2. Spectrophotometric Analysis of the Phytochemical Content and Antioxidant Capacity of the Inflorescences
All of the the tested samples had a similar or higher amount of total phenolics than the commonly used flower buds of
Capparis spinosa [14]. The highest amount of total phenolics (53.12 ± 0.79 mg GAE/g DW), flavonoids (38.89 ± 4.04 mg CE/g DW) and nonflavonoids (31.32 ± 0.71 mg GAE/g DW) were recorded in
PsKss (
Table 1). Because only about 10% of the medicinal plant material has total phenolics in a concentration higher than 5% DW of GAE
[7][15],
PsKss inflorescence is among the plant materials with the highest concentration of these compounds. Compared to the concentration of the total flavonoids in many other commonly used medicinal plants
[15], the tested inflorescences had a similar or higher concentration, suggesting their potential for the food industry. Moreover, very recently, it was shown that inflorescences of
Pa have far more total phenolics and flavonoids than stems and kernels
[16].
Table 1. Concentration (in mg/g dry weight) of the total phenolics (TP), total flavonoids (TF), total nonflavonoids (TNF), total tannins (TT), condensed tannins (CT) and soluble sugars (SS), and the antioxidant capacity (ABTS, FRAP and DPPH) of Rosaceae inflorescences.
| |
Prunus avium |
Prunus serrulata |
Prunus serrulata ‘Kiku Shidare Zakura’ |
Prunus yedoensis |
Malus purpurea |
Malus floribunda |
Chaenomeles japonica |
| TP (mg GAE/g DW) |
27.83 ± 0.69 f |
46.84 ± 0.75 b |
53.12 ± 0.79 a |
37.31 ± 0.48 e |
40.29 ± 0.64 c |
46.74 ± 0.93 b |
39.42 ± 0.97 d |
| TF (mg CE/g DW) |
13.57 ± 0.84 f |
32.35 ± 2.13 b |
38.89 ± 4.04 a |
25.78 ± 1.03 d |
13.43 ± 0.82 f |
23.83 ± 0.88 e |
29.45 ± 0.65 c |
| TNF (mg GAE/g DW) |
16.40 ± 0.91 g |
29.35 ± 0.83 b |
31.32 ± 0.71 a |
22.21 ± 0.86 e |
23.52 ± 0.56 d |
28.85 ± 0.58 c |
18.73 ± 0.48 f |
| TT (mg CE/g DW) |
27.26 ± 0.22 g |
59.44 ± 1.32 f |
71.59 ± 0.33 d |
83.55 ± 0.55 b |
64.32 ± 0.45 e |
107.85 ± 1.09 a |
80.27 ± 0.33 c |
| CT (mg CE/g DW) |
4.25 ± 0.33 e |
7.74 ± 1.02 d |
6.99 ± 0.17 d |
16.52 ± 0.01 b |
15.45 ± 0.10 b |
10.98 ± 0.24 c |
51.68 ± 0.38 a |
| SS (mg SE/g DW) |
3.37 ± 0.06 c |
3.04 ± 0.05 d |
2.41 ± 0.10 e |
8.61 ± 0.12 a |
1.56 ± 0.08 f |
3.58 ± 0.06 b |
3.27 ± 0.07 c |
| ABTS (mg TE/g DW) |
22.86 ± 4.61 e |
49.41 ± 7.23 b |
61.32 ± 5.84 a |
36.63 ± 4.32 c |
28.78 ± 2.52 d |
47.78 ± 6.26 b |
35.05 ± 4.25 c |
| FRAP (mg TE/g DW) |
27.89 ± 0.60 g |
51.68 ± 0.12 b |
58.06 ± 0.78 a |
40.28 ± 1.18 d |
29.12 ± 0.83 f |
44.36 ± 0.85 c |
36.36 ± 1.50 e |
| DPPH (mg TE/g DW) |
25.47 ± 2.57 d |
52.95 ± 4.22 b |
69.42 ± 3.27 a |
39.21 ± 4.86 c |
25.10 ± 3.25 d |
40.38 ± 3.4 c |
39.61 ± 3.95 c |
In order to reduce the global rise of overweightness and obesity, the content of sugars, as a source of calories, in food should be diminished. As expected, the level of sugars in the inflorescences was lower than that in the more often-used fruits. The interval of the concentrations in the inflorescences was between 1.56 ± 0.08 mg/g DW in
Mp and 8.61 ± 0.12 mg/g DW in
Py (
Table 1), which is relatively low compared to the concentrations recorded in
M. domestica fruits
[17] and
Pa fruits
[10]. Namely, the ripe flesh and skin of cultivated stone and pome fruits usually contain soluble sugars in the amount of 70–90% of the DW
[18]. This is one of the main reasons why
Rosaceae inflorescences have a high potential in the food industry, and should be considered a potent biomaterial.
The antioxidant potential was assessed using three standard methods (ABTS, FRAP and DPPH), and each of them revealed
PsKss as the most potent sample (
Table 1). Because this taxon had significantly more total phenolics, flavonoids, nonflavonoids (
Table 1), and total identified hydroxycinnamic acids than the other samples (
Table 2), with the predominant ones being caffeic acid hexoside 1,3-caffeoylquinic acid, 4-caffeoylquinic acid, 5-caffeoylquinic acid 1 (chlorogenic acid), and
p-coumaric acid hexoside 1 and 2, we assume that these compounds significantly contributed to its antioxidant potential. The same relations between the content of phenolic compounds and antioxidant activity between different parts of
Pa have already been revealed as well
[16][19]. The antioxidant activity of
Cj inflorescences was similar to that of its fruits
[20]. However, inflorescences of Pa showed higher antioxidant potential than the stems and kernels
[16].
Mf had a higher antioxidant capacity than
Mp (
Table 1), and this is probably related to the higher amount of identified hydroxycinnamic acids, especially chlorogenic acid, flavanols, flavanones, flavonols and procyanidins (
Table 2). Both
Mf and
Mp dry inflorescences showed higher antioxidant activity than the fresh flowers of different
Malus cultivars
[21], highlighting their potential for use in the food industry.
