LivHer cirrhosis is a frequent condition with high impact on patients’ life expectancy and health care systems. Cirrhotic portal hypertension (PH) gradually develops with deteriorating liver function and can lead to life-threatening complications. Other than an increase in intrahepatic flow resistance due to morphological remodeling of the organ, a functional dysre, we review the role of phosphodiesterases in regulation of the sinusoids, the smallest functional units of liver vasculature, plays a pivotal role. The current view of pathophysiology of PH has led to the hypothesis of the “NO-paradox” which describes a reduced NO availability inside the liver and an elevated NO production in the peripheral systemic circulation, necessitating a tailored therapeutic approach.
The two cyclic portal pressure in healthy liver anucleotides cyclic adenosine monophosphate (cAMP) and cyclic guanosine monophosphate (cGMP) are intracellular second messengers regulating important metabolic or regulatory pathways. They are formed by adenylate cyclases (ACs) or guanylate cyclases (GCs) and degraded by phosphodiesterases (PDEs). Thus, PDEs affect various metabolic processes, inflammatory mediator production and action or function of ion channels, muscle contraction, and myocardial contractility. The best known target of pharmacological modulation is PDE-5. An increasing number of PDE-5 inhibitors with different pharmacological profiles have been developed. Known indications are erectile dysfunction, pulmonal arterial hypertension, high altitude edema, and lower urinary tract syndromes. Potential emerging applications include heart failure, stroke, neurodegenerative diseases, diabetic nephropathy, peripheral arterial disease, peripheral neuropathy, intestinal motility disorders, COVID-19 (adjunct therapy only), and—as we propose—c in liver cirrhosis and it presents data, that inhibitors of phosphodiesterase-5 (PDE-5) might be a promising novel therapeutic approach in cirrhotic portal hypertension (PH).
Vascular tone is primarily regulated by the nitric oxide-cyclic guanosine monophosphate (NO-cGMP) pathway, wherein soluble guanylate cyclase (sGC) and phosphodiesterase-5 (PDE-5) are key enzymes. Data about pathobiochemistry of the sinusoidal regulation in healthy and cirrhotic liver are reviewed. .
- portal hypertension
- liver cirrhosis
- phosphodiesterase-5 inhibitors
- nitric oxide
- cGMP
- metabolic zonation
Results
1. Introduction
Liver cirrhosis is a frequent condition with high impact on patients' life expectancy and health care systems. Cirrhotic portal hypertension (PH) gradually develops with deteriorating liver function and can lead to life-threatening complications. Other than an increase in intrahepatic flow resistance due to morphological remodeling of the organ, a functional dysregulation of the sinusoids, the smallest functional units of liver vasculature, plays a pivotal role. The current view of pathophysiology of PH has led to the hypothesis of the "NO-paradox" which describes a reduced NO availability inside the liver and an elevated NO production in the peripheral systemic circulation, necessitating a tailored therapeutic approach.
The two cyclic nucleotides cyclic adenosine monophosphate (cAMP) and cyclic guanosine monophosphate (cGMP) are intracellular second messengers regulating important metabolic or regulatory pathways. They are formed by adenylate cyclases (ACs) or guanylate cyclases (GCs) and degraded by phosphodiesterases (PDEs). Thus, PDEs affect various metabolic processes, inflammatory mediator production and action or function of ion channels, muscle contraction, and myocardial contractility. The best known target of pharmacological modulation is PDE-5. An increasing number of PDE-5 inhibitors with different pharmacological profiles have been developed. Known indications are erectile dysfunction, pulmonal arterial hypertension, high altitude edema, and lower urinary tract syndromes. Potential emerging applications include heart failure, stroke, neurodegenerative diseases, diabetic nephropathy, peripheral arterial disease, peripheral neuropathy, intestinal motility disorders, COVID-19 (adjunct therapy only), and—as we propose—cirrhotic portal hypertension (PH).
Recent data showed characteristic alterations in the expression of these regulatory enzymes or metabolite levels in liver cirrhosis. Additionally, a disturbed zonation of the components of this pathway along the sinusoids was detected.
