
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Yoon-Yen Yow | + 3664 word(s) | 3664 | 2022-01-12 07:23:04 | | | |
| 2 | Peter Tang | Meta information modification | 3664 | 2022-01-20 08:48:17 | | |
Video Upload Options
Cosmeceuticals are topical cosmetic-pharmaceutical hybrids which refer to a cosmetic product with active ingredients claiming to have medicinal or drug-like benefits to skin health. Marine algae are rich in bioactive substances that have shown to exhibit strong benefits to the skin, particularly in overcoming rashes, pigmentation, aging, and cancer.
1. Introduction
1.1. Synthetic Versus Natural Ingredients in Cosmetic Industry
1.2. Current Applications of Algae-Derived Metabolites in Cosmeceutical Industrial
1.3. UV Radiation and Skin-Related Diseases
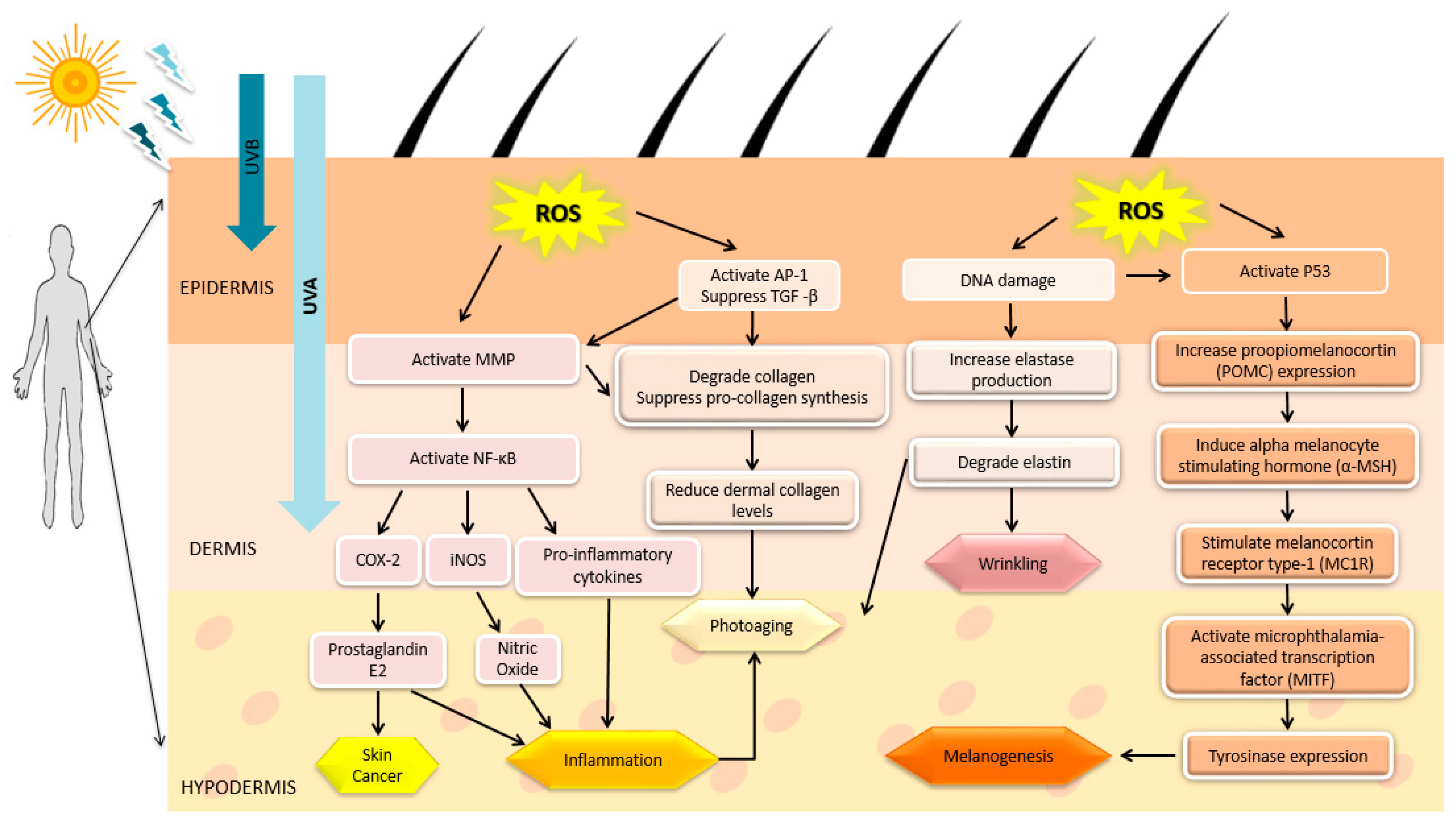
2. Marine Algae-Derived Compounds in Cosmeceutical Application
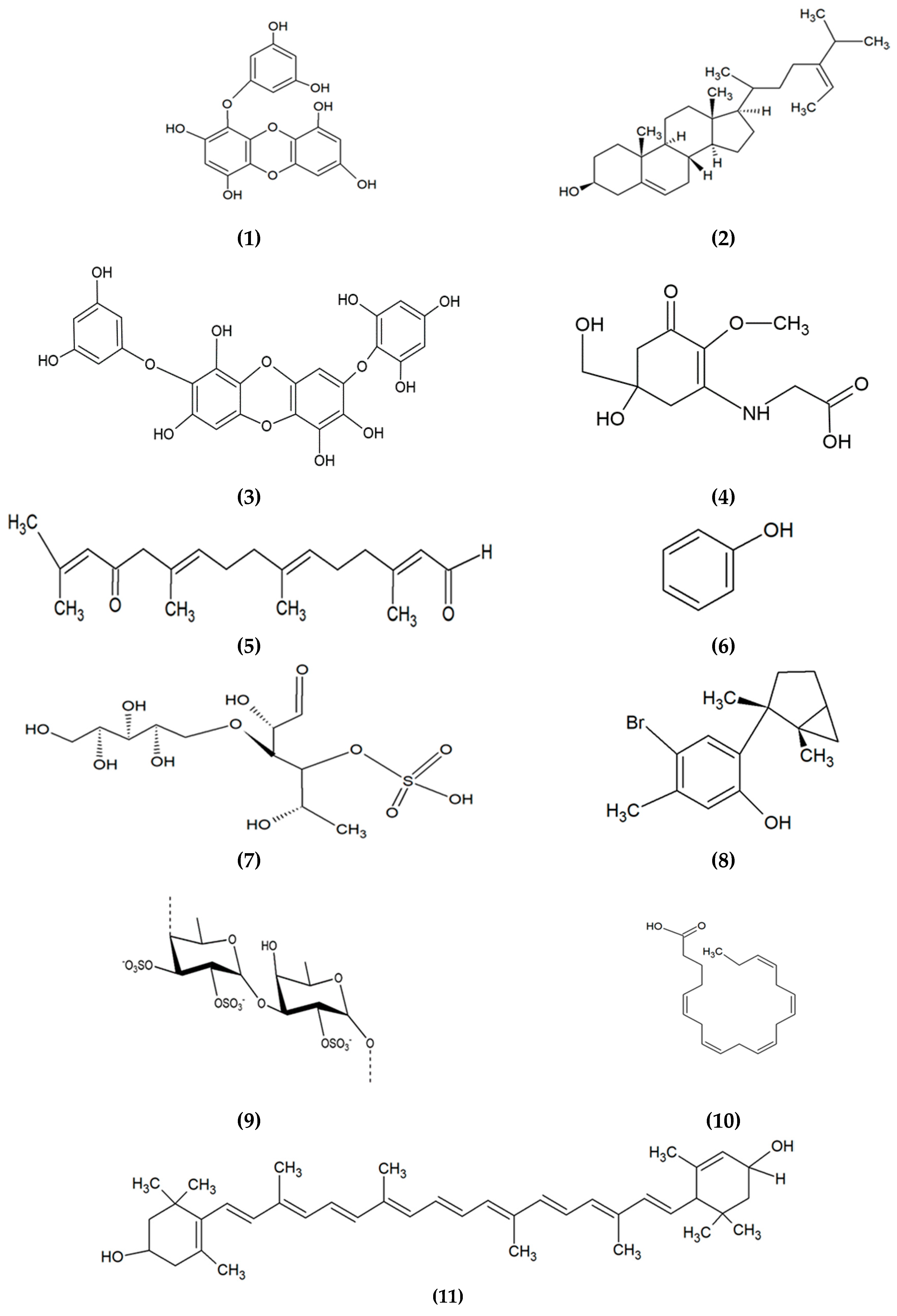
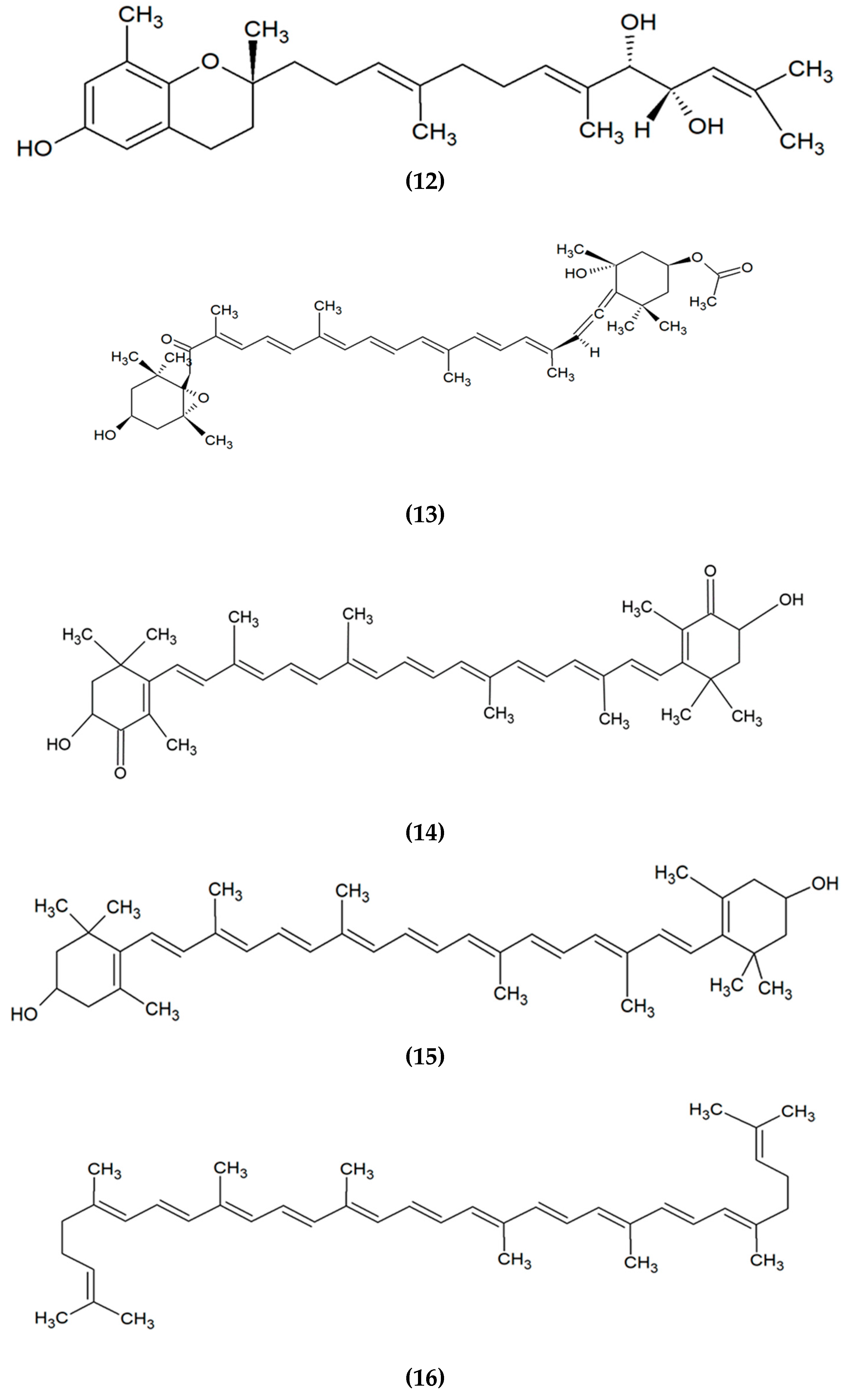
|
Algae Species |
Bioactive Compound/Extract |
Beneficial Activity |
Mechanism of Action |
Experimental Model |
Reference |
|---|---|---|---|---|---|
|
Brown algae |
|||||
|
Ascophyllum nodosum |
Ascophyllan |
Anticancer |
Inhibit MMP expression |
B16 melanoma cells |
[54] |
|
Bifurcaria bifurcata |
Eleganonal |
Antioxidant |
DPPH inhibition |
In vitro |
[55] |
|
Chnoospora implexa |
Ethanol extract |
Antimicrobial |
Bacterial growth inhibition |
Staphylococcus aureus, Staphylococcus pyogenes |
[56] |
|
Chnoospora minima |
Fucoidan |
Anti-inflammation |
Inhibition of LPS-induced NO production, iNOS, COX-2, and PGE2 levels |
RAW macrophages |
[47] |
|
Cladosiphon okamuranus |
Fucoxanthin |
Antioxidant |
DPPH inhibition |
In vitro |
[49] |
|
Colpomenia sinuosa |
Ethanol extract |
Antimicrobial |
Bacterial growth inhibition |
S. aureus, S. pyogenes |
[56] |
|
Cystoseira barbata |
Fat-soluble vitamin and carotenoids |
Antioxidant |
High fat-soluble vitamin and carotenoid content |
In vitro |
[57] |
|
Cystoseira foeniculacea |
Polyphenol |
Antioxidant |
DPPH inhibition (EC50 = 5.27 mg/mL) |
In vitro |
[58] |
|
Cystoseira hakodatensis |
Phenol and fucoxanthin |
Antioxidant |
High total phenolic and fucoxanthin content |
In vitro |
[59] |
|
Cystoseira osmundacea |
Ethanol extract |
Antimicrobial |
Bacterial growth inhibition |
S. pyogenes |
[56] |
|
Dictyopteris delicatula |
Ethanol extract |
Antimicrobial |
Bacterial growth inhibition |
S. aureus, S. pyogenes |
[56] |
|
Dictyota dichotoma |
Algae extract |
Antimicrobial |
Inhibit the synthesis of the peptidoglycan layer of bacterial cell walls |
Penicillium purpurescens, Candida albicans, Aspergillus flavus |
[60] |
|
Ecklonia cava |
Dieckol |
Anti-inflammation |
Suppression of iNOS and COX-2 |
Murine BV2 microglia |
[61] |
|
Phlorotannin |
Anti-melanogenic |
Inhibit melanin production |
B16F10 melanoma cells |
[44] |
|
|
Phlorotannin |
Antioxidant |
ROS scavenging potential |
Chinese hamster lung fibroblast (V79-4) |
[62] |
|
|
Ecklonia kurome |
Phlorotannin |
Anti-inflammation |
Inhibit hyaluronidase |
Assay of HAase (In vitro) |
[42] |
|
Ecklonia Stolonifera |
Phlorotannin |
Anti-aging |
Inhibit MMP expression |
Human dermal fibroblast cell |
[43] |
|
Phlorofucofuroeckol A and B |
Anti-inflammation |
Inhibition of NO production by downregulating iNOS and prostaglandin E2 |
LPS stimulated RAW 264.7 cells |
[63] |
|
|
Eisenia arborea |
Phlorotannin |
Anti-inflammation |
Inhibit release of histamine |
Rat basophile leukemia cells (RBL-2HE) |
[64] |
|
Eisenia bicyclis |
Phlorotannin |
Anti-inflammation |
Inhibit hyaluronidase |
Assay of HAase (In vitro) |
[42] |
|
Fucus evanescens |
Fucoidan |
Anticancer |
Inhibit cell proliferation |
Human malignant melanoma cells |
[45] |
|
Fucus vesiculosus |
Extract |
Anti-aging |
Stimulate collagen production |
N/A |
[8] |
|
Fucoidan |
Anti-melanogenic |
Inhibit tyrosinase and melanin |
B16 murine melanoma cells |
[46] |
|
|
Fucoidan |
Anticancer |
Decrease melanoma growth |
Mice |
[65] |
|
|
Fucoxanthin |
Antioxidant |
Prevent oxidation formation |
In vitro, RAW 264.7 macrophage, Mouse (ex vivo) |
[66] |
|
|
Halopteris scoparia |
Ethanol extract |
Anti-inflammation |
COX-2 inhibition |
COX inhibitory screening assay kit |
[67] |
|
Himanthalia elongota |
Fatty acid and Phenol |
Antimicrobial |
Bacterial growth inhibition |
Escherichia coli, Staphylococcus aureus |
[68] |
|
Hizikia fusiformis |
Fucosterol |
Anti-aging |
Inhibit MMP expression |
Human dermal fibroblast |
[18] |
|
Ethyl acetate extract |
Anti-melanogenic |
Inhibit tyrosinase and melanin |
B16F10 mouse melanoma cells |
[69] |
|
|
Fucoxanthin |
Antioxidant |
DPPH inhibition |
In vitro |
[70] |
|
|
Hydroclathrus clathratus |
Ethanol extract |
Antimicrobial |
Bacterial growth inhibition |
S. aureus, S. pyogenes |
[56] |
|