Table 2. Concentration (mg/g DW ± SD) of the individual phenolic compounds in Rosaceae inflorescences.
| |
|
Prunus avium |
Prunus serrulata |
Prunus serrulata ‘Kiku Shidare Zakura’ |
Prunus yedoensis |
Malus purpurea |
Malus floribunda |
Chaenomeles japonica |
| 1 |
Gallic acid |
0.28 ± 0.06 b |
0.15 ± 0.04 c |
0.06 ± 0.01 d |
0.38 ± 0.03 a |
0.17 ± 0.04 c |
0.03 ± 0.01 d |
nd |
| |
Total identified
hydroxybenzoic acids |
0.28 ± 0.06 b |
0.15 ± 0.04 c |
0.06 ± 0.01 d |
0.38 ± 0.03 a |
0.17 ± 0.04 c |
0.03 ± 0.01 d |
nd |
| 2 |
Caffeic acid |
1.95 ± 0.27 c |
1.25 ± 0.14 c |
2.50 ± 0.08 b |
3.90 ± 0.10 a |
nd |
nd |
0.53 ± 0.08 d |
| 3 |
Caffeic acid hexoside 1 |
0.14 ± 0.03 b |
0.39 ± 0.06 b |
15.48 ± 2.23 a |
0.10 ± 0.01 b |
0.05 ± 0.01 b |
0.23 ± 0.02 b |
0.75 ± 0.04 b |
| 4 |
Caffeic acid hexoside 2 |
nd |
nd |
nd |
5.62 ± 0.25 a |
nd |
nd |
0.02 ± 0.00 b |
| 5 |
Caffeic acid dihexoside |
0.29 ± 0.01 b |
0.19 ± 0.04 c |
0.41 ± 0.02 a |
nd |
nd |
nd |
nd |
| 6 |
3-caffeoylquinic acid |
nd |
0.36 ± 0.04 b |
1.70 ± 0.25 a |
0.54 ± 0.03 b |
nd |
nd |
0.02 ± 0.00 c |
| 7 |
4-caffeoylquinic acid |
nd |
0.27 ± 0.08 a |
0.35 ± 0.02 a |
nd |
0.13 ± 0.06 b |
0.15 ± 0.02 b |
0.07 ± 0.01 b |
| 8 |
5-caffeoylquinic acid 1 |
1.59 ± 0.18 d |
0.59 ± 0.17 e |
0.47 ± 0.02 e |
5.75 ± 0.15 c |
1.41 ± 0.30 d |
8.41 ± 0.69 a |
7.04 ± 0.23 b |
| 9 |
5-caffeoylquinic acid 2 |
0.43 ± 0.36 a |
nd |
nd |
0.27 ± 0.01 a |
0.31 ± 0.13 a |
nd |
0.14 ± 0.02 a |
| 10 |
di-caffeoylquinic acid 1 |
3.12 ± 0.08 b |
0.15 ± 0.03 c |
0.31 ± 0.02 c |
7.06 ± 0.82 a |
0.65 ± 0.14 c |
0.50 ± 0.03 c |
2.83 ± 0.30 b |
| 11 |
di-caffeoylquinic acid 2 |
0.13 ± 0.01 b |
nd |
nd |
0.29 ± 0.01 b |
0.16 ± 0.06 b |
1.02 ± 0.15 a |
nd |
| 12 |
di-caffeoylquinic acid 3 |
0.17 ± 0.03 a |
nd |
nd |
nd |
nd |
nd |
nd |
| 13 |
3-feruloylquinic acid |
0.24 ± 0.04 a |
0.03 ± 0.00 c |
0.05 ± 0.00 bc |
0.08 ± 0.00 b |
nd |
nd |
0.004 ± 0.001 c |
| 14 |
5-feruloylquinic acid |
0.26 ± 0.03 a |
0.06 ± 0.01 d |
0.11 ± 0.00 bc |
0.09 ± 0.01 cd |
0.01 ± 0.00 e |
0.13 ± 0.04 b |
0.01 ± 0.00 e |
| 15 |
3-p-coumaroylquinic acid |
0.52 ± 0.08 b |
0.14 ± 0.02 d |
0.21 ± 0.01 c |
0.73 ± 0.03 a |
0.03 ± 0.01 e |
0.15 ± 0.01 cd |
0.001 ± 0.000 e |
| 16 |
4-p-coumaroylquinic acid |
nd |
0.27 ± 0.08 a |
0.35 ± 0.02 a |
nd |
0.13 ± 0.06 b |
0.15 ± 0.02 b |
0.07 ± 0.02 b |
| 17 |
5-p-coumaroylquinic acid 1 |
0.39 ± 0.02 a |
0.09 ± 0.01 d |
0.07 ± 0.00 d |
0.33 ± 0.03 b |
0.10 ± 0.01 d |
0.19 ± 0.03 c |
0.12 ± 0.03 d |
| 18 |
5-p-coumaroylquinic acid 2 |
0.03 ± 0.00 c |
0.09 ± 0.01 b |
0.08 ± 0.01 bc |
0.10 ± 0.01 b |
0.07 ± 0.06 bc |
0.06 ± 0.02 bc |
0.25 ± 0.04 a |
| 19 |
p-coumaric acid hexoside 1 |
0.23 ± 0.06 b |
0.21 ± 0.04 b |
0.31 ± 0.01 a |
0.09 ± 0.00 c |
0.04 ± 0.01 cd |
0.22 ± 0.04 b |
0.001 ± 0.000 d |
| 20 |
p-coumaric acid hexoside 2 |
0.37 ± 0.06 b |
1.42 ± 0.15 a |
1.34 ± 0.05 a |
0.21 ± 0.01 c |
0.02 ± 0.00 d |
0.11 ± 0.01 cd |
0.13 ± 0.00 cd |
| |
Total identified
hydroxycinnamic acids |
9.87 ± 0.08 b |
5.51 ± 0.06 c |
23.70 ± 0.18 a |
25.16 ± 0.10 a |
3.12 ± 0.06 d |
11.31 ± 0.09 b |
11.93 ± 0.05 b |
| 21 |
Catechin |
0.23 ± 0.04 e |
2.61 ± 0.27 a |
2.46 ± 0.09 a |
0.91 ± 0.02 d |
nd |
1.99 ± 0.16 b |
1.67 ± 0.06 c |
| 22 |
Epicatechin |
1.10 ± 0.15 d |
0.91 ± 0.10 d |
1.81 ± 0.06 b |
6.92 ± 0.18 a |
1.18 ± 0.18 d |
1.38 ± 0.22 c |
0.40 ± 0.06 e |
| |
Total identified flavanols |
1.33 ± 0.09 e |
3.52 ± 0.18 cd |
4.27 ± 0.07 b |
7.83 ± 0.10 a |
1.18 ± 0.18 e |
3.38 ± 0.19 bc |
2.08 ± 0.06 de |
| 23 |
Eriodictyol hexoside 1 |
0.03 ± 0.00 b |
nd |
nd |
nd |
0.63 ± 0.04 b |
2.92 ± 0.86 a |
nd |
| 24 |
Eriodictyol hexoside 2 |
nd |
nd |
nd |
nd |
0.35 ± 0.09 b |
1.02 ± 0.15 a |
nd |
| 25 |
Naringenin hexoside |
nd |
nd |
nd |
nd |
nd |
nd |
0.62 ± 0.04 a |
| |
Total identified flavanones |
0.03 ± 0.00 b |
nd |
nd |
nd |
0.98 ± 0.06 b |
3.93 ± 0.50 a |
0.62 ± 0.04 b |
| 26 |
Quercetin-glycoside |
0.18 ± 0.01 c |
0.13 ± 0.01 d |
0.21 ± 0.02 b |
0.36 ± 0.02 a |
nd |
nd |
nd |
| 27 |
Quercetin-3-rutinoside |
2.78 ± 0.28 b |
0.58 ± 0.10 d |
4.79 ± 0.13 a |
1.93 ± 0.03 c |
0.08 ± 0.03 e |
0.21 ± 0.03 e |
0.72 ± 0.04 d |
| 28 |
Quercetin-3-rhamnoside hexoside |
nd |
nd |
nd |
nd |
0.58 ± 0.21 a |
0.41 ± 0.06 a |
nd |
| 29 |
Quercetin-hexoside pentoside |
nd |
0.25 ± 0.08 b |
0.59 ± 0.01 a |
nd |
nd |
nd |
nd |
| 30 |
Quercetin-rhamnoside dihexoside 1 |
0.15 ± 0.01 b |
nd |
nd |
0.20 ± 0.00 a |
