Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | NOBUTAKA YAMANAKA | + 395 word(s) | 395 | 2022-01-20 02:35:46 | | | |
| 2 | Jessie Wu | + 1201 word(s) | 1596 | 2022-02-07 02:57:23 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Yamanaka, N. Ni-Based Bimetallic Catalysts. Encyclopedia. Available online: https://encyclopedia.pub/entry/19035 (accessed on 07 February 2026).
Yamanaka N. Ni-Based Bimetallic Catalysts. Encyclopedia. Available at: https://encyclopedia.pub/entry/19035. Accessed February 07, 2026.
Yamanaka, Nobutaka. "Ni-Based Bimetallic Catalysts" Encyclopedia, https://encyclopedia.pub/entry/19035 (accessed February 07, 2026).
Yamanaka, N. (2022, January 31). Ni-Based Bimetallic Catalysts. In Encyclopedia. https://encyclopedia.pub/entry/19035
Yamanaka, Nobutaka. "Ni-Based Bimetallic Catalysts." Encyclopedia. Web. 31 January, 2022.
Copy Citation
Metallic Ni shows high activity for a variety of hydrogenation reactions due to its intrinsically high capability for H2 activation, but it suffers from low chemoselectivity for target products when two or more reactive functional groups are present on one molecule. Modification by other metals changes the geometric and electronic structures of the monometallic Ni catalyst, providing an opportunity to design Ni-based bimetallic catalysts with improved activity, chemoselectivity, and durability.
Ni-based bimetallic catalysts
1. Introduction
Catalysis has emerged as an important branch of energy and sustainability research because it allows for chemical transformations to be carried out at relatively low temperatures while minimizing or avoiding the formation of byproducts [1][2]. Catalysts can be broadly classified into two groups: homogeneous and heterogeneous catalysts. Homogeneous catalysts have some advantages over heterogeneous catalysts, such as the possibility of carrying out a reaction under relatively mild conditions, higher activity and selectivity, ease of spectroscopic monitoring, and controlled and tunable reaction sites [3]. The main drawback of homogeneous catalysts is the difficulty in separating them from the products after completion of the reaction [4]. Heterogeneous catalysts can overcome this drawback [4]. To date, heterogeneous catalysts based on transition metals have been found to be effective in a number of processes. In particular, hydrogenation is of great importance in petroleum refining and processing and in the manufacture of fine and bulk chemicals [5]. Although most catalytic hydrogenations today rely on precious metals such as Pd and Pt, the high cost and low availability of these metals have caused scientific interest to shift from such precious metals to nonprecious metals for hydrogenation catalysts [6]. Earth-abundant first-row transition metals such as Fe, Co, and Ni have received much more attention due to their specific advantages, such as high abundance on earth, low price, low or no toxicity, and unique catalytic properties [7]. Ni has a long history in the field of catalysis, and its first application for hydrogenation led P. Sabatier to earn the Nobel Prize in chemistry in 1912 [8]. Therefore, Ni is a fascinating alternative to precious metals such as Pd and Pt. However, the chemoselective hydrogenation of a target functional group in the presence of other reactive functional groups in a molecule is difficult to achieve because most transition metal catalysts cannot recognize and preferentially interact with the target group [9]. For this reason, great efforts have been made to seek heterogeneous Ni-based catalysts with high activity for chemoselective hydrogenation reactions.
2. Hydrogenation of Alkynes
Selective hydrogenation of alkynes to alkenes, while avoiding over-hydrogenation to undesired alkanes, represents an industrially important chemical transformation in the manufacturing of polymers as well as fine chemicals [10][11]. For example, the selective hydrogenation of acetylene to ethylene has been used to remove trace acetylene in ethylene feed streams in the production of polyethylene [12]. The most commonly used industrial catalyst for this reaction is based on supported Pd nanoparticles modified by Ag additives, although Lindlar catalyst (Pd poisoned with Pb supported on CaCO3) is not used because of its toxicity [13]. However, this system leaves ample room for improvement, particularly in terms of cost-effectiveness in catalyst design, over-hydrogenation to ethane, and oligomerization to higher hydrocarbons [10][14]. Therefore, it is highly desirable to develop more cost-effective and efficient substituents from both industrial and academic perspectives.