Ishige foliacea |
Phlorotannin |
Anti-melanogenic |
Downregulation of tyrosinase and melanin synthesis |
B16F10 cells Zebrafish embryo |
|
|
Ishige okamurae |
Diphlorethohydroxycarmalol |
Anti-inflammation |
Down-regulation of iNOS and COX-2 expression and NF-κβ activation |
Human umbilical vein endothelial cells |
[73] |
|
Laminaria japonica |
Fucoxanthin |
Anti-melanogenic |
Suppress tyrosinase activity |
UVB- irradiated guinea pig |
[48] |
|
Laminaria ochroleuca |
Polyphenol |
Antioxidant |
High total phenolic content and antioxidant capacity |
In vitro |
[74] |
|
Macrocystis pyrifera |
Phlorotannin |
Antioxidant |
ROS scavenging potential |
In vitro |
[8] |
|
Hyaluronic acid |
Anti-aging |
Enhance the production of syndecan-4 |
N/A |
[75] |
|
|
Padina concrescens |
Ethanol extract |
Antimicrobial |
Bacterial growth inhibition |
S. aureus, S. pyogenes |
[56] |
|
Padina pavonica |
Polyphenol |
Antimicrobial |
Bacterial growth inhibition |
Candida albicans and Mucor ramaniannus |
[17] |
|
Acetone extract |
Antioxidant |
Free radical scavenging activity (IC50 = 691.56 µg L−1) |
In vitro |
[60] |
|
|
Padina tetrastromatic |
Diterpenes |
Antioxidant |
DPPH (IC50 = 1.73) & ABTS (IC50 = 2.01) inhibitions |
In vitro |
[76] |
|
Sulfated polysaccharide |
Anti-inflammation |
COX-2 and iNOS inhibitions |
Paw edema in rats |
[77] |
|
|
Petalonia binghamiae |
Ethanol extract |
Anti-melanogenic |
Inhibit tyrosinase and melanin |
B16F10 murine melanoma cells |
[78] |
|
Aqueous extract |
Antioxidant Anti-inflammation |
DPPH inhibition COX-2 inhibition |
In vitro In vitro |
[67] |
|
|
Rosenvingea intrincata |
Ethanol extract |
Antimicrobial |
Bacterial growth inhibition |
S. aureus, S. pyogenes |
[56] |
|
Saccharina latissima |
Phenol |
Antioxidant |
High total phenolic content, DPPH scavenging activity and FRAP |
In vitro |
[79] |
|
Sargassum fulvellum |
Fucoxanthin |
Antioxidant |
DPPH inhibition |
In vitro |
[70] |
|
Sargassum furcatum |
Methanol extract |
Antioxidant |
DPPH (EC50 = 0.461) & ABTS (EC50 = 0.266) inhibitions |
In vitro |
[80] |
|
Sargassum hemiphyllum |
Sulfated polysaccharide |
Anti-inflammation |
Inhibit LPS-induced inflammatory response |
RAW 264.7 macrophage cells |
[81] |
|
Sargassum henslowianum |
Sulfated polysaccharide |
Anticancer |
Activation of caspase-3 |
B16 melanoma cells |
[82] |
|
Sargassum horridum |
Ethanol extract |
Antimicrobial |
Bacterial growth inhibition |
S. aureus, S. pyogenes |
[56] |
|
Sargassum horneri |
Sargachromanol.E |
Anti-aging |
Inhibit MMP expression |
UVA irradiated dermal fibroblast |
[83] |
|
Alginic acid |
Anti-inflammation |
Inhibit inflammatory response |
HaCaT cells |
[84] |
|
|
Sargassum muticum |
Tetraprenyltoluquinol chromane meroterpenoid |
Anti-aging |
ROS scavenging potential |
Human dermal fibroblast |
[85] |
|
Sargassum polycystum |
Ethanol extract |
Anti-melanogenic |
Inhibit tyrosinase and melanin production |
B16F10 melanoma cells |
[39] |
|
Sargassum serratifolium |
Sargachromenol |
Anti-melanogenic |
Downregulation of microphthalmia-associated transcription factor |
B16F10 melanoma cells |
[39] |
|
Sargassum siliquastrum |
Fucoxanthin |
Antioxidant |
Reduced UVB-induced ROS production |
Human fibroblast |
[86] |
|
Sargassum thunbergi |
Thunbergols |
Antioxidant |
DPPH inhibition |
In vitro |
[87] |
|
Sargassum vulgare |
Methanol extract |
Antioxidant |
β-carotene bleaching activity |
In vitro |
[88] |
|
Stoechospermum marginatum |
Spatane diterpenoids |
Anticancer |
Cell growth inhibition |
Murine B16F10 melanoma cells |
[89] |
|
Turbinaria conoides |
Laminarin, alginate, fucoidan |
Antioxidant |
ROS scavenging potential |
N/A |
[33] |
|
Turbinaria ornata |
Fucoxanthin |
Antioxidant |
High FRAP value (>10 µM/µg of extract) |
In vitro |
[90] |
|
Undaria pinnatifida |
Fucoxanthin |
Anti-aging |
MMP expression reduction, VEGF |
Mouse |
[50] |
|
Ethyl acetate extract |
Anti-melanogenic |
Down regulate melanin and inhibit tyrosinase |
Mouse B16 melanoma cells |
[91] |
|
|
Polyunsaturated fatty acid |
Anti-inflammation |
N/A |
Mouse ear edema and erythema |
[92] |
|
|
Fucoxanthin |
Antioxidant |
DPPH inhibition |
In vitro |
[70] |
|
|
Red algae |
|||||
|
Alsidium corallinum |
Methanol extract |
Antimicrobial |
Bacterial growth inhibition |
Escherichia coli, Klebsiella pneumoniae, Staphylococcus aureus |
[93] |
|
Bangia |
Algae extract |
Antioxidant |
Induce peroxidase and superoxide dismutase to reduce oxidative stress |
In vitro |
[94] |
|
Bryothamnion triquetrum |
Methanol extract |
Antioxidant |
DPPH (EC50 = 0.357) & ABTS (EC50 = 0.370) inhibitions |
In vitro |
[80] |
|
Ceramium rubrum |
Methanol extract |
Antimicrobial |
Bacterial growth inhibition |
Escherichia coli, Enterococcus faecalis, Staphylococcus aureus |
[93] |
|
Chondrocanthus acicularis |
Methanol extract |