nd |
nd |
nd |
| 31 |
Quercetin-rhamnoside dihexoside 2 |
nd |
0.02 ± 0.01 c |
0.05 ± 0.00 b |
0.47 ± 0.01 a |
nd |
nd |
nd |
| 32 |
Quercetin-3-galactoside |
0.26 ± 0.02 c |
nd |
nd |
0.98 ± 0.01 b |
1.21 ± 0.27 a |
0.309 ± 0.070 c |
0.17 ± 0.01 c |
| 33 |
Quercetin-3-glucoside |
0.03 ± 0.00 d |
0.19 ± 0.02 a |
0.12 ± 0.01 b |
nd |
0.02 ± 0.00 d |
0.089 ± 0.004 c |
0.02 ± 0.00 d |
| 34 |
Quercetin-3-rhamnoside |
nd |
nd |
nd |
nd |
2.26 ± 0.38 b |
4.31 ± 0.51 a |
nd |
| 35 |
Quercetin-3-xyloside |
0.01 ± 0.00 e |
0.02 ± 0.00 de |
0.06 ± 0.00 c |
0.56 ± 0.04 a |
0.09 ± 0.01 b |
0.05 ± 0.01 cd |
0.02 ± 0.00 de |
| 36 |
Quercetin-arabinofuranoside |
0.13 ± 0.04 c |
0.15 ± 0.02 c |
0.25 ± 0.03 b |
0.17 ± 0.00 c |
0.03 ± 0.01 d |
0.31 ± 0.04 a |
0.004 ± 0.000 d |
| 37 |
Quercetin-arabinopyranoside |
0.01 ± 0.00 c |
nd |
nd |
nd |
2.07 ± 0.20 a |
1.61 ± 0.05 b |
nd |
| 38 |
Quercetin-acetyl hexoside 1 |
nd |
4.00 ± 0.40 a |
1.53 ± 0.07 b |
0.22 ± 0.00 c |
nd |
nd |
nd |
| 39 |
Quercetin-acetyl hexoside 2 |
nd |
0.14 ± 0.02 b |
0.08 ± 0.00 c |
0.35 ± 0.02 a |
nd |
nd |
nd |
| 40 |
Kaempferol trihexoside |
nd |
1.27 ± 0.11 a |
0.81 ± 0.08 b |
nd |
nd |
nd |
nd |
| 41 |
Kaempferol-3-rutinoside |
0.92 ± 0.02 a |
0.06 ± 0.00 f |
0.05 ± 0.01 f |
0.54 ± 0.01 b |
0.37 ± 0.08 c |
0.24 ± 0.03 d |
0.15 ± 0.01 e |
| 42 |
Kaempferol acetyl hexoside 1 |
nd |
1.50 ± 0.15 a |
0.32 ± 0.01 b |
0.09 ± 0.01 a |
nd |
nd |
nd |
| 43 |
Kaempferol acetyl hexoside 2 |
nd |
0.07 ± 0.01 a |
nd |
0.33 ± 0.04 a |
nd |
nd |
nd |
| 44 |
Kaempferol dihexoside |
nd |
0.12 ± 0.01 b |
0.44 ± 0.03 a |
nd |
nd |
nd |
nd |
| 45 |
Kaempferol pentoside 1 |
nd |
nd |
nd |
1.34 ± 0.09 a |
nd |
nd |
nd |
| 46 |
Kaempferol pentoside 2 |
nd |
nd |
nd |
0.12 ± 0.01 a |
nd |
nd |
nd |
| 47 |
Kaempferol rhamnoside |
0.06 ± 0.01 b |
nd |
nd |
nd |
0.32 ± 0.08 b |
4.43 ± 0.63 a |
nd |
| 48 |
Kaempferol hexoside 1 |
0.01 ± 0.00 e |
0.20 ± 0.02 b |
0.08 ± 0.00 d |
0.27 ± 0.02 a |
0.03 ± 0.01 e |
0.02 ± 0.00 e |
0.14 ± 0.01 c |
| 49 |
Kaempferol hexoside 2 |
0.69 ± 0.23 a |
nd |
nd |
0.38 ± 0.01 b |
0.01 ± 0.00 c |
0.34 ± 0.04 b |
nd |
| 50 |
Kaempferol rhamnosyl hexoside |
nd |
nd |
nd |
nd |
0.02 ± 0.01 b |
0.22 ± 0.03 a |
nd |
| 51 |
Isorhamnetin hexoside |
nd |
0.01 ± 0.00 c |
0.04 ± 0.00 c |
0.04 ± 0.00 c |
0.28 ± 0.04 b |
3.12 ± 0.21 a |
0.22 ± 0.00 b |
| 52 |
Isorhamnetin dihexoside |
0.31 ± 0.02 a |
nd |
nd |
nd |
nd |
nd |
nd |
| 53 |
Isorhamnetin acetyl hexoside 1 |
nd |
nd |
nd |
nd |
nd |
nd |
0.98 ± 0.12 a |
| 54 |
Isorhamnetin acetyl hexoside 2 |
nd |
nd |
nd |
nd |
nd |
nd |
0.04 ± 0.00 a |
| 55 |
Isorhamnetin-3-rutinoside |
0.02 ± 0.00 a |
0.01 ± 0.00 c |
0.02 ± 0.00 b |
0.02 ± 0.00 b |
nd |
nd |
nd |
| 56 |
Myricetin rutinoside |
nd |
nd |
nd |
nd |
nd |
nd |
0.004 ± 0.000 a |
| 57 |
Laricitrin glucuronide |
0.07 ± 0.01 a |
nd |
nd |
nd |
nd |
nd |
nd |
| 58 |
Syringetin hexoside 1 |
0.02 ± 0.00 b |
nd |
nd |
nd |
0.30 ± 0.03 b |
5.18 ± 0.62 a |
nd |
| 59 |
Syringetin hexoside 2 |
0.29 ± 0.10 a |
nd |
nd |
nd |
nd |
nd |
nd |
| 60 |
Syringetin acetyl hexoside 1 |
nd |
nd |
nd |
nd |
1.61 ± 0.41 a |
0.78 ± 0.14 b |
0.30 ± 0.02 b |
| 61 |
Syringetin acetyl hexoside 2 |
nd |
nd |
nd |
nd |
nd |
nd |
0.07 ± 0.00 a |
| |
Total identified flavonols |
5.93 ± 0.05 b |
8.71 ± 0.06 b |
9.42 ± 0.03 b |
8.37 ± 0.02 b |
9.29 ± 0.11 b |
21.60 ± 0.16 a |
2.84 ± 0.02 c |
| 62 |
Apigenin hexoside |
nd |
0.02 ± 0.00 b |
0.01 ± 0.00 b |
0.04 ± 0.00 a |
nd |
nd |
nd |
| |
Total identified flavones |
nd |
0.02 ± 0.00 b |
0.01 ± 0.00 b |
0.04 ± 0.00 a |
nd |
nd |
nd |
| 63 |
Phloretin xylosylglucoside |
nd |
nd |
nd |
nd |
0.18 ± 0.03 a |
0.09 ± 0.02 b |
nd |
| 64 |
Phloridzin |
nd |
nd |
nd |
nd |
5.14 ± 0.74 a |
5.23 ± 0.52 a |
nd |
| 65 |
Trilobatin |
nd |
nd |
nd |
nd |
0.30 ± 0.08 b |
1.47 ± 0.20 a |
nd |
| |
Total identified chalcones |
nd |
nd |
nd |
nd |
5.61 ± 0.28 a |
6.80 ± 0.24 a |
nd |
| 66 |
Procyanidin dimer 1 |
nd |
0.33 ± 0.03 c |
0.52 ± 0.03 c |
1.24 ± 0.04 c |
1.45 ± 0.42 bc |
2.52 ± 0.36 b |
7.90 ± 1.48 a |
| 67 |
Procyanidin dimer 2 |
nd |
nd |
nd |
5.07 ± 0.19 a |
0.75 ± 0.31 c |
2.93 ± 0.47 b |
5.30 ± 0.27 a |
| 68 |
Procyanidin dimer 3 |
nd |
nd |
nd |
nd |
0.02 ± 0.01 b |
3.43 ± 0.40 a |
0.36 ± 0.02 b |
| 69 |
Procyanidin dimer 4 |
nd |
nd |
nd |
nd |
0.03 ± 0.01 c |
3.43 ± 0.39 b |
6.31 ± 0.25 a |
| 70 |
Procyanidin dimer 5 |
nd |
nd |
nd |
nd |
