It is still uncertain whether recipients of solid organ transplant (SOT) are at increased risk of SARS-CoV-2 infection and/or poor outcomes due to COVID-19 in comparison to the general population. In this study, we report the cumulative incidence and outcomes of SARS-CoV-2 infection in a cohort of 291 SOT recipients. The COVID-19 cumulative incidence in SOT recipients resulted slightly higher compared to that of age-matched population during the study period. Moreover, the SARS-CoV-2 antibody frequency was around 2.6-fold higher than the incidence of cases who tested positive for SARS-CoV-2 RT-PCR, suggesting that the number of SOT recipients infected with SARS-CoV-2 is likely higher than described. In symptomatic recipients, kidney transplant was associated with a higher risk of developing moderate/critical disease, while common risk factors, including age and comorbidities, resulted less relevant for COVID-19 severity. Due to the high estimated crude mortality, symptomatic SOT recipients should be considered at high risk in case of SARS-CoV-2 infection.
1. Introduction
On 21 February 2020 the first diagnosed case of COVID-19 was confirmed in Lombardy, a region of Northern Italy. On 8 March 2020, the entire Lombardy region went into lockdown with the rest of the country, and it quickly became a hotspot on the wold map of the COVID-19 pandemic. On 3 June 2020, free movement within the entire national territory was restored, de facto signaling the end of the SARS-CoV-2 associated disease first wave. Starting in July 2020, Italy witnessed a new progressive rise in COVID-19 cases, resulting in a second wave in November 2020 and a third wave in March 2021, as a result of the spreading of the Delta variant of SARS-CoV-2. Reports from the Italian National Institute of Health (Istituto Superiore di Sanità-ISS) document that most patients with COVID-19 show no or mild symptoms (52% asymptomatic, 17% pauci-symptomatic and 21% with mild symptoms). Ten percent of the patients suffer disease at the severe end of the spectrum, with 3% progressing to critical disease with an overall case fatality rate of approximately 3% (reaching >20% in individuals >80 year old)
[1][2]. A more severe SARS-CoV-2 infection has been documented in patients with older age and with coexisting premorbid conditions, such as hypertension, morbid obesity, chronic kidney disease and diabetes
[3]. Solid organ transplantation (SOT) recipients may be at increased risk for severe disease and mortality from COVID-19 disease due to immunosuppression and prolonged end-stage organ disease
[4]. While some studies have suggested higher morbidity in SOT recipients
[5][6][7][8][9][10][11][12][13][14][15], others did not confirm this evidence
[15][16][17][18][19][20]. We report here the incidence and outcomes of SARS-CoV-2 infection in a cohort of 291 patients with kidney, pancreas or islet transplant, all of whom received regular follow-ups at the IRCCS Ospedale San Raffaele between February 2020 and April 2021.
2. Current Researches and Results
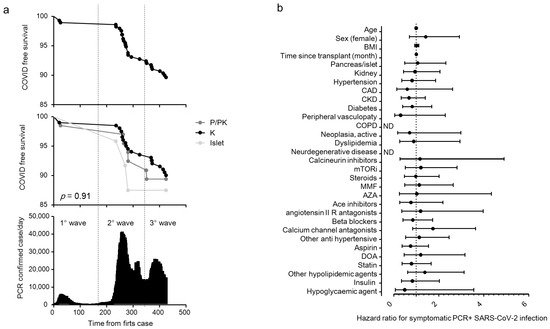
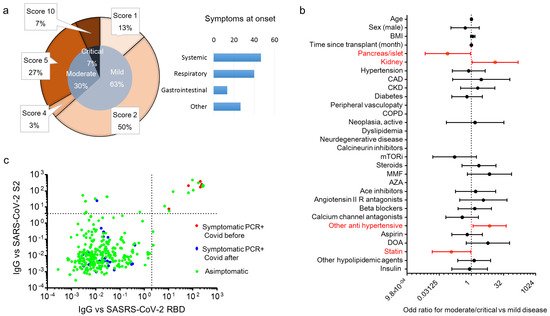
We enrolled 290 patients with a kidney (K, n = 201), a pancreas (pancreas alone, PA = 10; pancreas-kidney, PK = 56) or islet transplant alone (ITA, n = 24) from the cohort of SOT recipients attending a regular follow-up at the IRCCS Ospedale San Raffaele. The baseline characteristics of the entire cohort are summarized in Table 1. The overall median age of the cohort was 56 (47–65) years, and 179 were male (61.5%). Of the 291 SOT recipients, 30 (10.3%) tested positive for SARS-CoV-2 RT-PCR during the study period (21 February 2020 to 24 April 2021, Figure 1a) and their characteristics are reported in Table 2. Most of the cases of positivity occurred during the second and third pandemic waves, while during the first wave the SOT recipients were mainly spared. COVID-19 prevalence was not different among different transplants: 20 out 201 for kidney (10%), 7 out 66 (10.7%) for pancreas ± kidney and 3 out 24 (12.5%) for islet (Figure 1a). The region of residence of SOT recipients did not influence COVID-19 prevalence: 10.6% of patients were form Northern Italy, 10.3% from Southern Italy and 5.9% from Central Italy (p = 0.825). There was no statistically significant association between the positivity for SARS-CoV-2 and age, body mass index, time since transplantation, type of transplant, comorbidities and ongoing therapies (Figure 1b) or