Vascular tone is primarily regulated by the nitric oxide-cyclic guanosine monophosphate (NO-cGMP) pathway, wherein soluble guanylate cyclase (sGC) and phosphodiesterase-5 (PDE-5) are key enzymes. Data about pathobiochemistry of the sinusoidal regulation in healthy and cirrhotic liver are reviewed.
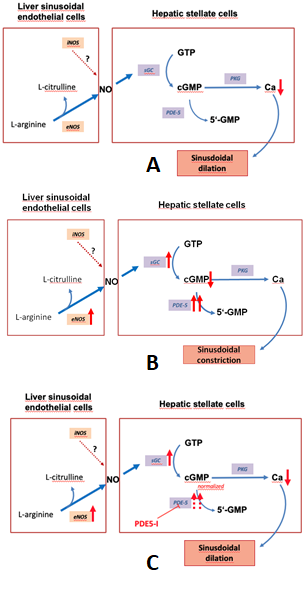
Figure 1 depicts the regulation of the NO-cGMP pathway in liver cirrhosis.
2. Results and Biochemical Model
Recent data showed characteristic alterations in the expression of these regulatory enzymes or metabolite levels in liver cirrhosis. Additionally, a disturbed zonation of the components of this pathway along the sinusoids was detected.
Figure 1. The nitric oxide-cyclic guanosine monophosphate (NO-cGMP) pathway, a regulator of
sinusoidal tone (adapted from Schaffner).(A):Regulation of sinusoidal tone in healthy livers:
the activation of the NO-cGMP pathway takes place once NO is generated by eNOS in sinusoidal
endothelial cells and diffuses into the neighboring hepatic stellate cells, where it binds to the enzyme sGC.
The following activation of sGC, in turn, catalyzes the conversation of GTP to cGMP. cGMP, an intracellular
second messenger, triggers distinct downstream signaling effects, which eventually exert vasodilation.
As a negative feedback mechanism, rising cGMP concentrations initiate the activation of the enzyme
PDE-5 which mediates cGMP inactivation. (B): Disturbed regulation of sinusoidal tone in liver cirrhosis:
altered expression of key enzymes in the NO-cGMP pathway lead to reduced cGMP concentrations
and thus sinusoidal constriction. (C): Effects of PDE-5 inhibitors in liver cirrhosis: application of PDE-5
inhibitors lead to a normalization of cGMP concentrations and thus sinusoidal dilation. (↑ increased
expression; ↑↑ markedly increased expression; ↓ decreased concentration; ⊥ inhibition).
Figure 1 depicts the regulation of the NO-cGMP pathway in liver cirrhosis.
Figure 1. The nitric oxide-cyclic guanosine monophosphate (NO-cGMP) pathway, a regulator of sinusoidal tone (adapted from Schaffner).(A):Regulation of sinusoidal tone in healthy livers: the activation of the NO-cGMP pathway takes place once NO is generated by eNOS in sinusoidal endothelial cells and diffuses into the neighboring hepatic stellate cells, where it binds to the enzyme sGC. The following activation of sGC, in turn, catalyzes the conversation of GTP to cGMP. cGMP, an intracellular second messenger, triggers distinct downstream signaling effects, which eventually exert vasodilation. As a negative feedback mechanism, rising cGMP concentrations initiate the activation of the enzyme PDE-5 which mediates cGMP inactivation. (B): Disturbed regulation of sinusoidal tone in liver cirrhosis: altered expression of key enzymes in the NO-cGMP pathway lead to reduced cGMP concentrations and thus sinusoidal constriction. (C): Effects of PDE-5 inhibitors in liver cirrhosis: application of PDE-5 inhibitors lead to a normalization of cGMP concentrations and thus sinusoidal dilation. (↑ increased expression; ↑↑ markedly increased expression; ↓ decreased concentration; ⊥ inhibition).