Antimicrobial |
Bacterial growth inhibition |
E. coli, K. pneumoniae, E. faecalis, S. aureus |
[93] |
|
Chondrus canaliculatus |
Polysaccharide |
Antioxidant |
DPPH inhibition |
In vitro |
[95] |
|
Chondrus crispus |
Aqueous extract |
Antimicrobial |
Bacterial growth inhibition |
Salmonella Enteritidis |
[96] |
|
Corallina pilulifera |
Methanol extract |
Anti-aging Antioxidant |
Reduce the expression of gelatinase Inhibit free radical oxidation |
Human dermal fibroblast Human fibrosarcoma (HT-1080) |
[97] |
|
Corallina vancouverensis |
Ethanol extract |
Antimicrobial |
Bacterial growth inhibition |
S. aureus, S. pyogenes |
[56] |
|
Ganonema farinosum |
Ethanol extract |
Antimicrobial |
Bacterial growth inhibition |
S. aureus, S. pyogenes |
[56] |
|
Gelidium crinaale |
Fat-soluble vitamin and carotenoids |
Antioxidant |
High fat-soluble vitamin and carotenoid content |
In vitro |
[57] |
|
Gelidium robustum |
Ethanol extract |
Antimicrobial |
Bacterial growth inhibition |
S. aureus, S. pyogenes |
[56] |
|
Gracilaria gracilis |
Phenol |
Antioxidant |
ROS scavenging potential |
In vitro |
[98] |
|
Gracilariopsis lemaneiformis |
Sulfated polysaccharide |
Antioxidant |
DPPH, Superoxide radical assay, hydroxyl radical assay (EC50 = 2.45 mg/mL) |
In vitro |
[99] |
|
Gracilaria salicornia |
2H- chromenyl |
Antioxidant Anti-inflammation |
DPPH and ABTS inhibitions COX-1 inhibition |
In vitro |
[100] |
|
Jania rubens |
Glycosaminoglycan |
Anti-aging |
Collagen synthesis |
Unknown |
[75] |
|
Laurencia caspica |
Phenol Ethanol extract |
Antioxidant Antimicrobial |
DPPH inhibition Bacterial growth inhibition |
In vitro Klebsiella pneumonia, Pseudomonas aeroginosa |
[101] |
|
Laurencia luzonensis |
Sesquiterpenes |
Antimicrobial |
Bacterial growth inhibition |
Bacillus megaterium |
[12] |
|
Laurenicia obtusa |
Polysaccharide |
Antioxidant |
DPPH (IC50 = 24 µg/mL), FRAP (IC50 = 92 µg/mL), Hydroxyl radical scavenging activity (IC50 = 113 µg/mL) |
In vitro |
[102] |
|
Laurenicia pacifica |
Laurinterol |
Antimicrobial |
Bacterial growth inhibition |
Staphylococcus aureus |
[9] |
|
Laurencia rigida |
Sesquiterpenes |
Antimicrobial |
Bacterial growth inhibition |
Bacillus megaterium |
[12] |
|
Meristotheca dakarensis |
Glycosaminoglycan |
Anti-aging |
Collagen synthesis |
Unknown |
[75] |
|
Osmundaria obtusilo |
Methanol extract |
Antioxidant |
DPPH (EC50 = 0.041 mg/mL), ABTS (EC50 = 0.031 mg/mL), Metal chelating (EC50 = 0.1 mg/mL), folin ciocalteu (EC50 = 0.128 mg/mL) |
In vitro |
[80] |
|
Palisada flagellifera |
Methanol extract |
Antioxidant |
β-carotene bleaching activity |
In vitro |
[88] |
|
Palmaria palmata |
MAA |
Anti-aging |
Collagenase inhibition |
Clostridium histolyticum |
[53] |
|
Polysiphonia howei |
Fucoxanthin |
Antioxidant |
High FRAP value (>5 µM/µg of extract) |
In vitro |
[90] |
|
Porphyra haitanensis |
Sulfated Polysaccharide |
Antioxidant |
ROS scavenging potential |
Mice |
[103] |
|
Porphyra umbilicalis |
MAA |
Anti-aging |
Control expression of MMP |
Human dermal fibroblast |
[16] |
|
Porphyra sp. |
MAA |
Anti-aging |
Collagenases inhibition |
Clostridium histolyticum |
[53] |
|
Porphyra yezoensis |
MAA Polyphenol Phycoerythrin |
Antioxidant Anticancer Anti-inflammation |
ROS scavenging potential and MMP expression Induce apoptosis Suppression of mast cells |
Human skin fibroblast HaCaT cells Rat |
[51] |
|
Pterocladia capillacea |
Sulfated polysaccharide |
Antimicrobial |
N/A |
Staphylococcus aureus Escherichia coli |
[104] |
|
Pyropia columbia |
Phenol |
Antioxidant |
DPPH, β-carotene bleaching and ABTS inhibitions |
Piaractus mesopotamicus |
[105] |
|
Pyropia yezoensis |
Polysaccharide |
Anti-aging |
Promote collagen synthesis |
Human dermal fibroblast |
[106] |
|
Rhodomela confervoides |
Polyphenol |
Antimicrobial |
Bacterial growth inhibition |
Candida albicans Mucor ramaniannus |
[17] |
|
Bromophenol |
Antioxidant |
DPPH inhibition |
In vitro |
[107] |
|
|
Schizymenia dubyi |
Phenol |
Anti-melanogenic |
Inhibit tyrosinase activity |
In vitro |
[39] |
|
Green algae |
|||||
|
Bryopsis plumose |
Polysaccharide |
Antioxidant |
ROS scavenging potential |
In vitro |
[108] |
|
Chaetomorpha antennia |
Fucoxanthin |
Antioxidant |
DPPH inhibition (63.77%) |
In vitro |
[109] |
|
Chlamydomonas hedleyi |
MAA |
Antioxidant Anti-aging Anti-inflammation |
ROS scavenging potential Increase UV-suppressed genes (procollagen C proteinase enhancer and elastin) expression Reduce COX-2 and involucrin expression |
In vitro HaCaT cells HaCaT cells |
[52] |
|
Cladophora sp. |
Ethanol extract |
Antimicrobial |
Bacterial growth inhibition |
S. aureus, S. pyogenes |
[56] |
|
Codium amplivesiculatum |
Ethanol extract |
Antimicrobial |
Bacterial growth inhibition |
S. aureus, S. pyogenes |
[56] |
|
Codium cuneatum |
Ethanol extract |
Antimicrobial |
