inhibitor udenafil lowers portal pressure in compensated preascitic liver cirrhosis. A dose-finding phase-II-study. Dig. Liver Dis. 2015, 47, 144–150, doi:10.1016/j.dld.2014.10.018.
- Uschner, F.E.; Glückert, K.; Paternostro, R.; Gnad, T.; Schierwagen, R.; Mandorfer, M.; Magdaleno, F.; Ortiz, C.; Schwarzkopf, K.M.; Kamath, P.S.; et al. Combination of phosphodiesterase‐5‐inhibitors and beta blockers improves experimental portal hypertension and erectile dysfunction. Liver Int. 2020, doi:10.1111/liv.14586.
- Brusilovskaya, K.; Konigshofer, P.; Lampach, D.; Szodl, A.; Supper, P.; Bauer, D.; Beer, A.; Stift, J.; Timelthaler, G.; Oberhuber, G.; Podesser, B.-K.; Seif1, M.; Zinober, K.; Rohr-Udilova, N.; Trauner, M.; Reiberger, T.; Schwabl, P. Soluble guanylyl cyclase stimulation and phosphodiesterase-5 inhibition improve portal hypertension and reduce liver fibrosis in bile duct-ligated rats [Internet]. United European gastroenterology journal2020 [cited 2020 Sep 10];[PMID: 32878579 DOI: 10.1177/2050640620944140]Available from: https://pubmed.ncbi.nlm.nih.gov/32878579/.
- Kirstetter P, Moreau R, Vachiery F, Gadano A, Soupison T, Pilette C, Pussard E, Cailmail S, Takahashi H, Lebrec D. Plasma concentrations of cyclic 3’, 5’-guanosine monophosphate in patients with cirrhosis: relationship with atrial natriuretic peptide and haemodynamics. J Gastroenterol Hepatol 1997;12:233–6 [PMID: 9142641].
- Corbalán, R.; Montoliu, C.; Minana, M.D.; Del Olmo, J.A.; Serra, M. Á.; Aparisi, L.; Rodrigo, R.; Felipo, V. Altered Modulation of Soluble Guanylate Cyclase by Nitric Oxide in Patients with Liver Disease. Metab. Brain Dis. 2002, 17, 295–301, doi:10.1023/a:1021953717331.
- Felipo, V.; Urios, A.; Giménez-Garzó, C.; Cauli, O.; Andrés-Costa, M.-J.; González, O.; A Serra, M.; Sánchez-González, J.; Aliaga, R.; Giner-Durán, R.; et al. Non invasive blood flow measurement in cerebellum detects minimal hepatic encephalopathy earlier than psychometric tests. World J. Gastroenterol. 2014, 20, 11815–25, doi:10.3748/wjg.v20.i33.11815.