F. Studt et al. used density functional theory (DFT) calculations to determine why Ag showed the high selectivity to ethylene for the hydrogenation of acetylene [14]. The process is as follows: acetylene adsorbs exothermically, and the transition state energies for the first and second steps are below the energy of gas-phase acetylene. The ethylene formed on the surface is subjected to desorption or reaction to undesired ethane. Ethylene from the gas phase can also adsorb on the surface and be hydrogenated to ethane. For PdAg(111), the barrier for desorption is smaller than that of Pd(111). This result explains the reason for the addition of Ag to Pd in the industrial catalyst. These researchers also developed a series of Ni-Zn alloy catalysts on MgAl2O4 spinel supports and evaluated their catalytic performance in the hydrogenation of acetylene in a gas mixture of ethylene, acetylene, and hydrogen [14]. Ni-Zn catalyst with the highest Zn content of 75% showed an even greater selectivity to ethylene than the well-established Pd-Ag system. The DFT calculation for Ni-Zn(110) revealed that there was no obvious difference between the adsorption energy of ethylene and the energy of gas-phase ethylene.
Y. Liu et al. discovered that intermetallic NixMy (M = Ga and Sn) nanocrystals exhibited much higher selectivity for the semi-hydrogenation of acetylene to ethylene than Pd-based catalysts (i.e., Pd and PdAg) and could be applied to different substrates containing terminal and internal alkynes [10]. A DFT study was carried out to identify why these intermetallic compounds can be used as alternatives to precious metal-based catalysts. Figure 1 illustrates the full potential energy diagram for the semi-hydrogenation of acetylene to ethane on Ni3Ga. The barrier for desorption of ethylene from Ni3Ga is 0.31 eV, smaller than that from PdAg [14]. More importantly, the transition state energy of ethylene hydrogenation is above the energy of gas-phase ethylene, which indicates that ethylene is subjected to desorption rather than over-hydrogenation to ethane. The excellent selectivity to ethylene in the hydrogenation of acetylene can be assigned to the partial isolation and modified electronic structure of the active metal.
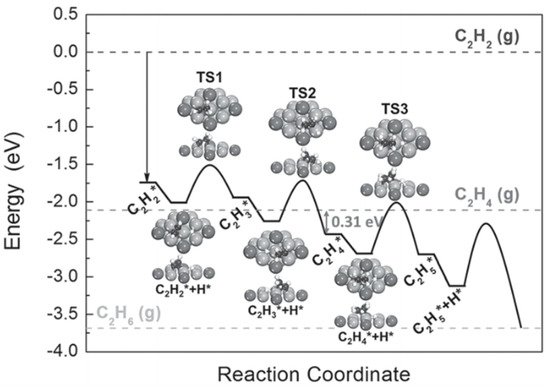
Figure 1. Potential energy diagram obtained from DFT calculations for the hydrogenation of acetylene to ethane on (111) of Ni3Ga. The geometries of the reaction intermediates, containing acetylene and ethylene, and transition states along the reaction pathway are displayed in the inset. The Ni, Ga, C, and H atoms are shown by the gray, dark gray, black, and white balls, respectively. Adapted from [10] with permission of Wiley-VCH, copyright 2016.
Komatsu and coworkers studied the catalytic properties of Ni-Sn intermetallic compounds for the hydrogenation of acetylene compared with those of pure Ni [15]. The activity descended in the order of Ni >> Ni3Sn > Ni3Sn2 >> Ni3Sn4. The successive hydrogenation of acetylene into ethane via ethylene seemed to occur over the parent active metal, and ethane was the main final product. However, Ni3Sn yielded ethylene as the main final product along with only a trace amount of ethane. In the case of Ni3Sn2, ethane was not observed as a product of the reaction. Therefore, Ni3Sn and Ni3Sn2 showed a high selectivity to ethylene for acetylene hydrogenation. These authors presumed, based on the H-D exchange between C2D4 and H2, that the inhibition of ethylene hydrogenation on Ni3Sn2 was due to no formation of ethylidyne species by the geometric restriction. The same theoretical prediction might be applied to Ni3Sn. However, it should be considered that the electronic effect might cause the inhibition of ethylene hydrogenation because the electron density of Ni3Sn2 at the Fermi level was found to be less than that of Ni according to X-ray photoelectron spectroscopy (XPS).