Bacterial growth inhibition |
S. aureus, S. pyogenes |
[56] |
|
Codium fragile |
Sterol |
Anti-inflammation |
Reduces the expression of COX-2, iNOS, and TNF-α |
Mice |
[110] |
|
Codium simulans |
Ethanol extract |
Antimicrobial |
Bacterial growth inhibition |
S. aureus, S. pyogenes |
[56] |
|
Entromorpha intestinalis |
Chloroform and methanol extract |
Antioxidant |
SOD activity is reduced |
Labidochromis caeruleus |
[111] |
|
Enteromorpha linza |
Polysaccharide |
Antioxidant |
ROS scavenging potential |
In vitro |
[108] |
|
Gayralia oxysperma |
Fucoxanthin |
Antioxidant |
High FRAP value (>6 µM/µg of extract) |
In vitro |
[90] |
|
Ulva dactilifera |
Ethanol extract |
Antimicrobial |
Bacterial growth inhibition |
S. aureus, Streptococcus pyogenes |
[56] |
|
Ulva fasciata |
Fucoxanthin |
Antioxidant |
DPPH inhibition (83.95%) |
In vitro |
[109] |
|
Ulva lactuca |
Phycocolloids |
Anti-inflammation |
N/A |
N/A |
[75] |
|
Ulva pertusa |
Polysaccharide |
Antioxidant |
ROS scavenging potential |
In vitro |
[108] |
|
Ulva prolifera |
Phenol and flavonoid |
Antioxidant |
DPPH inhibition, high phenolic and flavonoid contents |
In vitro |
[112] |
|
Ulva rigida |
Phenol |
Antioxidant |
DPPH inhibition |
In vitro |
[113] |
|
Ulva sp. |
Sulfated polysaccharide |
Anti-aging |
Increase hyaluronan production |
Human dermal fibroblast |
[114] |
|
Microalgae/Cyanobacteria |
|||||
|
Anabaena vaginicola |
Lycopene |
Antioxidant Anti-aging |
N/A |
In vitro |
[115] |
|
Arthrospira platensis |
Methanol extracts of exopolysaccharides |
Antioxidant |
N/A |
In vitro |
[115] |
|
Chlorella fusca |
Sporopollenin |
Anti-aging |
Protect cells from UV radiation |
N/A |
[116] |
|
Chlorella minutissima |
MAA |
Anti-aging |
Protect cells from UV radiation |
N/A |
[116] |
|
Chlorella sorokiniana |
MAA |
Anti-aging |
Protect cells from UV radiation |
N/A |
[116] |
|
Lutein |
Anti-aging |
Reduce UV induced damage |
N/A |
[33] |
|
|
Chlorella vulgaris |
Hot water extract |
Anti-aging |
Reduced activity of SOD |
Human diploid fibroblast |
[117] |
|
Anti-inflammation |
Downregulated mRNA expression levels of IL-4 and IFN-γ |
NC/Nga mice |
[118] |
||
|
Dunaliella salina |
β-carotene |
Antioxidant |
Protect against oxidative stress |
Rat |
[119] |
|
β-cryptoxanthin |
Anti-inflammation |
Reduced the production of IL-1β, IL-6, TNF-α, the protein expression of iNOS and COX-2 |
LPS-stimulated RAW 264.7 cells |
[120] |
|
|
Haematococcus pluvialis |
Astaxanthin (carotenoid) |
Anti-aging |
Inhibit MMP expression |
Mice and human dermal fibroblasts |
[121] |
|
Anticancer |
ROS scavenging potential |
Mice |
[122] |
||
|
Nannochloropsis granulata |
Carotenoid |
Antioxidant |
DPPH inhibition |
In vitro |
[123] |
|
Nannochloropsis oculata |
Zeaxanthin |
Anti-melanogenic |
Inhibit tyrosinase |
In vitro |
[124] |
|
Nitzschia sp. |
Fucoxanthin |
Antioxidant |
Reduced oxidative stress |
Human Glioma Cells |
[125] |
|
Nostoc sp. |
MAA |
Antioxidant |
ROS scavenging potential |
In vitro |
[126] |
|
Odontella aurita |
EPA |
Antioxidant |
Reduce oxidative stress |
Rat |
[127] |
|
Planktochlorella nurekis |
Fatty acid |
Antimicrobial |
Bacterial growth inhibition |
Campylobacter jejuni, E. coli, Salmonella enterica var. |
[128] |
|
Porphyridium sp. |
Sulfated polysaccharide |
Anti-inflammation Antioxidant |
Inhibit proinflammatory modulator Inhibited oxidative damage |
Unknown 3T3 cells |
[103] |
|
Rhodella reticulata |
Sulfated polysaccharide |
Antioxidant |
ROS scavenging potential |
In vitro |
[103] |
|
Skeletonema marinoi |
Polyunsaturated aldehyde and fatty acid |
Anticancer |
Inhibit cell proliferation |
Human melanoma cells (A2058) |
[129] |
|
Spirulina platensis |
β-carotene and phycocyanin |
Antioxidant Anti-inflammation |
Inhibit lipid peroxidation Inhibit TNF-α and IL-6 expressions |
Mouse Human dermal fibroblast cells (CCD-986sk) |
[130] |
|
Ethanol extract |
Antimicrobial |
Bacterial growth inhibition |
E. coli, Pseudomonas aeruginosa, Bacillus subtilis, and Aspergillus niger |
[131] |
|
|
Synechocystis spp. |
Fatty acids and phenols |
Antimicrobial |
Bacterial growth inhibition |
E. coli and S. aureus |
[68] |
References
- Kligman, D. Cosmeceuticals. Dermatol. Clin. 2000, 18, 609–615.
- Dureja, H.; Kaushik, D.; Gupta, M.; Kumar, V.; Lather, V. Cosmeceuticals: An emerging concept. Indian J. Pharm. 2005, 37, 155–159.
- Smit, N.; Vicanova, J.; Pavel, S. The Hunt for Natural Skin Whitening Agents. Int. J. Mol. Sci. 2009, 10, 5326–5349.
- Bos, J.D.; Meinardi, M.M. The 500 Dalton rule for the skin penetration of chemical compounds and drugs. Exp. Dermatol. 2000, 9, 165–169.
- Kerdudo, A.; Burger, P.; Merck, F.; Dingas, A.; Rolland, Y.; Michel, T.; Fernandez, X. Development of a natural ingredient–Natural preservative: A case study. Comptes Rendus Chimie 2016, 19, 1077–1089.