nd |
nd |
1.21 ± 0.08 a |
| 71 |
Procyanidin dimer 6 |
nd |
nd |
nd |
nd |
nd |
nd |
0.50 ± 0.23 a |
| 72 |
Procyanidin trimer 1 |
nd |
1.18 ± 0.09 d |
0.86 ± 0.05 d |
11.05 ± 0.30 a |
2.15 ± 0.31 c |
4.76 ± 0.39 b |
0.02 ± 0.00 e |
| 73 |
Procyanidin trimer 2 |
nd |
2.63 ± 0.18 c |
2.68 ± 0.16 c |
5.97 ± 0.55 a |
0.12 ± 0.05 d |
0.07 ± 0.01 d |
4.52 ± 0.16 b |
| 74 |
Procyanidin trimer 3 |
nd |
nd |
nd |
nd |
0.42 ± 0.04 b |
8.66 ± 1.08 a |
0.02 ± 0.00 b |
| 75 |
Procyanidin trimer 4 |
nd |
nd |
nd |
nd |
2.33 ± 0.58 a |
1.72 ± 0.28 a |
nd |
| 76 |
Procyanidin trimer 5 |
nd |
nd |
nd |
nd |
nd |
4.10 ± 1.60 a |
nd |
| 77 |
Procyanidin tetramer |
nd |
nd |
nd |
nd |
nd |
4.11 ± 1.60 a |
2.89 ± 0.18 a |
| |
Total identified condensed tannins |
nd |
4.14 ± 0.10 c |
4.06 ± 0.08 c |
23.33 ± 0.27 b |
7.27 ± 0.22 c |
35.73 ± 0.66 a |
29.03 ± 0.27 b |
| |
Total identified compounds |
16.44 ± 0.06 d |
22.05 ± 0.07 d |
41.54 ± 0.06 c |
65.10 ± 0.09 b |
27.62 ± 0.14 d |
82.77 ± 0.26 a |
46.49 ± 0.09 c |
3. LC-DAD-MS Analysis of the Individual Phenolics in the Inflorescences
Detailed studies on the phytochemical profile of
Rosaceae plant parts other than the fruits (roots, stems, leaves, and flowers) are very scarce. The identification and quantification of chemical compounds in the inflorescences of
Rosaceae contributes to the understanding of the basis of their bioactivity. Just recently, the first results of the phytochemical analyses of the stems, leaves and flowers of
Pa were published by Jesus et al.
[8]; however, inflorescences were not included. Our study is the first to present a comprehensive LC-DAD-MS polyphenolic profile of selected
Rosaceae inflorescences, and it provides a fingerprint for the future quality control of the selected inflorescences.
We identified, in total, 77 phenolic compounds (
Table 2,
Supplementary Table S1, the supplementary are available online at
https://www.mdpi.com/article/10.3390/plants11030271/s1). The representative chromatograms, at different wavelengths, of each of the species are presented in
Supplementary Figure S1; the highest number of individual compounds (46), as well as the highest concentration of total identified compounds (82.77 ± 0.26 mg/g DW), were recorded in
Mf (
Table 2;
Supplementary Figure S2). This species also contained the highest concentration of procyanidins, with 35.73 ± 0.66 mg/g DW (
Table 1). On the other hand,
Pa had the lowest concentration of total identified compounds, 18.05 ± 2.34 mg/g DW. In the inflorescences of this species, we identified 36 compounds, which is significantly more than were identified in its stems, leaves and fruits
[8][9][19][22][23], emphasising the wealth of different compounds in inflorescences. However, this was the only sample in which we did not identify individual procyanidins (
Table 2). The most individual compounds represented with the highest concentration among the samples were detected in
Py (20), representing 49% of all of the identified compounds in this species (
Table 2,
Supplementary Figure S2).
The individual phenolic with the highest concentration in
Pa was di-caffeoylquinic acid (3.12 ± 0.08 mg/g DW), in
Ps it was quercetin-acetyl hexoside (4.00 ± 0.40 mg/g DW), in
PsKss it was caffeic acid hexoside (15.48 ± 2.23 mg/g DW), in
Py and
Mf it was procyanidin trimer (11.05 ± 0.30 mg/g DW and 8.66 ± 1.08 mg/g DW, respectively), in
Mp it was phloridzin (5.14 ± 0.74 mg/g DW), and in
Cj the most represented phenolic was procyanidin dimer (7.90 ± 1.48 mg/g DW) (
Table 2). In each of the
Prunus inflorescence samples, the concentration of quercetin-3-rutinoside was present with a higher concentration than that of quercetin-3-galactoside, which is opposite to the inflorescence of
P. serotina [24], and
P. avium inflorescence had a similar concentration of chlorogenic acid to the flowers of
P. padus [25].
In
Pa,
PsKss and
Py, the predominant identified compounds were hydroxycinnamic acids in
Ps and
Mp flavonols, and in
Mf and
Cj they were procyanidins (
Table 1). In
Pa, epicatechin was the main flavanol, while catechin was present in a smaller concentration, and the same ratio had already been recorded for fruits as well
[23]. Among hydroxybenzoic acids, we identified gallic acid in the concentration of 0.28 ± 0.06 mg/g DW. This was previously found in
Pa stems and fruits as well; however, it was not found in leaves and flowers
[8].