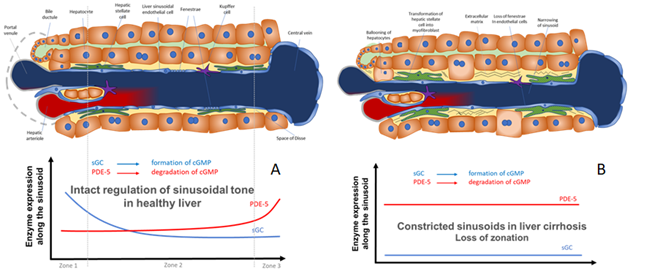
Figure 2. Model for the regulation of sinusoidal tone by the key enzymes soluble guanylate (sGC) and PDE-5 in healthy and diseased liver. The role of zonation of sGC and PDE-5 in healthy and cirrhotic liver. (
A
) In healthy livers an opposing zonation of sGC and PDE-5 may lead to a high cGMP production in the peripheral parts of the hepatic lobule, in which cGMP may exert its physiological function inside the sinusoids. However, excess cGMP might be degraded by high PDE-5 presence (zone 3) before entering the extrahepatic vasculature. (
B) In cirrhotic livers the loss of physiological hepatic zonation, marked PDE-5 overexpression, and resulting increase in cGMP degradation might lead to sinusoidal constriction.
) In cirrhotic livers the loss of physiological hepatic zonation, marked PDE-5 overexpression, and resulting increase in cGMP degradation might lead to sinusoidal constriction and elevation of portal pressure.
Experimental and clinical data showed, that inhibitors of PDE-5 induced a reduction of portal pressure that may predict a beneficial effect in terms of reduced rate of esophageal variceal bleeding or rebleeding and other sequelae of liver cirrhosis. The reviewed data suggest that deranged cGMP availability better explains the contrasting findings of intrahepatic vasoconstriction and peripheral systemic vasodilation than the mere focus on NO. Thus, we suggest considering the term “cGMP-paradox” to describe the circulatory findings in liver cirrhosis: eNOS and sGC are overexpressed in cirrhosis. However, this effect is overridden by a very marked overexpression of PDE-5. This results in low levels of cGMP inside the cirrhotic liver leading to sinusoidal constriction. Inhibition of PDE-5 normalizes cGMP levels and lowers portal pressure. In peripheral arteries both eNOS and sGC are upregulated, however PDE-5 is downregulated. This results in high peripheral cGMP and systemic vasodilation.
In Figure 2 we show a biochemical model how the opposing zonation of the enzymes sGC and PDE-5 and the loss of correct zonation in liver cirrhosis may regulate the sinusoidal diameter, thus regulating portal pressure.
Targeting components of the NO-cGMP pathways for therapy of portal hypertension
Experimental and clinical data showed, that inhibitors of PDE-5 induced a reduction of portal pressure that may predict a beneficial effect in terms of reduced rate of esophageal variceal bleeding or rebleeding and other sequelae of liver cirrhosis. The reviewed data suggest that deranged cGMP availability better explains the contrasting findings of intrahepatic vasoconstriction and peripheral systemic vasodilation than the mere focus on NO. Thus, we suggest considering the term "cGMP-paradox" to describe the circulatory findings in liver cirrhosis: eNOS and sGC are overexpressed in cirrhosis. However, this effect is overridden by a very marked overexpression of PDE-5. This results in low levels of cGMP inside the cirrhotic liver leading to sinusoidal constriction. Inhibition of PDE-5 normalizes cGMP levels and lowers portal pressure. In peripheral arteries both eNOS and sGC are upregulated, however PDE-5 is downregulated. This results in high peripheral cGMP and systemic vasodilation.
Since altered activities and/or zonation of sGC and PDE-5 may play a pivotal role in this process, these enzymes should be investigated more systematically as potential targets in medical therapy of PH. Moreover, since there are first hints showing antifibrotic effects induced by sGC and PDE-5, these might represent interesting targets for the medical therapy of liver fibrosis/cirrhosis.
3. Conclusions
Since altered activities and/or zonation of sGC and PDE-5 may play a pivotal role in this process, these enzymes should be investigated more systematically as potential targets in medical therapy of PH. Moreover, since there are first hints showing antifibrotic effects induced by sGC and PDE-5, these might represent interesting targets for the medical therapy of liver fibrosis/cirrhosis.