immunosuppression intensity (triple vs. double/single regimen: HR 1.17 (0.56–2.42). p = 0.68). All positive SOT recipients were classified for disease severity following the WHO severity classification at diagnosis: 19 (63.3%) were mild, 9 (30%) were moderate, and 2 were critical and died (Figure 2a) with a crude mortality rate of 6.7%. No patient lost organ function during the SARS-CoV-2 infection. The most common symptoms at presentation were systemic (53.3%: fever, fatigue/malaise, myalgia/arthralgia) and respiratory (40%: cough, dyspnea, sore throat, chest pain). Gastrointestinal symptoms were less prevalent (13.3%: diarrhea, vomiting/plasma, abdominal) as well as other symptoms (26.7%: headache, conjunctivitis, hypo/anosmia, hypo/dysgeusia, skin rash). Kidney transplant (OR 12.9 (1.1–150) p = 0.041) and anti-hypertensive therapy other than ACE system blockers, beta blockers and calcium channel antagonists (OR 7.07 (1.18–42.3) p = 0.032) were associated with an increased risk to develop moderate/critical disease. Statin therapy (OR 0.116 (0.015–0.926) p = 0.042) and pancreas/islet transplant (OR 0.077 (0.007–0.906) p = 0.041) were protective against moderate/critical disease (Figure 2b). Immunosuppression intensity was not significantly associated with an increased risk to develop moderate/critical disease (triple vs. double/single regimen: OR 2.25 (0.36–13.9); p = 0.38). To estimate the prevalence of asymptomatic disease in our cohort, we analyzed the antibody response of the IgG class to the SARS-CoV-2 Spike protein (both RBD and S2) in all 291 patients at the time of their first study visit (median time after the first case in Italy: 242 days, IQR 201–292). Specific antibody response (positivity for both RBD and S2) was present in 16 out 291 patients (5.5%). Of these, 6 participants were symptomatic and tested positive for SARS-CoV-2 RT-PCR before the study sampling (123 days (IQR 39–220) before), while no history of symptoms and/or positivity for SARS-CoV-2 RT-PCR were reported in the remaining 10 participants (62.5%) (Figure 2c). As expected, all 24 participants who developed symptomatic disease and tested positive for SARS-CoV-2 RT-PCR after the study sampling (96 days (IQR 42–166) after) were negative for the antibody response against the virus.
Figure 1. COVID-19 RT-PCR positive subjects in solid organ transplantation (SOT) recipient. Kaplan–Meier COVID-free survival estimates for SOT recipients are in (panel a). Survival rate was estimated for all 291 SOT recipients (top panel) or according to the transplant type (kidney (K, n = 201), pancreas (pancreas alone, PA = 10; pancreas-kidney, PK = 56), islet transplant alone (ITA: 24)) (middle panel) in relationship with the development of the COVID-19 pandemic in Italy (bottom panel). The log-rank test was used to test differences in the estimated survival rates among transplant types. The forest plot (panel b) shows the hazard ratios (HR) for positive RT-PCR for each factor tested. The univariate Cox regression analysis was adjusted for sex and age. Dots represent the HR, lines represent 95% confidence interval (CI).
Figure 2. Clinical characteristic and SARS-CoV-2 antibody prevalence in COVID-19 RT-PCR positive subjects in solid organ transplantation (SOT) recipient. WHO severity classification
[21] of COVID-19 RT-PCR positive subjects and prevalence of symptoms at diagnosis are in (panel
a). Of the 291 SOT recipients, 30 (10.3%) tested positive for SARS-CoV-2 RT-PCR during the study period (21 February 2020–24 April 2021). Presenting symptoms were classified as systemic (fever, fatigue/malaise, myalgia/arthralgia), respiratory (cough, dyspnea, sore throat, chest pain), gastrointestinal (diarrhea, vomiting/plasma, abdominal) and others (headache, conjunctivitis, hypo/anosmia, hypo/dysgeusia, skin rash). The forest plot (panel
b) shows the odd ratios (OR) for moderate/critical disease for each factor tested. The univariate logistic regression analysis was adjusted for sex and age. Dots represent the HR, lines represent 95% confidence interval (CI), and red dots indicate
p < 0.05. The dot plot (panel
c) shows the value of IgG antibodies to the virus receptor binding domain (RBD) and the S2 domain of the spike protein. Patients were classified as asymptomatic (green), and symptomatic with a positive SARS-CoV-2 RT-PCR before the antibody test (red) and after the antibody test (blue). Dotted lines indicate the cut-off of antibody test positivity.