Komatsu’s group prepared Ge-containing Ni-based intermetallic compound, Ni3Ge, for the hydrogenation of acetylene and found that it exhibited greater selectivity to ethylene than Ni [16]. This greater selectivity is due to the expanding atomic distance between adjacent Ni atoms and the relatively low electron density of Ni atoms in Ni3Ge. A large atomic distance will slow the formation of ethylidyne species adsorbed on three-fold Ni sites; ethylidyne is known to be the intermediate in the direct hydrogenation of acetylene into ethane [17]. In addition to the geometric effect, the researchers mentioned the electronic effect on the high selectivity to ethylene. The lower electron density of Ni in Ni3Ge prevents further hydrogenation to ethane on Ni because there is less back donation [16].
G. X. Pei et al. employed a series of Ag-Ni/SiO2 bimetallic catalysts with varied Ni/Ag atomic ratios of 1, 0.5, 0.25, and 0.125 for the semi-hydrogenation of acetylene in an ethylene-rich stream [18]. When the loading of Ni was relatively high (Ni/Ag = 1 and 0.5), extremely low ethylene selectivity was displayed, similar to that of monometallic Ni/SiO2 catalyst. With the decreased loading of Ni, the ethylene selectivity gradually increased. These authors analyzed the structure of Ag-Ni/SiO2 bimetallic catalysts to determine the reasons for the enhanced ethylene selectivity. Scanning transmission electron microscopy– energy dispersive X-ray spectroscopy (STEM-EDS) analysis of the AgNi0.5/SiO2 catalyst with relatively high Ni loading revealed that Ni and Ag were not uniformly distributed in the particles. It is known that it is difficult to form Ag-Ni alloy, and only a small amount of Ni can be alloyed with Ag [19][20]. However, in the AgNi0.25/SiO2 catalyst with a relatively low Ni content, most of the Ni interacted closely with Ag because of decreased Ni amounts. Thus, the interaction between Ag and Ni was believed to be responsible for the enhanced ethylene selectivity.
Transition metal silicides have unique chemical properties, such as the lower electronegativity of silicon compared to carbon and the strong modification of the electronic structure around the Fermi level of transition metals [21][22]. However, there have been few reports describing their catalytic applications. C. Liang and coworkers achieved the selective hydrogenation of phenylacetylene to styrene by Ni-Si intermetallics [22]. Adding Si altered the Ni coordination, leading to a strong modification of the electronic structure around the Fermi level compared to metallic Ni; this electronic structure modification influenced styrene adsorption. Ni-Si intermetallic compound prepared by direct silicification at 723 K showed excellent selectivity for styrene (approximately 93%) before the complete conversion of phenylacetylene. Phenylacetylene was very strongly adsorbed on the catalyst surface and blocked the sites for styrene hydrogenation. However, styrene hydrogenation to ethylbenzene occurred when the concentration of phenylacetylene was significantly low. C. Liang group extended the use of Ni-Si intermetallic compounds to other hydrogenation reactions, as shown below.
References
- Corma, A.; Serna, P.; Concepcićn, P.; Calvino, J.J. Transforming nonselective into chemoselective metal catalysts for the hydrogenation of substituted nitroaromatics. J. Am. Chem. Soc. 2008, 130, 8748–8753.
- Zhou, P.; Li, D.; Jin, S.; Chen, S.; Zhang, Z. Catalytic transfer hydrogenation of nitro compounds into amines over magnetic graphene oxide supported Pd nanoparticles. Int. J. Hydrogen Energy 2016, 41, 15218–15224.
- Available online: https://www.sciencedirect.com/topics/engineering/homogeneous-catalyst (accessed on 8 February 2021).
- Mei, N.; Liu, B. Pd nanoparticles supported on : An effective heterogeneous catalyst for the transfer hydrogenation of nitro compounds into amines. Int. J. Hydrogen Energy 2016, 41, 17960–17966.
- Niu, H.; Lu, J.; Song, J.; Pan, L.; Zhang, X.; Wang, L.; Zou, J.-J. Iron oxide as a catalyst for nitroarene hydrogenation: Important role of oxygen vacancies. Ind. Eng. Chem. Res. 2016, 55, 8527–8533.
- Shesterkina, A.A.; Kustov, L.M.; Strekalova, A.A.; Kazansky, V.B. Heterogeneous iron-containing nanocatalysts—Promising systems for selective hydrogenation and hydrogenolysis. Catal. Sci. Technol. 2020, 10, 3160–3174.
- Su, B.; Cao, Z.-C.; Shi, Z.-J. Exploration of earth-abundant transition metals (Fe, Co, and Ni) as catalysts in unreactive chemical bond activations. Acc. Chem. Res. 2015, 48, 886–896.