Mf had a significantly higher concentration of total identified flavonols and procyanidins than other samples, at 21.60 ± 0.16 mg/g DW and 35.73 ± 0.66 mg/g DW, respectively (
Table 1). It had far more chlorogenic acid than its whole fruit, flesh or peel
[26]. Chalcones were identified in
Mf and
Mp only, in total concentrations of 6.80 ± 0.24 mg/g DW and 5.61 ± 0.28 mg/g DW, respectively (
Table 1). Among them, phloridzin predominated, with 23 ± 0.52 mg/g DW and 5.14 ± 0.74 mg/g DW, respectively. Phloretin xylosylglucoside and trilobatin were represented in inverse proportions in
Mf and
Mp; in
Mf trilobatin predominated with a five-times-higher concentration than in
Mp, while in
Mp phloretin xylosylglucoside predominated with a two-times-higher concentration than in
Mf (
Table 1). This suggests that the ratio of phloretin xylosylglucoside and trilobatin could be used as a phytochemical differentiator between these two species.
In
Cj, we identified 43 polyphenolic compounds (
Table 2), compared to the 20 compounds identified in the fruits
[27]. This emphasises the value of inflorescences in the richness and variety of polyphenolic compounds. The representation of flavanols, flavonols and flavanones in
Cj inflorescences was similar to that in
Chaenomeles maulei fruit juices
[28]. The most important polyphenol group was procyanidins (
Table 1 and
Table 2), which was similar to its leaves
[12] and fruits
[27].
4. Antidiabetic Activity of the Inflorescences
The antidiabetic activity of the samples was assessed via the inhibition of α-amylase and α-glucosidase, which are enzymes required for carbohydrate digestion. These enzymes are targets not only when attempting to alleviate diabetes but also hyperlipidemia, obesity and caries
[29]. The potential of
Pa flower, stem and leaf extracts in the inhibition of α-glucosidase has been recognised
[8]. Stem extract inhibited α-glucosidase significantly more than extracts of
P. avium Saco and
Hedelfinger fruits
[22], which shows the biopotential of
Rosaceae plant parts other than the commonly consumed fruits.
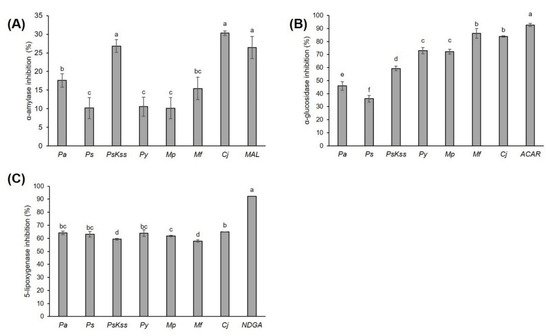
This work is the first report on α-amylase and α-glucosidase inhibition by Rosaceae inflorescences. Each of the tested samples more efficiently inhibited α-glucosidase than α-amylase (Figure 1A,B).
Figure 1. Inhibition of (A) α-amylase, (B) α-glucosidase, and (C) 5-lipoxygenase activity by Rosaceae inflorescences’ extracts (0.80 mg/mL, 0.55 mg/mL and 1.45 mg/mL, respectively). The values represent the mean ± standard deviation of three replicates. Different letters indicate a significant difference among the values (ANOVA, Duncan test, p ≤ 0.05). Pa = P. avium, Ps = P. serrulata, PsKss = P. serrulata ‘Kiku Shidare Zakura’, Py = P. yedoensis, Mp = M. purpurea, Mf = M. floribunda, Cj = Chaenomeles japonica, MAL = maltose 0.80 mg/mL, ACAR = acarbose 0.55 mg/mL, NDGA = nordihydroguaiaretic acid 0.15 mg/mL.
The same tendency was recorded with extracts of
Chaenomeles fruits as well
[20]. However, the fact that the inhibitions of α-amylase,
Cj and
PsKss were equally efficient to the standard maltose at the same concentration is very interesting and promising. Moreover, both samples even showed a tendency to be more effective than maltose. This emphasizes the high antidiabetic potential of the mentioned inflorescences, and we suggest further
in vivo investigations of the antidiabetic activity of these biomaterials and the mechanisms behind this activity. One of the possible intermediates in this α-amylase inhibitory activity could be phenolic compounds. Indeed, phenolic acids and flavonoids bind covalently to α-amylase, forming quinones or lactones that react with nucleophilic groups of the enzyme, thus altering the enzyme’s activity.
Cj had the highest concentration of total condensed tannins, 51.68 ± 0.38 mg CE/g DW, while
PsKss had the highest concentration of total phenolics, flavonoids and nonflavonoid compounds, at 53.12 ± 0.79 mg GAE/g DW, 38.89 ± 4.04 mg CE/g DW and 31.32 ± 0.71 mg GAE/g DW, respectively (
Table 1). Therefore, we assume that, among those measured, these compounds mostly contributed to the inhibition of α-amylase. Moreover, α-amylase showed the highest positive Pearson’s correlation coefficient in its total condensed tannins and total flavonoids, with r = 0.585 and r = 0.458, respectively (
Table 3).
Table 3. Pearson’s correlation coefficient (r) between the groups of metabolites, antioxidant capacity, cytotoxicity, and hypoglycemic and anti-inflammatory potential of Rosaceae inflorescences.