Table 1. Baseline characteristics of the entire transplant cohort, and of patients with different type of transplant.
| Items |
All |
Islet |
Pancreas ± Kidney |
Kidney |
p |
| N |
291 |
24 |
66 |
201 |
|
| Age in years, median (IQR) |
56 (47–65) |
51 (36–60) |
54 (47–59) |
57 (49–66) |
0.001 |
| Sex M/F |
179/112 |
11/13 |
41/25 |
127/74 |
0.264 |
| Race Caucasian (N (%)) |
284 (97.6) |
24 (100) |
64 (97) |
196 (97.5) |
0.631 |
| Body mass index (kg/m2) |
24.2 (21.8–26.6) |
22.2 (17.8–23.5) |
23.2 (20–26.7) |
25 (22.5–27) |
<0.001 |
| Months since transplant, median (IQR) |
53.4 (17–121) |
79 (34–131) |
75 (24–160) |
48 (12–106) |
0.005 |
| Comorbidities (N (%)) |
|
|
|
|
|
| Hypertension |
227 (78) |
11 (45.8) |
42 (63.6) |
174 (86.6) |
<0.001 |
| Coronary artery disease |
35 (12) |
2 (8.3) |
11 (16.7) |
22 (10.9) |
0.392 |
| Chronic kidney disease |
156 (53.6) |
2 (8.3) |
29 (43.9) |
125 (62.2) |
<0.001 |
| Diabetes |
140 (48.1) |
24 (100) |
66 (100) |
50 (24.9) |
<0.001 |
| Peripheral vasculopathy |
30 (10.3) |
1 (4.2) |
7 (10.6) |
22 (10.9) |
0.585 |
| Chronic obstructive pulmonary disease |
1 (0.3) |
0 (0) |
0 (0) |
1 (0.5) |
0.799 |
| Neoplasia active |
27 (9.3) |
1 (4.2) |
3 (4.5) |
23 (11.4) |
0.164 |
| Dyslipidemia |
35 (12) |
2 (8.3) |
5 (7.6) |
28 (13.9) |
0.327 |
| Neuro degenerative disease |
2 (0.7) |
1 (4.2) |
1 (1.5) |
0 (0) |
0.043 |
| Baseline therapy |
|
|
|
|
|
| Calcineurin inhibitor (CNI) |
270 (92.8) |
21 (87.5) |
64 (97) |
185 (92) |
0.235 |
| Mammalian target of rapamycin inhibitors (mTORi) |
58 (19.9) |
9 (37.5) |
3 (4.5) |
46 (22.9) |
<0.001 |
| Steroids |
133 (45.7) |
1 (4.2) |
31 (47) |
101 (50.2) |
<0.001 |
| Mycophenolate mofetil |
218 (74.9) |
11 (45.8) |
59 (89.4) |
148 (73.6) |
<0.001 |
| Azathioprine |
18 (6.2) |
6 (25) |
4 (6.1) |
8 (4) |
<0.001 |
| “Intensity” of immunosuppression |
|
|
|
|
|
| - Triple regimen |
122 (41.9) |
1 (4.2) |
30 (45.5) |
91 (45.3) |
|
| ○ CNI+antimetabolite+steroid |
102 (83.6) |
0 (0) |
28 (93.3) |
74 (81.3) |
|
| ○ CNI+mTORi+steroid |
14 (11.5) |
0 (0) |
1 (3.3) |
13 (14.3) |
|
| ○ mTORi+antimetabolite+steroid |
5 (4.1) |
0 (0) |
1 (3.3) |
4 (4.4) |
|
| ○ mTORi+CNI+antimetabolite |
1 (0.8) |
1 (100) |
0 (0) |
0 (0) |
|
| - Double regimen |
162 (55.7) |
22 (91.7) |
36 (54.5) |
104 (51.7) |
|
| ○ CNI+antimetabolite |
118 (72.8) |
13 (59.1) |
34 (94.4) |
71 (68.3) |
|
| ○ CNI+mTORi |
28 (17.3) |
6 (27.3) |
0 (0) |
22 (21.2) |
|
| ○ CNI+steroid |
5 (3.1) |
1 (4.5) |
1 (2.8) |
3 (2.9) |
|
| ○ mTORi+steroid |
5 (3.1) |
0 (0) |
0 (0) |
5 (4.8) |
|
| ○ mTORi+antimetabolite |
4 (2.5) |
2 (9.1) |
1 (2.8) |
1 (1) |
|
| ○ antimetabolite+steroid |
2 (1.2) |