- Barrett, J. Chemical Exposures: The Ugly Side of Beauty Products. Environ Health Perspect. 2005, 113, A24.
- Global Market Value for Natural Cosmetics in 2018–2027|Statista. Available online: https://www.statista.com/statistics/673641/global-market-value-for-natural-cosmetics/ (accessed on 18 November 2019).
- Ariede, M.B.; Candido, T.M.; Jacome, A.L.M.; Velasco, M.V.R.; de Carvalho, J.C.M.; Baby, A.R. Cosmetic attributes of algae—A review. Algal Res. 2017, 25, 483–487.
- Wang, H.M.D.; Chen, C.C.; Huynh, P.; Chang, J.S. Exploring the potential of using algae in cosmetics. Bioresour. Technol. 2015, 184, 355–362.
- Fu, W.; Nelson, D.R.; Yi, Z.; Xu, M.; Khraiwesh, B.; Jijakli, K.; Chaiboonchoe, A.; Alzahmi, A.; Al-Khairy, D.; Brynjolfsson, S.; et al. Bioactive compounds from microalgae: Current development and prospects. Stud. Nat. Prod. Chem. 2017, 54, 199–225.
- Freile-Pelegrín, Y.; Robledo, D. Bioactive phenolic compounds from algae. In Bioactive Compounds from Marine Foods: Plant and Animal Sources, 1st ed.; Hernández-Ledesma, B., Herrero, M., Eds.; John Wiley & Sons, Ltd.: Chichester, UK, 2014; pp. 113–129.
- Thomas, N.V.; Kim, S.K. Beneficial effects of marine algal compounds in cosmeceuticals. Mar. Drugs 2013, 11, 146–164.
- Fernando, I.S.; Nah, J.W.; Jeon, Y.J. Potential anti-inflammatory natural products from marine algae. Environ. Toxicol. Pharm. 2016, 48, 22–30.
- Peng, J.; Yuan, J.-P.; Wu, C.-F.; Wang, J.-H. Fucoxanthin, a marine carotenoid present in brown seaweeds and diatoms: Metabolism and bioactivities relevant to human health. Mar. Drugs 2011, 9, 1806–1828.
- Talero, E.; García-Mauriño, S.; Ávila-Román, J.; Rodríguez-Luna, A.; Alcaide, A.; Motilva, V. Bioactive compounds isolated from microalgae in chronic inflammation and cancer. Mar. Drugs 2015, 13, 6152–6209.
- Wang, H.M.D.; Li, X.C.; Lee, D.J.; Chang, J.S. Potential biomedical applications of marine algae. Bioresour. Technol. 2017, 244, 1407–1415.
- Saidani, K.; Bedjou, F.; Benabdesselam, F.; Touati, N. Antifungal activity of methanolic extracts of four Algerian marine algae species. Afr. J. Biotechnol. 2012, 11, 9496–9500.
- Hwang, E.; Park, S.Y.; Sun, Z.W.; Shin, H.S.; Lee, D.G.; Yi, T.H. The protective effects of fucosterol against skin damage in UVB-irradiated human dermal fibroblasts. Mar. Biotechnol. 2014, 16, 361–370.
- Martins, A.; Vieira, H.; Gaspar, H.; Santos, S. Marketed marine natural products in the pharmaceutical and cosmeceutical industries: Tips for success. Mar. Drugs 2014, 12, 1066–1101.
- Nayaka, S.; Toppo, K.; Verma, S. Adaptation in Algae to Environmental Stress and Ecological Conditions. In Plant Adaptation Strategies in Changing Environment; Springer: Singapore, 2017; pp. 103–115.
- Katz, A.; Waridel, P.; Shevchenko, A.; Pick, U. Salt-induced changes in the plasma membrane proteome of the halotolerant alga Dunaliella salina as revealed by blue native gel electrophoresis and nano-LC-MS/MS analysis. Mol. Cell. Proteom. 2007, 6, 1459–1472.
- Sydney, E.B.; Sydney, A.C.N.; de Carvalho, J.C.; Soccol, C.R. Potential carbon fixation of industrially important microalgae. In Biofuels from Algae; Elsevier: Amsterdam, The Netherlands, 2019; pp. 67–88.
- Usher, P.K.; Ross, A.B.; Camargo-Valero, M.A.; Tomlin, A.S.; Gale, W.F. An overview of the potential environmental impacts of large-scale microalgae cultivation. Biofuels 2014, 5, 331–349.
- Álvarez-Gómez, F.; Korbee, N.; Casas-Arrojo, V.; Abdala-Díaz, R.T.; Figueroa, F.L. UV photoprotection, cytotoxicity and immunology capacity of red algae extracts. Molecules 2019, 24, 341.
- Khanra, S.; Mondal, M.; Halder, G.; Tiwari, O.N.; Gayen, K.; Bhowmick, T.K. Downstream processing of microalgae for pigments, protein and carbohydrate in industrial application: A review. Food Bioprod. Process. 2018, 110, 60–84.
- Pagels, F.; Guedes, A.C.; Amaro, H.M.; Kijjoa, A.; Vasconcelos, V. Phycobiliproteins from cyanobacteria: Chemistry and biotechnological applications. Biotechnol. Adv. 2019, 37, 422–443.
- Ambati, R.R.; Gogisetty, D.; Aswathanarayana, R.G.; Ravi, S.; Bikkina, P.N.; Bo, L.; Yuepeng, S. Industrial potential of carotenoid pigments from microalgae: Current trends and future prospects. Crit. Rev. Food Sci. Nutr. 2019, 59, 1880–1902.
- Galasso, C.; Corinaldesi, C.; Sansone, C. Carotenoids from marine organisms: Biological functions and industrial applications. Antioxidant 2017, 6, 96.
- Torres, P.; Santos, J.P.; Chow, F.; dos Santos, D.Y. A comprehensive review of traditional uses, bioactivity potential, and chemical diversity of the genus Gracilaria (Gracilariales, Rhodophyta). Algal Res. 2019, 37, 288–306.
- Jahan, A.; Ahmad, I.Z.; Fatima, N.; Ansari, V.A.; Akhtar, J. Algal bioactive compounds in the cosmeceutical industry: A review. Phycologia 2017, 56, 410–422.
- Siezen, R.J. Microbial sunscreens. Microb. Biotechnol. 2011, 4, 1–7.
- Muñoz, R.; Gonzalez-Fernandez, C. (Eds.) Microalgae-Based Biofuels and Bioproducts: From Feedstock Cultivation to End-Products; Woodhead Publishing: Duxford, UK, 2017.
- Berthon, J.Y.; Nachat-Kappes, R.; Bey, M.; Cadoret, J.P.; Renimel, I.; Filaire, E. Marine algae as attractive source to skin care. Free Radic. Res. 2017, 51, 555–567.
- Tabassum, N.; Hamdani, M. Plants used to treat skin diseases. Pharm. Rev. 2014, 8, 52–60.
- Skin Cancer|Skin Cancer Facts|Common Skin Cancer Types. Available online: https://www.cancer.org/cancer/skin-cancer.html (accessed on 27 August 2018).
- Tan, L.T.; Mahendra, C.K.; Yow, Y.Y.; Chan, K.G.; Khan, T.M.; Lee, L.H.; Goh, B.H. Streptomyces sp. MUM273b: A mangrove-derived potential source for antioxidant and UVB radiation protectants. Microbiol. Open 2019, 8, e859.
- D’Orazio, J.; Jarrett, S.; Amaro-Ortiz, A.; Scott, T. UV radiation and the skin. Int. J. Mol. Sci. 2013, 14, 12222–12248.
- Amaro-Ortiz, A.; Yan, B.; D’Orazio, J.A. Ultraviolet radiation, aging and the skin: Prevention of damage by topical cAMP manipulation. Molecules 2014, 19, 6202–6219.
- Azam, M.S.; Choi, J.; Lee, M.S.; Kim, H.R. Hypopigmenting effects of brown algae-derived phytochemicals: A review on molecular mechanisms. Mar. Drugs 2017, 15, 297.
- Hwang, H.; Chen, T.; Nines, R.G.; Shin, H.C.; Stoner, G.D. Photochemoprevention of UVB-induced skin carcinogenesis in SKH-1 mice by brown algae polyphenols. Int. J. Cancer 2006, 119, 2742–2749.
- Mahendra, C.K.; Tan, L.T.H.; Yap, W.H.; Chan, C.K.; Pusparajah, P.; Goh, B.H. An optimized cosmetic screening assay for ultraviolet B (UVB) protective property of natural products. Prog. Drug Discov. Biomed. Sci. 2019, 2.
- Shibata, T.; Fujimoto, K.; Nagayama, K.; Yamaguchi, K.; Nakamura, T. Inhibitory activity of brown algal phlorotannins against hyaluronidase. Int. J. Food Sci. Technol. 2002, 37, 703–709.
- Joe, M.J.; Kim, S.N.; Choi, H.Y.; Shin, W.S.; Park, G.M.; Kang, D.W.; Kim, Y.K. The inhibitory effects of eckol and dieckol from Ecklonia stolonifera on the expression of matrix metalloproteinase-1 in human dermal fibroblasts. Biol. Pharm. Bull. 2006, 29, 1735–1739.