| |
TP |
TF |
TNF |
TT |
CT |
SS |
ABTS |
FRAP |
DPPH |
HepG2 |
HCT116 |
HaCaT |
α-Amyl |
α-Glucos |
5-Lipoxy |
| TP |
1.000 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| TF |
0.761 |
1.000 |
|
|
|
|
|
|
|
|
|
|
|
|
|
| TNF |
0.938 |
0.629 |
1.000 |
|
|
|
|
|
|
|
|
|
|
|
|
| TT |
0.471 |
0.014 |
0.333 |
1.000 |
|
|
|
|
|
|
|
|
|
|
|
| CT |
−0.065 |
0.178 |
−0.367 |
0.314 |
1.000 |
|
|
|
|
|
|
|
|
|
|
| SS |
0.194 |
−0.210 |
0.251 |
0.262 |
0.045 |
1.000 |
|
|
|
|
|
|
|
|
|
| ABTS |
0.937 |
0.875 |
0.908 |
0.160 |
−0.140 |
0.115 |
1.000 |
|
|
|
|
|
|
|
|
| FRAP |
0.873 |
0.913 |
0.862 |
0.016 |
−0.176 |
−0.022 |
0.979 |
1.000 |
|
|
|
|
|
|
|
| DPPH |
0.830 |
0.952 |
0.762 |
−0.015 |
−0.094 |
−0.170 |
0.945 |
0.969 |
1.000 |
|
|
|
|
|
|
| HepG2 |
−0.459 |
−0.567 |
−0.396 |
−0.336 |
−0.353 |
0.059 |
−0.451 |
−0.489 |
−0.393 |
1.000 |
|
|
|
|
|
| HCT116 |
−0.416 |
−0.353 |
−0.327 |
−0.602 |
−0.171 |
0.454 |
−0.250 |
−0.274 |
−0.265 |
0.689 |
1.000 |
|
|
|
|
| HaCaT |
−0.148 |
−0.067 |
−0.029 |
−0.670 |
−0.512 |
−0.059 |
−0.004 |
0.050 |
0.104 |
0.729 |
0.623 |
1.000 |
|
|
|
| α-amyl |
0.169 |
0.458 |
−0.116 |
−0.038 |
0.585 |
−0.064 |
0.237 |
0.191 |
0.377 |
0.146 |
0.234 |
0.117 |
1.000 |
|
|
| α-glucos |
0.077 |
−0.083 |
−0.101 |
0.645 |
0.627 |
0.429 |
−0.065 |
−0.205 |
−0.213 |
−0.312 |
−0.121 |
−0.786 |
0.263 |
1.000 |
|
| 5-lipoxy |
−0.345 |
0.020 |
−0.371 |
−0.530 |
0.431 |
0.318 |
−0.172 |
−0.114 |
−0.152 |
−0.077 |
0.534 |
0.154 |
0.232 |
0.011 |
1.000 |
As in the case of the total condensed tannins, as determined by the spectrophotometric method, which were most represented in the
Cj (
Table 1), the LC-DAD-MS method also revealed the highest concentrations of individual procyanidins in this species (
Table 2). Compared to the other samples,
Cj had significantly more procyanidin dimer 1, 2, 4, 5, 6 and procyanidin tetramer, and also 5-
p-coumaroylquinic acid 2, naringenin hexoside, isorhamnetin acetyl hexoside 1 and 2, myricetin rutinoside and syringetin acetyl hexoside 2 (
Table 2), so we hypothesyze that among these compounds one might potentially find new strong inhibitor/s of α-amylase. Here, we would especially emphasise procyanidins, as they were present in higher amounts—procyanidin dimer 1 even being present at 7.90 ± 1.48 mg/g DW—than the other compounds, and probably mainly contributed to the inhibition of this enzyme. As a support, the significance of tannins for α-amylase inhibition was recognised in work with sorghum as well
[30]. An extract of pinhão coat (
Araucaria angustifolia) rich in condensed tannins also effectively inhibited α-amylase
[29]. On the other hand, in
PsKss extract, the predominant compound was caffeic acid hexoside 1 with 15.48 ± 2.23 mg/g DW, which is more than 37% of all of the identified compounds in this species, and this might be one of the key contributors to the inhibition of α-amylase activity. Moreover, recently, fruits from the genus
Prunus have been suggested for the preparation of extracts with antidiabetic activities
[31].
The potential of
Pa fruits to inhibit α-glucosidase is known
[22]; however, there are no data on their or other
Rosaceae inflorescences’ antidiabetic activity. In our study,
Mf and
Cj showed a significantly higher rate of α-glucosidase inhibition than the other samples (
Figure 1B). Moreover, the inhibition percentages (86.29 ± 3.78% and 83.91 ± 0.48%, respectively) were very close to the value of the standard acarbose at the same concentration, 92.70 ± 1.21% (
Figure 1). Compared to the inhibition percentages of common vegetables
[32],
Rosaceae inflorescences can justifiably be considered relevant natural α-glucosidase inhibitors. Acarbose, a pseudotetrasaccharide, is otherwise a highly effective inhibitor of intestinal α-glucosidases; however, it is not effectively absorbed into the bloodstream, but rather retained in the intestine, and may cause gastrointestinal side effects
[29]. Therefore, the plant-based α-glucosidase inhibitors with lower side effects are very welcome. This indicates the high potential of
Mf and
Cj inflorescences to attenuate hyperglycemia, and should definitely be investigated further in
in vivo models. For example, just recently, Kumar et al.
[33] revealed that the extract of
P. amygdalus seed coat applied to diabetic rats significantly reduced the level of blood glucose, and down-regulated hyperglycemic stress, oxidative stress and hyperlipidaemia. Moreover, they found out that the
in vivo antidiabetic activity of the extract was accomplished via the inhibition of dipeptidyl peptidase IV (DPP-IV) protein. This is a hydrolase distributed in various tissues and the circulation, which quickly metabolizes glucagon-like peptide-1 (GLP-1). GLP-1, otherwise, maintains the blood glucose level, supporting insulin secretion and β-cell masses, reducing glucagon secretion, and changing the rate of gastric emptying
[34][35]. By inhibiting the DPP-IV, the level of GLP-1 can be maintained; in this way the blood glucose level can be maintained as well. We hypothesize that
Mf and
Cj inflorescences’ extracts might also affect DPP-IV activity
in vivo, and this would be good to test in future. Considering the content of phytochemicals,
Mf had the highest concentration of total tannins among the samples, 107.85 ± 1.09 mg CE/g DW, and
Cj—as mentioned earlier—had the highest concentration of total condensed tannins and individual identified procyanidins (
Table 1,
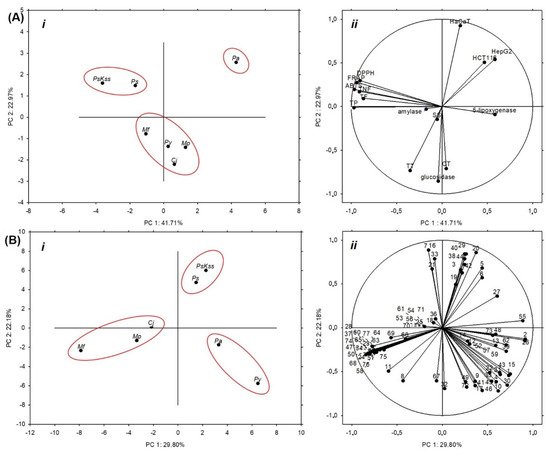
Table 2). Therefore, these groups of compounds were probably responsible for the strong α-glucosidase inhibition. Furthermore, PCA revealed that the total and condensed tannins mostly contributed to the inhibition of α-glucosidase (
Figure 2Aii).