0 (0) |
0 (0) |
2 (1.9) |
|
| - Single regimen |
7 (2.4) |
1 (4.2) |
0 (0) |
6 (3) |
|
| ○ Antimetabolite |
4 (57.1) |
1 (100) |
0 (0) |
3 (50) |
|
| ○ CNI |
2 (28.6) |
0 (0) |
0 (0) |
2 (33.3) |
|
| ○ mTORi |
1 (14.3) |
0 (0) |
0 (0) |
1 (16.7) |
|
| Ace inhibitors |
51 (17.5) |
6 (25) |
7 (10.6) |
38 (18.9) |
0.185 |
| Angiotensin II receptor type 1 antagonists |
26 (8.9) |
1 (4.2) |
5 (7.6) |
20 (10) |
0.584 |
| Beta blockers |
157 (54) |
6 (25) |
33 (50) |
118 (58.7) |
0.006 |
| Calcium channel antagonists |
125 (43) |
3 (12.5) |
26 (39.4) |
96 (47.8) |
0.003 |
| Other anti-hypertensive |
106 (36.4) |
2 (8.3) |
20 (30.3) |
84 (41.8) |
0.003 |
| Aspirin |
185 (63.6) |
6 (25) |
45 (68.2) |
134 (66.7) |
<0.001 |
| Direct oral anticoagulant |
44 (15.1) |
4 (16.7) |
12 (18.2) |
28 (13.9) |
0.688 |
| Statin |
134 (46) |
7 (29.2) |
27 (40.9) |
100 (49.8) |
0.102 |
| Other hypolipidemic agents |
61 (21) |
2 (8.3) |
8 (12.1) |
51 (25.4) |
0.02 |
| Insulin |
69 (23.7) |
18 (75) |
19 (28.8) |
32 (15.9) |
<0.001 |
| Hypoglycemic agent |
19 (6.5) |
0 (0) |
5 (7.6) |
14 (7) |
0.395 |
Table 2. Characteristics of SOT recipients that tested positive for SARS-CoV-2 RT-PCR during the study period.
| Items |
SARS-CoV-2 RT-PCR Negative |
SARS-Cov-2 RT-PCR Positive |
p |
| N |
261 |
30 |
|
| Age in years, median (IQR) |
56 (47–65) |
52 (48–61) |
0.341 |
| Sex M/F |
163/98 |
16/14 |
0.331 |
| Race Caucasian (N (%)) |
256 (98.1) |
28 (93.3) |
0.156 |
| Body mass index (kg/m2) |
24.2 (21.8–26.6) |
24 (21.9–26.9) |
0.817 |
| Type of transplant |
|
|
|
| - Kidney |
181 (69.3) |
20 (66.7) |
0.924 |
| - Pancreas ± kidney |
59 (22.6) |
7 (23.3) |
| - Islets |
21 (8) |
3 (10) |
| Comorbidities (N (%)) |
|
|
|
| - Hypertension |
205 (78.5) |
22 (73.3) |
0.492 |
| - Coronary artery disease |
33 (12.6) |
2 (6.7) |
0.552 |
| - Chronic kidney disease |
143 (54.8) |
13 (43.3) |
0.251 |
| - Diabetes |
127 (48.7) |
13 (43.3) |
0.7 |
| - Peripheral vasculopathy |
29 (11.1) |
1 (3.3) |
0.337 |
| - Chronic obstructive pulmonary disease |
1 (0.4) |
0 (0) |
1 |
| - Neoplasia active |
25 (9.6) |
2 (6.7) |
1 |
| - Dyslipidemia |
32 (12.3) |
3 (10) |
1 |
| - Neuro degenerative disease |
2 (0.8) |
0 (0) |
1 |
| Baseline therapy |
|
|
|
| - Calcineurin inhibitor (CNI) |
242 (92.7) |
28 (93.3) |
1 |
| - Mammalian target of rapamycin inhibitors (mTORi) |
51 (19.5) |
7 (23.3) |
0.631 |
| - Steroids |
119 (45.6) |
14 (46.7) |
1 |
| - Antimetabolites |
211 (80.8) |
25 (83.3) |
1 |
| “Intensity” of immunosuppression |
|
|
|
| - Triple regimen |
108 (41.4) |
14 (46.7) |
0.421 |
| - Double regimen |
146 (55.9) |
16 (50.2) |
| - Single regimen |
7 (2.7) |
0 (0) |