- Yoon, N.Y.; Eom, T.K.; Kim, M.M.; Kim, S.K. Inhibitory effect of phlorotannins isolated from Ecklonia cava on mushroom tyrosinase activity and melanin formation in mouse B16F10 melanoma cells. J. Agric. Food Chem. 2009, 57, 4124–4129.
- Anastyuk, S.; Shervchenko, N.; Ermakova, S.; Vishchuk, O.; Nazarenko, E.; Dmitrenok, P.; Zvyagintseva, T. Anticancer activity in vitro of a fucoidan from the brown algae Fucus evanescens and its low-molecular fragments, structurally characterized by tandem mass-spectrometry. Carbohydr. Polym. 2012, 87, 186–194.
- Wang, Z.-J.; Xu, W.; Liang, J.-W.; Wang, C.-S.; Kang, Y. Effect of fucoidan on B16 murine melanoma cell melanin formation and apoptosis. Afr. J. Tradit. Complement. Altern. Med. 2017, 14, 149–155.
- Fernando, I.S.; Sanjeewa, K.A.; Samarakoon, K.W.; Lee, W.W.; Kim, H.S.; Kang, N.; Ranasinghe, P.; Lee, H.S.; Jeon, Y.J. A fucoidan fraction purified from Chnoospora minima; a potential inhibitor of LPS-induced inflammatory responses. Int. J. Boil. Macromol. 2017, 104, 1185–1193.
- Shimoda, H.; Tanaka, J.; Shan, S.J.; Maoka, T. Anti-pigmentary activity of fucoxanthin and its influence on skin mRNA expression of melanogenic molecules. J. Pharm. Pharm. 2010, 62, 1137–1145.
- Mise, T.; Ueda, M.; Yasumoto, T. Production of fucoxanthin-rich powder from Cladosiphon okamuranus. Adv. J. Food Sci. Technol. 2011, 3, 73–76.
- Urikura, I.; Sugawara, T.; Hirata, T. Protective effect of fucoxanthin against UVB-induced skin photoaging in hairless mice. Biosci. Biotechnol. Biochem. 2011, 75, 757–760.
- Sakai, S.; Komura, Y.; Nishimura, Y.; Sugawara, T.; Hirata, T. Inhibition of mast cell degranulation by phycoerythrin and its pigment moiety phycoerythrobilin, prepared from Porphyra yezoensis. Food Sci. Technol. Res. 2011, 17, 171–177.
- Suh, S.S.; Hwang, J.; Park, M.; Seo, H.; Kim, H.S.; Lee, J.; Moh, S.; Lee, T.K. Anti-inflammation activities of mycosporine-like amino acids (MAAs) in response to UV radiation suggest potential anti-skin aging activity. Mar. Drugs 2014, 12, 5174–5187.
- Hartmann, A.; Gostner, J.; Fuchs, J.E.; Chaita, E.; Aligiannis, N.; Skaltsounis, L.; Ganzera, M. Inhibition of collagenase by mycosporine-like amino acids from marine sources. Planta Medica 2015, 81, 813–820.
- Abu, R.; Jiang, Z.; Ueno, M.; Isaka, S.; Nakazono, S.; Okimura, T.; Cho, K.; Yamaguchi, K.; Kim, D.; Oda, T. Anti-metastatic effects of the sulfated polysaccharide ascophyllan isolated from Ascophyllum nodosum on B16 melanoma. Biochem. Biophys. Res. Commun. 2015, 458, 727–732.
- Silva, J.; Alves, C.; Freitas, R.; Martins, A.; Pinteus, S.; Ribeiro, J.; Gaspar, H.; Alfonso, A.; Pedrosa, R. Antioxidant and Neuroprotective Potential of the Brown Seaweed Bifurcaria bifurcata in an in vitro Parkinson’s Disease Model. Mar. Drugs 2019, 17, 85.
- Muñoz-Ochoa, M.; Murillo-Álvarez, J.I.; Zermeño-Cervantes, L.A.; Martínez-Díaz, S.; Rodríguez-Riosmena, R. Screening of extracts of algae from Baja California Sur, Mexico as reversers of the antibiotic resistance of some pathogenic bacteria. Eur. Rev. Med. Pharmacol. Sci. 2010, 14, 739–747.
- Panayotova, V.; Merzdhanova, A.; Dobreva, D.A.; Zlatanov, M.; Makedonski, L. Lipids of black sea algae: Unveiling their potential for pharmaceutical and cosmetic applications. J. IMAB–Ann. Proc. Sci. Pap. 2017, 23, 1747–1751.
- Messina, C.M.; Renda, G.; Laudicella, V.A.; Trepos, R.; Fauchon, M.; Hellio, C.; Santulli, A. From ecology to biotechnology, study of the defense strategies of algae and halophytes (from Trapani Saltworks, NW Sicily) with a focus on antioxidants and antimicrobial properties. Int. J. Mol. Sci. 2019, 20, 881.
- Airanthi, M.W.A.; Hosokawa, M.; Miyashita, K. Comparative antioxidant activity of edible Japanese brown seaweeds. J. Food Sci. 2011, 76, C104–C111.
- Kosanić, M.; Ranković, B.; Stanojković, T. Brown macroalgae from the Adriatic Sea as a promising source of bioactive nutrients. J. Food Meas. Charact. 2019, 13, 330–338.
- Jung, W.K.; Heo, S.J.; Jeon, Y.J.; Lee, C.M.; Park, Y.M.; Byun, H.G.; Choi, Y.H.; Park, S.G.; Choi, I.W. Inhibitory effects and molecular mechanism of dieckol isolated from marine brown alga on COX-2 and iNOS in microglial cells. J. Agric. Food Chem. 2009, 57, 4439–4446.
- Kang, K.A.; Lee, K.H.; Chae, S.; Koh, Y.S.; Yoo, B.S.; Kim, J.H.; Ham, Y.M.; Baik, J.S.; Lee, N.H.; Hyun, J.W. Triphlorethol-A from Ecklonia cava protects V79-4 lung fibroblast against hydrogen peroxide induced cell damage. Free Radic. Res. 2005, 39, 883–892.
- Lee, M.S.; Kwon, M.S.; Choi, J.W.; Shin, T.; No, H.K.; Choi, J.S.; Byun, D.S.; Kim, J.I.; Kim, H.R. Anti-inflammatory activities of an ethanol extract of Ecklonia stolonifera in lipopolysaccharide-stimulated RAW 264.7 murine macrophage cells. J. Agric. Food Chem. 2012, 60, 9120–9129.
- Sugiura, Y.; Takeuchi, Y.; Kakinuma, M.; Amano, H. Inhibitory effects of seaweeds on histamine release from rat basophile leukemia cells (RBL-2H3). Fish. Sci. 2006, 72, 1286–1291.
- Teas, J.; Irhimeh, M.R. Melanoma and brown seaweed: An integrative hypothesis. J. Appl. Phycol. 2017, 29, 941–948.
- Zaragozá, M.C.; López, D.P.; Sáiz, M.; Poquet, M.; Pérez, J.; Puig-Parellada, P.; Marmol, F.; Simonetti, P.; Gardana, C.; Lerat, Y.; et al. Toxicity and antioxidant activity in vitro and in vivo of two Fucus vesiculosus extracts. J. Agric. Food Chem. 2008, 56, 7773–7780.
- Campos, A.M.; Matos, J.; Afonso, C.; Gomes, R.; Bandarra, N.M.; Cardoso, C. Azorean macroalgae (Petalonia binghamiae, Halopteris scoparia and Osmundea pinnatifida) bioprospection: A study of fatty acid profiles and bioactivity. Int. J. Food Sci. Technol. 2018, 54, 880–890.
- Plaza, M.; Santoyo, S.; Jaime, L.; Reina, G.G.B.; Herrero, M.; Señoráns, F.J.; Ibáñez, E. Screening for bioactive compounds from algae. J. Pharm. Biomed. Anal. 2010, 51, 450–455.
- Choi, E.O.; Kim, H.S.; Han, M.H.; Choi, Y.H.; Park, C.; Kim, B.W.; Hwang, H.J. Effects of Hizikia fusiforme fractions on melanin synthesis in B16F10 melanoma cells. J. Life Sci. 2013, 23, 1495–1500.
- Yan, X.; Chuda, Y.; Suzuki, M.; Nagata, T. Fucoxanthin as the major antioxidant in Hijikia fusiformis, a common edible seaweed. Biosci. Biotechnol. Biochem. 1999, 63, 605–607.
- Kim, K.N.; Yang, H.M.; Kang, S.M.; Kim, D.; Ahn, G.; Jeon, Y.J. Octaphlorethol A isolated from Ishige foliacea inhibits α-MSH-stimulated induced melanogenesis via ERK pathway in B16F10 melanoma cells. Food Chem. Toxicol. 2013, 59, 521–526.
- Kim, K.N.; Yang, H.M.; Kang, S.M.; Ahn, G.N.; Roh, S.W.; Lee, W.; Kim, D.K.; Jeon, Y.J. Whitening effect of octaphlorethol A isolated from Ishige foliacea in an in vivo zebrafish model. J. Microbiol. Biotechnol. 2015, 25, 448–451.