Figure 2. Principal component analysis of (A) the groups of metabolites, antioxidant capacity, cytotoxicity, antidiabetic and anti-inflammatory potential of Rosaceae inflorescences: (i) score plot separating the inflorescence samples based on the measured groups of metabolites, antioxidant capacity, cytotoxicity, and antidiabetic and anti-inflammatory potential, and (ii) loading plot of the measured variables; (B) the individual identified phenolic compounds in Rosaceae inflorescences: (i) score plot separating the inflorescence samples based on the individual identified phenolic compounds they contain, and (ii) the loading plot of the individual phenolics as variables. Pa = P. avium, Ps = P. serrulata, PsKss = P. serrulata ‘Kiku Shidare Zakura’, Py = P. yedoensis, Mp = M. purpurea, Mf = M. floribunda, Cj = Chaenomeles japonica, TP = total phenolics, TF = total flavonoids, TNF = total nonflavonoids, TT = total tannins, CT = condensed tannins, SS = soluble sugars, ABTS = 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt, FRAP = ferric reducing antioxidant power, DPPH = 2,2-diphenyl-1-picrylhydrazyl, 1–77 = numbers related to the individual identified phenolics, as depicted in the Table 2.
The significance of condensed tannins in α-glucosidase inhibition has also been detected in the analysis of
P. persica pulp
[13]. The individual identified compounds predominating in
Cj we have already emphasized, and in
Mf the predominant compounds were chlorogenic acid (8.41 ± 0.69 mg/g DW), di-caffeoylquinic acid 2, eriodictyol hexoside 1 and 2, quercetin-3-rhamnoside, quercetin-arabinofuranoside, kaempferol rhamnoside, isorhamnetin hexoside, syringetin hexoside 1, phloridzin, trilobatin, procyanidin dimers, procyanidin trimers, and procyanidin tetramer (
Table 2). At the same time,
Mf had the highest concentration of total identified compounds, total identified procyanidins, chalcones, flavonols and flavanones (
Table 1). As with α-amylase, we would also like to draw attention to the identified procyanidins, especially procyanidin trimer 3, which was present in
Mf with more than 10% of the total identified compounds in that species, and probably significantly affected the activity of α-glucosidase. In both of these species, in addition to the identified procyanidins, the predominant compound among the individual compounds was chlorogenic acid, at 8.41 ± 0.69 mg/g DW in
Mf and 7.04 ± 0.23 mg/g DW in
Cj (
Table 2); as such, we assume that these compounds may be responsible for potent α-glucosidase inhibition. Another thing that we detected is that
Mf and
Cj had high concentrations of procyanidins similar to hawthorn (
Crataegus spp.) fruits, which are—due to the preventive activity of these compounds toward oxidative stress after ischemia repercussion injury and myocardial infarction—included in the European pharmacopeia as a complementary treatment for chronic heart failure
[36]. Therefore, we think, in the future, that it would be wise to test the potential of
Mf and
Cj inflorescences’ extracts for the prevention or alleviation of cardiovascular diseases.
6. Cytotoxic Activity of the Inflorescences
In this study, we evaluated the in vitro anti-proliferative effect of Rosaceae inflorescences on human hepatocellular (HepG2) and colorectal (HCT 116) carcinoma cells, and non-tumorigenic skin keratinocytes (HaCaT). There is evidently a variability in the cell’s metabolic response to the extracts (Table 4).
Table 4. In vitro antiproliferative activity (IC50 expressed in μg/mL) of Rosaceae inflorescence exstracts tested on hepatocellular carcinoma (HepG2), colorectal cancer (HCT 116) and keratinocyte (HaCaT) cell lines.
| |
Cell Type (IC50 μg/mL) |
| |
HepG2 |
HCT 116 |
HaCaT |
| Prunus avium |
300.89 ± 0.21 c A |
261.97 ± 13.12 c A |
323.84 ± 46.61 c A |
| Prunus serrulata |
473.59 ± 35.69 ab A |
517.42 ± 37.10 a A |
377.66 ± 34.85 bc B |
| Prunus serrulata 2018Kiku Shidare Zakura’ |
409.71 ± 103.52 b A |
464.01 ± 57.31 a A |
385.20 ± 7.27 bc A |
| Prunus yedoensis |
508.09 ± 26.28 a A |
537.92 ± 43.0 a A |
521.64 ± 67.29 a A |
| Malus purpurea |
386.2 ± 19.92 b B |
539.66 ± 45.19 a A |
461.39 ± 71.56 ab AB |
| Malus floribunda |
445.78 ± 27.42 ab A |
361.83 ± 31.19 b B |
459.28 ± 43.69 ab A |
| Chaenomeles japonica |
452.48 ± 15.18 ab A |
470.66 ± 48.16 a A |
473.27 ± 92.54 ab A |
The most potent cytotoxic activity shown by
Pa toward HCT 116 cell line IC
50 was 261.97 ± 13.12 μg/mL, toward HepG2 IC
50 was 300.89 ± 0.21 μg/mL, and toward HaCaT cells IC
50 was 323.84 ± 46.61 μg/mL (
Table 4). Jesus et al.
[8] speculated that the high concentration of chlorogenic acid in the leaves of
Pa might be the reason of their anticancer activity. However, among the tested samples in our work,
Pa was not the one with the highest concentration of this acid (
Table 2), and still showed the most potent anticancer activity (
Table 4). Therefore, we presume that chlorogenic acid, in itself, may not be crucial for the antiproliferative activity of
Pa inflorescences, but in combination with other compounds could act synergistically and enhance the cytotoxicity. Interestingly,
Pa stem extracts up to the concentration of 400 μg/mL did not show cytotoxicity toward different cancer cell types, including HepG2, while extracts of the fruits revealed selectivity against colon carcinoma HCT-15
[19]. The cytotoxic effects of isolated catechins are known from both
in vitro and
in vivo investigations; however, in combinations with the other compounds in the extract, their effects might be different
[37]. What surprised us was that
Pa had the lowest concentrations of catechins and their oligo- and polymers (
Supplementary Figure S3). The cytotoxic activity of these phenolics is well known; however, in the extracts of
Pa,
Ps,
PsKss and
Py, some chemical interactions occur between compounds which lead to unexpected effects—the highest cytotoxicity was found in samples with the lowest concentration of catechins, and this was especially emphasized with HaCaT cells, suggesting the cell-based specificity of the extracts as well. Moreover, we detected a strong negative linear correlation between the viability of HaCaT cells, and the content of total tannins (
R2 = 0.780) and condensed tannins (
R2 = 0.977), and the total identified flavan-3-ols (
R2 = 0.952) and procyanidins (
R2 = 0.990) among the
Prunus samples (
Supplementary Figure S3). We hypothesize that these compounds in some way interfere with the cytotoxic effect of
Prunus inflorescences’ extracts toward HaCaT. When we looked at the individual components, we detected that epicatechin, procyanidin dimer 2 and procyanidin trimer 1 had strong negative correlations (
r = −0.622,
r = −0.810, and
r = −0.686, respectively) with the HaCaT viability (
Supplementary Table S2), so we assume that these compounds might attenuate the cytotoxicity of the
Prunus extracts. One possible explanation could be that flavan-3-ols (catechins), and their oligomers and polymers, bind the cytotoxic components of the extracts and act as their antagonists. The higher the concentration of the total and condensed tannins, and the total identified procyanidins and flavanols in
Prunus inflorescences, the higher the number of viable HaCaT cells. This is useful information for the possible application of the tested
Prunus extracts in dermal wound healing.