- Che, M. Nobel Prize in chemistry 1912 to Sabatier: Organic chemistry or catalysis? Catal. Today 2013, 218–219, 162–171.
- Wei, H.; Ren, Y.; Wang, A.; Liu, X.; Liu, X.; Zhang, L.; Miao, S.; Li, L.; Liu, J.; Wang, J.; et al. Remarkable effect of alkalis on the chemoselective hydrogenation of functionalized nitroarenes over high-loading Pt/FeOx catalysts. Chem. Sci. 2017, 8, 5126–5131.
- Liu, Y.; Liu, X.; Feng, Q.; He, D.; Zhang, L.; Lian, C.; Shen, R.; Zhao, G.; Ji, Y.; Wang, D.; et al. Intermetallic NixMy (M = Ga and Sn) nanocrystals: A non-precious metal catalyst for semi-hydrogenation of alkynes. Adv. Mater. 2016, 28, 4747–4754.
- Borodzinski, A.; Bond, G.C. Selective Hydrogenation of Ethyne in Ethene-Rich Streams on Palladium Catalysts, Part 2: Steady-State Kinetics and Effects of Palladium Particle Size, Carbon Monoxide, and Promoters. Catal. Rev. 2008, 50, 379–469.
- Studt, F.; Abild-Pedersen, F.; Bligaard, T.; Sørensen, R.Z.; Christensen, C.H.; Nørskov, J.K. On the role of surface modifications of palladium catalysts in the selective hydrogenation of acetylene. Angew. Chem. Int. Ed. 2008, 47, 9299–9302.
- Chan, C.W.A.; Mahadi, A.H.; Li, M.M.-J.; Corbos, E.C.; Tang, C.; Jones, G.; Kuo, W.C.H.; Cookson, J.; Brown, C.M.; Bishop, P.T.; et al. Interstitial modification of palladium nanoparticles with boron atoms as a green catalyst for selective hydrogenation. Nat. Commun. 2014, 5, 5787–5807.
- Studt, F.; Abild-Pedersen, F.; Bligaard, T.; Sørensen, R.Z.; Christensen, C.H.; Nørskov, J.K. Identification of Non-Precious Metal Alloy Catalysts for Selective Hydrogenation of Acetylene. Science 2008, 320, 1320–1322.
- Onda, A.; Komatsu, T.; Yashima, T. Characterization and catalytic properties of Ni-Sn intermetallic compounds in acetylene hydrogenation. Phys. Chem. Chem. Phys. 2000, 2, 2999–3005.
- Komatsu, T.; Kishi, T.; Gorai, T. Preparation and catalytic properties of uniform particles of Ni3Ge intermetallic compound formed inside the mesopores of MCM-41. J. Catal. 2008, 259, 174–182.
- Molnár, Á.; Sárkány, A.; Varga, M. Hydrogenation of carbon–carbon multiple bonds: Chemo-, regio- and stereo-selectivity. J. Mol. Catal. A Chem. 2001, 173, 185–221.
- Pei, G.X.; Liu, X.Y.; Wang, A.; Su, Y.; Li, L.; Zhang, T. Selective hydrogenation of acetylene in an ethylene-rich stream over silica supported Ag-Ni bimetallic catalysts. Appl. Catal. A Gen. 2017, 545, 90–96.
- Kabir, L.; Mandal, A.; Mandal, S. Polymer stabilized Ni–Ag and Ni–Fe alloy nanoclusters: Structural and magnetic properties. J. Magn. Magn. Mater. 2010, 322, 934–939.
- Hansen, M.; Anderko, K.; Salzberg, H.W. Constitution of Binary Alloys. J. Electrochem. Soc. 1958, 105, 260C.
- Franciosi, A.; Weaver, J.H.; Schmidt, F.A. Electronic structure of nickel silicides Ni2Si, NiSi, and NiSi2. Phys. Rev. B 1982, 26, 546–553.
- Chen, X.; Li, M.; Guan, J.; Wang, X.; Williams, C.T.; Liang, C. Nickel-silicon intermetallics with enhanced selectivity in hydrogenation reactions of cinnamaldehyde and phenylacetylene. Ind. Eng. Chem. Res. 2012, 51, 3604–3611.
More
Information
Subjects:
Chemistry, Applied
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.3K
Entry Collection:
Organic Synthesis
Revisions:
2 times
(View History)
Update Date:
07 Feb 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