- Heo, S.J.; Hwang, J.Y.; Choi, J.I.; Lee, S.H.; Park, P.J.; Kang, D.H.; Oh, C.; Kim, D.W.; Han, J.S.; Jeon, Y.J.; et al. Protective effect of diphlorethohydroxycarmalol isolated from Ishige okamurae against high glucose-induced-oxidative stress in human umbilical vein endothelial cells. Food Chem. Toxicol. 2010, 48, 1448–1454.
- Del Olmo, A.; Picon, A.; Nuñez, M. High pressure processing for the extension of Laminaria ochroleuca (kombu) shelf-life: A comparative study with seaweed salting and freezing. Innov. Food Sci. Emerg. Technol. 2019, 52, 420–428.
- SpecialChem—The Universal Selection Source: Cosmetics Ingredients. Available online: https://cosmetics.specialchem.com/ (accessed on 5 May 2020).
- Antony, T.; Chakraborty, K. Xenicanes attenuate pro-inflammatory 5-lipoxygenase: Prospective natural anti-inflammatory leads from intertidal brown seaweed Padina tetrastromatica. Med. Chem. Res. 2019, 28, 591–607.
- Mohsin, S.; Kurup, G.M. Mechanism underlying the anti-inflammatory effect of sulphated polysaccharide from Padina tetrastromatica against carrageenan induced paw edema in rats. Biomed. Prev. Nutr. 2011, 1, 294–301.
- Yoon, H.S.; Koh, W.B.; Oh, Y.S.; Kim, I.J. The Anti-Melanogenic Effects of Petalonia binghamiae extarcts in α-melanocyte stimulating hormone-induced B16/F10 murine melanoma cells. J. Korean Soc. Appl. Biol. Chem. 2009, 52, 564–567.
- Sappati, P.K.; Nayak, B.; VanWalsum, G.P.; Mulrey, O.T. Combined effects of seasonal variation and drying methods on the physicochemical properties and antioxidant activity of sugar kelp (Saccharina latissima). J. Appl. Phycol. 2019, 31, 1311–1332.
- Vasconcelos, J.B.; de Vasconcelos, E.R.; Urrea-Victoria, V.; Bezerra, P.S.; Reis, T.N.; Cocentino, A.L.; Navarro, D.M.; Chow, F.; Areces, A.J.; Fujii, M.T. Antioxidant activity of three seaweeds from tropical reefs of Brazil: Potential sources for bioprospecting. J. Appl. Phycol. 2019, 31, 835–846.
- Hwang, P.A.; Chien, S.Y.; Chan, Y.L.; Lu, M.K.; Wu, C.H.; Kong, Z.L.; Wu, C.J. Inhibition of lipopolysaccharide (LPS)-induced inflammatory responses by Sargassum hemiphyllum sulfated polysaccharide extract in RAW 264.7 macrophage cells. J. Agric. Food Chem. 2011, 59, 2062–2068.
- Ale, M.T.; Maruyama, H.; Tamauchi, H.; Mikkelsen, J.D.; Meyer, A.S. Fucose-containing sulfated polysaccharides from brown seaweeds inhibit proliferation of melanoma cells and induce apoptosis by activation of caspase-3 in vitro. Mar. Drugs 2011, 9, 2605–2621.
- Kim, J.A.; Ahn, B.N.; Kong, C.S.; Kim, S.K. The chromene sargachromanol E inhibits ultraviolet A-induced ageing of skin in human dermal fibroblasts. Br. J. Dermatol. 2013, 168, 968–976.
- Fernando, I.S.; Jayawardena, T.U.; Sanjeewa, K.A.; Wang, L.; Jeon, Y.J.; Lee, W.W. Anti-inflammatory potential of alginic acid from Sargassum horneri against urban aerosol-induced inflammatory responses in keratinocytes and macrophages. Ecotoxicol. Environ. Saf. 2018, 160, 24–31.
- Balboa, E.M.; Li, Y.X.; Ahn, B.N.; Eom, S.H.; Domínguez, H.; Jiménez, C.; Rodríguez, J. Photodamage attenuation effect by a tetraprenyltoluquinol chromane meroterpenoid isolated from Sargassum muticum. J. Photochem. Photobiol. B Biol. 2015, 148, 51–58.
- Heo, S.-J.; Jeon, Y.-J. Protective effect of fucoxanthin isolated from Sargassum siliquastrum on UV-B induced cell damage. J. Photochem. Photobiol. B 2009, 95, 101–107.
- Seo, Y.; Park, K.E.; Kim, Y.A.; Lee, H.J.; Yoo, J.S.; Ahn, J.W.; Lee, B.J. Isolation of tetraprenyltoluquinols from the brown alga Sargassum thunbergii. Chem. Pharm. Bull. 2006, 54, 1730–1733.
- Santos, J.P.; Torres, P.B.; dos Santos, D.Y.; Motta, L.B.; Chow, F. Seasonal effects on antioxidant and anti-HIV activities of Brazilian seaweeds. J. Appl. Phycol. 2018, 31, 1333–1341.
- Velatooru, L.R.; Baggu, C.B.; Janapala, V.R. Spatane diterpinoid from the brown algae, Stoechospermum marginatum induces apoptosis via ROS induced mitochondrial mediated caspase dependent pathway in murine B16F10 melanoma cells. Mol. Carcinog. 2016, 55, 2222–2235.
- Kelman, D.; Posner, E.K.; McDermid, K.J.; Tabandera, N.K.; Wright, P.R.; Wright, A.D. Antioxidant activity of Hawaiian marine algae. Mar. Drugs 2012, 10, 403–416.
- Kim, M.; Kim, D.; Yoon, H.; Lee, W.; Lee, N.; Hyun, C. Melanogenesis inhibitory activity of Korean Undaria pinnatifida in mouse B16 melanoma cells. Interdiscip. Toxicol. 2014, 7, 89–92.
- Khan, M.N.A.; Yoon, S.J.; Choi, J.S.; Park, N.G.; Lee, H.H.; Cho, J.Y.; Hong, Y.K. Anti-edema effects of brown seaweed (Undaria pinnatifida) extract on phorbol 12-myristate 13-acetate-induced mouse ear inflammation. Am. J. Chin. Med. 2009, 37, 373–381.
- Rhimou, B.; Hassane, R.; José, M.; Nathalie, B. The antibacterial potential of the seaweeds (Rhodophyceae) of the Strait of Gibraltar and the Mediterranean Coast of Morocco. Afr. J. Biotechnol. 2010, 9, 6365–6372.
- Wang, W.J.; Li, X.L.; Zhu, J.Y.; Liang, Z.R.; Liu, F.L.; Sun, X.T.; Wang, F.J.; Shen, Z.G. Antioxidant response to salinity stress in freshwater and marine Bangia (Bangiales, Rhodophyta). Aquat. Bot. 2019, 154, 35–41.
- Jaballi, I.; Sallem, I.; Feki, A.; Cherif, B.; Kallel, C.; Boudawara, O.; Jamoussi, K.; Mellouli, L.; Nasri, M.; Amara, I.B. Polysaccharide from a Tunisian red seaweed Chondrus canaliculatus: Structural characteristics, antioxidant activity and in vivo hemato-nephroprotective properties on maneb induced toxicity. Int. J. Biol. Macromol. 2019, 123, 1267–1277.
- Kulshreshtha, G.; Borza, T.; Rathgeber, B.; Stratton, G.S.; Thomas, N.A.; Critchley, A.; Hafting, J.; Prithiviraj, B. Red seaweeds Sarcodiotheca gaudichaudii and Chondrus crispus down regulate virulence factors of Salmonella enteritidis and induce immune responses in Caenorhabditis elegans. Front. Microbiol. 2016, 7, 421.
- Ryu, B.; Qian, Z.J.; Kim, M.M.; Nam, K.W.; Kim, S.K. Anti-photoaging activity and inhibition of matrix metalloproteinase (MMP) by marine red alga, Corallina pilulifera methanol extract. Radiat. Phys. Chem. 2009, 78, 98–105.
- Francavilla, M.; Franchi, M.; Monteleone, M.; Caroppo, C. The red seaweed Gracilaria gracilis as a multi products source. Mar. Drugs 2013, 11, 3754–3776.
- Wang, X.; Zhang, Z.; Wu, Y.; Sun, X.; Xu, N. Synthesized sulfated and acetylated derivatives of polysaccharide extracted from Gracilariopsis lemaneiformis and their potential antioxidant and immunological activity. Int. J. Boil. Macromol. 2019, 124, 568–572.
- Antony, T.; Chakraborty, K. First report of antioxidative 2H-chromenyl derivatives from the intertidal red seaweed Gracilaria salicornia as potential anti-inflammatory agents. Nat. Prod. Res. 2019.
- Moshfegh, A.; Salehzadeh, A.; Shandiz, S.A.S.; Shafaghi, M.; Naeemi, A.S.; Salehi, S. Phytochemical analysis, antioxidant, anticancer and antibacterial properties of the Caspian Sea red macroalgae, Laurencia caspica. Iran. J. Sci. Technol. Trans. A Sci. 2019, 43, 49–56.