Ps and PsKss showed similar cytotoxic potentials to Pa toward HaCaT cells. Cell-specific cytotoxicity levels were ascertained for Ps (HaCaT was most susceptible), Mp (HepG2 was most sensitive), and Mf (HCT 116 was most sensitive) (Table 4).
So far, the anticancer properties of the fruits and stems of
Pa have been investigated against five human cancer cell lines, and only the extract of the fruits showed activity against colon carcinoma HCT-15
[19]. Because fruits contain anthocyanins, and stems do not, the presumption is that they might be the key to the anticancer properties of
Pa fruits. The inflorescences have not been tested so far.
A very strong positive correlation was found between cytotoxic activity against HepG2 and di-caffeoylquinic acid 3, isorhamnetin dihexoside, laricitrin glucuronide and syringetin hexoside 2 (
Supplementary Table S1), all of which are predominantly present in
Pa. As such, these compounds might be responsible for the inhibition of cell proliferation, and their cytotoxic potential should be investigated further. The cytotoxic activity toward HCT116 was very strongly correlated with di-caffeoylquinic acid 3,5-feruloylquinic acid, isorhamnetin dihexoside, laricitrin glucuronide and syringetin hexoside 2, which were again predominant in
Pa. On the other hand, caffeic acid dihexoside was the only compound that was very strongly correlated with an antiproliferative effect on HaCaT cells (
Supplementary Table S2).
7. Statistical Analysis
The principal component analysis (PCA) based on the measured groups of metabolites and the antioxidant capacity (Table 1), cytotoxicity (Table 4), and hypoglycemic and anti-inflammatory potential (Figure 1) explained 64.68% of the total variation among the samples, where PC1 accounted for 41.71% of the variance and PC2 accounted for 22.97% (Figure 2Ai). The samples were separated into three groups: Ps and PsKss formed one group; Mp, Cj, Py and Mf formed the other; and Pa was separated alone as the most specific sample (Figure 2Ai). The variables that mostly contributed to the group of Ps and PsKss were antioxidant capacity (ABTS, FRAP and DPPH), total phenolics, flavonoids, and nonflavonoid compounds (Figure 2Aii). The separation of Pa was due to the cytotoxic activity toward all of the three tested cell types. The group of Mp, Cj, Py and Mf mostly contributed tannins, soluble sugars, and the inhibition of α-glucosidase.
Based on the individual identified phenolics, the samples were similarly grouped: the only difference was in the grouping of Py, which was closer to Pa, and they grouped together (Figure 2Bi). This suggests that the representation of the individual identified phenolics and the bioactivity of the tested Rosaceae inflorescences are analogously distributed between all of the samples, except for Py. We also noticed that Cj, based on the measured parameters, was closer to the Malus than to the Prunus samples.
HC analysis, an algorithm that creates a dendrogram showing the hierarchical relationships between different datasets, showed the degree of similarities/dissimilarities between the samples. Based on their groups of metabolites, antioxidant capacity, cytotoxicity, and hypoglycemic and anti-inflammatory potential,
Ps and
PsKss were the most similar samples to each other. Other samples were close to them, while
Pa was the most distant from all of the samples (
Supplementary Figure S4A). These results indicate that greater genetic similarity does not imply a greater similarity in biological effects (
Supplementary Figure S4A).
Based on their individual identified phenolic compounds, the samples were, as expected, more similar (closer) to each other (
Supplementary Figure S4B); the most similar were
Pa and
Ps, while the most distant from them was
Py.
Pearson’s correlation coefficient (r) between the groups of metabolites, antioxidant capacity, cytotoxicity, and hypoglycemic and anti-inflammatory potential of the samples revealed a strong positive correlation between antioxidant capacity (ABTS, FRAP and DPPH) and the total phenolics, flavonoids and nonflavonoids (
Table 3). The inhibition of α-glucosidase was strongly positively correlated with cytotoxicity toward HaCaT cells (
Table 3). Cytotoxicity toward HepG2 was positively correlated with cytotoxicity toward both HCT 116 and HaCaT cells. Cytotoxic activities toward HCT 116 and HaCaT were also strongly correlated. The total tannins exhibited a strong negative correlation with cytotoxicity toward HCT 116 and HaCaT cells. This suggests that a removal of tannins from the extracts might increase the cytotoxic effect/s of the samples toward HCT 116 and HaCaT, along with their inhibition of α-glucosidase, which is worthy of further investigations. The inhibition of α-glucosidase was strongly positively correlated with the total and condensed tannins, which indicates that these compounds might be responsible for the inhibition of this enzyme. These results are in accordance with previous observations regarding the tannin effect on glucosidase activity
[38].
As far as individual compounds are concerned, according to Pearson’s correlation coefficient, the only compound that was very strongly correlated with the inhibition of α-glucosidase was chlorogenic acid (
Supplementary Table S2); as such, we suggest the further investigation of the antidiabetic potential of this phenolic compound. The results from all three antioxidant methods strongly correlated with caffeic acid hexoside 1, 3-caffeoylquinic acid, 4-caffeoylquinic acid, 3-
p-coumaroylquinic acid,
p-coumaric acid hexoside 2, catechin, quercetin-hexoside pentoside, quercetin-3-glucoside, kaempferol dihexoside and kaempferol trihexoside. Therefore, we assume that these compounds mostly contributed to the antioxidant activity of the inflorescences. The cytotoxic activity toward HepG2, HCT 116 and HaCaT strongly correlated with di-caffeoylquinic acid 3,3-feruloylquinic acid, 5-feruloylquinic acid, isorhamnetin dihexoside, laricitrin glucuronide and syringetin hexoside 2. The individual compounds that strongly correlated with the inhibition of both antidiabetic enzymes, α-amylase and α-glucosidase, were procyanidin dimer 1 and 4. With the inhibition of α-amylase, the only strong correlations were naringenin hexoside, isorhamnetin acetyl hexoside 1 and 2, myricetin rutinoside, syringetin acetyl hexoside 2, and procyanidin dimer 1, 4, 5 and 6. The inhibition of α-glucosidase strongly correlated with chlorogenic acid; procyanidin dimer 1, 2, 4; and procyanidin tetramer.
Based on the results, we propose the consideration of Prunus, Malus and Chaenomeles inflorescences as low-sugar plant foods rich in polyphenolic bioactive compounds, or at least as functional additives to regular food that could improve human health. In particular, we propose Cj inflorescences for further in vivo studies of their antidiabetic and anti-inflammatory activity, and for possible use as a functional food. Prunus inflorescences would be excellent candidates for further analyses of the influence of monomers, oligomers and polymers of flavanols on HaCaT cells’ proliferation. Finally, PsKss is a material with a respectable amount of total phenolics and flavonoids that shows strong antioxidant activity and α-amylase inhibition; therefore, it is worthy of further in vitro and in vivo investigations.