- Lajili, S.; Ammar, H.H.; Mzoughi, Z.; Amor, H.B.H.; Muller, C.D.; Majdoub, H.; Bouraoui, A. Characterization of sulfated polysaccharide from Laurencia obtusa and its apoptotic, gastroprotective and antioxidant activities. Int. J. Biol. Macromol. 2019, 126, 326–336.
- De Jesus Raposo, M.; de Morais, A.; de Morais, R. Marine polysaccharides from algae with potential biomedical applications. Mar. Drugs 2015, 13, 2967–3028.
- Pimentel, F.B.; Alves, R.C.; Rodrigues, F.; PP Oliveira, M.B. Macroalgae-derived ingredients for cosmetic industry—An Update. Cosmetics 2017, 5, 2.
- Cian, R.E.; Bacchetta, C.; Rossi, A.; Cazenave, J.; Drago, S.R. Red seaweed Pyropia columbina as antioxidant supplement in feed for cultured juvenile Pacú (Piaractus mesopotamicus). J. Appl. Phycol. 2019, 31, 1455–1465.
- Kim, C.R.; Kim, Y.M.; Lee, M.K.; Kim, I.H.; Choi, Y.H.; Nam, T.J. Pyropia yezoensis peptide promotes collagen synthesis by activating the TGF-β/Smad signaling pathway in the human dermal fibroblast cell line Hs27. Int. J. Mol. Med. 2017, 39, 31–38.
- Li, K.; Li, X.M.; Gloer, J.B.; Wang, B.G. New nitrogen-containing bromophenols from the marine red alga Rhodomela confervoides and their radical scavenging activity. Food chem. 2012, 135, 868–872.
- Zhang, Z.; Wang, F.; Wang, X.; Liu, X.; Hou, Y.; Zhang, Q. Extraction of the polysaccharides from five algae and their potential antioxidant activity in vitro. Carbohydr. Polym. 2010, 82, 118–121.
- Premalatha, M.; Dhasarathan, P.; Theriappan, P. Phytochemical characterization and antimicrobial efficiency of seaweed samples, Ulva fasciata and Chaetomorpha antennina. Int. J. Pharm. Biol. Sci. 2011, 2, 288–293.
- Lee, C.; Park, G.H.; Ahn, E.M.; Kim, B.A.; Park, C.I.; Jang, J.H. Protective effect of Codium fragile against UVB-induced pro-inflammatory and oxidative damages in HaCaT cells and BALB/c mice. Fitoterapia 2013, 86, 54–63.
- Pezeshk, F.; Babaei, S.; Abedian Kenari, A.; Hedayati, M.; Naseri, M. The effect of supplementing diets with extracts derived from three different species of macroalgae on growth, thermal stress resistance, antioxidant enzyme activities and skin colour of electric yellow cichlid (Labidochromis caeruleus). Aquac. Nutr. 2019, 25, 436–443.
- Farasat, M.; Khavari-Nejad, R.A.; Nabavi, S.M.B.; Namjooyan, F. Antioxidant properties of two edible green seaweeds from northern coasts of the Persian Gulf. Jundishapur. J. Nat. Pharm. Prod. 2013, 8, 47.
- Fernandes, H.; Salgado, J.M.; Martins, N.; Peres, H.; Oliva-Teles, A.; Belo, I. Sequential bioprocessing of Ulva rigida to produce lignocellulolytic enzymes and to improve its nutritional value as aquaculture feed. Bioresour. Technol. 2019, 281, 277–285.
- Adrien, A.; Bonnet, A.; Dufour, D.; Baudouin, S.; Maugard, T.; Bridiau, N. Pilot production of ulvans from Ulva sp. and their effects on hyaluronan and collagen production in cultured dermal fibroblasts. Carbohydr. Polym. 2017, 157, 1306–1314.
- Mourelle, M.L.; Gómez, C.P.; Legido, J.L. The potential use of marine microalgae and cyanobacteria in cosmetics and thalassotherapy. Cosmetics 2017, 4, 46.
- José de Andrade, C.; Maria de Andrade, L. An overview on the application of genus Chlorella in biotechnological processes. J. Adv. Res. Biotechnol. 2017, 2, 1–9.
- Makpol, S.; Yeoh, T.W.; Ruslam, F.A.C.; Arifin, K.T.; Yusof, Y.A.M. Comparative effect of Piper betle, Chlorella vulgaris and tocotrienol-rich fraction on antioxidant enzymes activity in cellular ageing of human diploid fibroblasts. BMC Complement. Altern. Med. 2013, 13, 210.
- Kang, H.; Lee, C.H.; Kim, J.R.; Kwon, J.Y.; Seo, S.G.; Han, J.G.; Kim, B.; Kim, J.; Lee, K.W. Chlorella vulgaris attenuates dermatophagoides farinae-induced atopic dermatitis-like symptoms in NC/Nga mice. Int. J. Mol. Sci. 2015, 16, 21021–21034.
- Murthy, K.; Vanitha, A.; Rajesha, J.; Swamy, M.; Sowmya, P.; Ravishankar, G. In vivo antioxidant activity of carotenoids from Dunaliella salina—A green microalga. Life Sci. 2005, 76, 1381–1390.
- Yang, D.J.; Lin, J.T.; Chen, Y.C.; Liu, S.C.; Lu, F.J.; Chang, T.J.; Wang, M.; Lin, H.W.; Chang, Y.Y. Suppressive effect of carotenoid extract of Dunaliella salina alga on production of LPS-stimulated pro-inflammatory mediators in RAW264. 7 cells via NF-κB and JNK inactivation. J. Funct. Foods 2013, 5, 607–615.
- Shin, J.; Kim, J.E.; Pak, K.J.; Kang, J.I.; Kim, T.S.; Lee, S.Y.; Yeo, I.H.; Park, J.H.Y.; Kim, J.H.; Kang, N.J.; et al. A Combination of soybean and Haematococcus extract alleviates ultraviolet B-induced photoaging. Int. J. Mol. Sci. 2017, 18, 682.
- Rao, A.R.; Sindhuja, H.N.; Dharmesh, S.M.; Sankar, K.U.; Sarada, R.; Ravishankar, G.A. Effective inhibition of skin cancer, tyrosinase, and antioxidative properties by astaxanthin and astaxanthin esters from the green alga Haematococcus pluvialis. J. Agric. Food Chem. 2013, 61, 3842–3851.
- Banskota, A.H.; Sperker, S.; Stefanova, R.; McGinn, P.J.; O’Leary, S.J. Antioxidant properties and lipid composition of selected microalgae. J. Appl. Phycol. 2019, 31, 309–318.
- Shen, C.T.; Chen, P.Y.; Wu, J.J.; Lee, T.M.; Hsu, S.L.; Chang, C.M.J.; Young, C.C.; Shieh, C.J. Purification of algal anti-tyrosinase zeaxanthin from Nannochloropsis oculate using supercritical anti-solvent precipitation. J. Supercrit. Fluids 2011, 55, 955–962.
- Wu, H.L.; Fu, X.Y.; Cao, W.Q.; Xiang, W.Z.; Hou, Y.J.; Ma, J.K.; Wang, Y.; Fan, C.D. Induction of apoptosis in human glioma cells by fucoxanthin via triggering of ROS-mediated oxidative damage and regulation of MAPKs and PI3K-AKT pathways. J. Agric. Food Chem. 2019, 67, 2212.
- Rastogi, R.P.; Sonani, R.R.; Madamwar, D.; Incharoensakdi, A. Characterization and antioxidant functions of mycosporine-like amino acids in the cyanobacterium Nostoc sp. R76DM. Algal Res. 2016, 16, 110–118.
- Haimeur, A.; Ulmann, L.; Mimouni, V.; Guéno, F.; Pineau-Vincent, F.; Meskini, N.; Tremblin, G. The role of Odontella aurita, a marine diatom rich in EPA, as a dietary supplement in dyslipidemia, platelet function and oxidative stress in high-fat fed rats. Lipids Health Dis. 2012, 11, 147.
- Shannon, E.; Abu-Ghannam, N. Antibacterial derivatives of marine algae: An overview of pharmacological mechanisms and applications. Mar. Drugs 2016, 14, 81.
- Lauritano, C.; Andersen, J.H.; Hansen, E.; Albrigtsen, M.; Escalera, L.; Esposito, F.; Helland, K.; Hanssen, K.Ø.; Romano, G.; Ianora, A. Bioactivity screening of microalgae for antioxidant, anti-inflammatory, anticancer, anti-diabetes, and antibacterial activities. Front. Mar. Sci. 2016, 3, 68.
- Wu, Q.; Liu, L.; Miron, A.; Klímová, B.; Wan, D.; Kuča, K. The antioxidant, immunomodulatory, and anti-inflammatory activities of Spirulina: An overview. Arch. Toxicol. 2016, 90, 1817–1840.
- El-Sheekh, M.M.; Daboor, S.M.; Swelim, M.A.; Mohamed, S. Production and characterization of antimicrobial active substance from Spirulina platensis. Iran. J. Microbiol. 2014, 6, 112–119.




