| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Stephen Fey | + 1580 word(s) | 1580 | 2022-01-04 10:34:41 | | | |
| 2 | Rita Xu | Meta information modification | 1580 | 2022-01-17 02:46:38 | | | | |
| 3 | Rita Xu | + 411 word(s) | 1991 | 2022-01-24 04:58:38 | | | | |
| 4 | Rita Xu | Meta information modification | 1991 | 2022-01-24 04:59:25 | | |
Video Upload Options
Thioxanthenes are one of the three major groups of antipsychotics (the others being phenothiazines and butyrophenones).
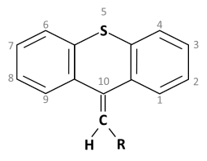
Thioxanthene is a chemical compound having a central triple ring structure closely related to one of the other group of antipsychotics (phenothiazines, which also has the triple ring). The major structural difference between the two classes is that the carbon in position 10 in thioxanthenes is replaced by a nitrogen atom in phenothiazines. In thioxanthenes, this C10 shares a double bond with the side chain.
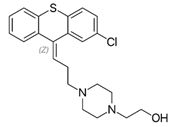
Thioxanthenes are represented in two geometric stereoisomers: Z- and E-compounds.
1. Thioxanthene Definition
1.1. Characteristic Structure
Thioxanthenes are one of the three major groups of antipsychotics (the others being phenothiazines and butyrophenones) [1].
Thioxanthene is a chemical compound having a central triple ring structure closely related to one of the other group of antipsychotics (phenothiazines, which also has the triple ring). The major structural difference between the two classes is that the carbon in position 10 in thioxanthenes is replaced by a nitrogen atom in phenothiazines. In thioxanthenes, this C10 shares a double bond with the side chain [2].
Thioxanthenes are represented in two geometric stereoisomers: Z- and E-compounds [3][4].
Basic ring structure:
1.2. Therapeutic Use
Thioxanthenes are prescribed for patients suffering from schizophrenia and other psychoses. They were synthesized and developed with the hope of eliminating the toxic effects of chlorpromazine, the first antipsychotic drug that was primarily used as an anesthetic agent in surgery [2].
Thioxanthenes block the action of D2 neuroreceptors in the dopamine pathway in the brain, resulting in a reduction in the release of dopamine and a large number of other hypothalamic and hypophyseal hormones [3].
1.3. Major Derivatives of Thioxanthenes
Several derivatives of thioxanthenes are used as typical antipsychotics in the treatment of schizophrenia and other psychoses. The first thioxanthene, chlorprothixene, was found to have an excellent effect in schizophrenic patients [5]. Some of the major used derivatives and their use are listed below.
Chlorprothixene: Treatment of neuroses with anxiety, tension, insomnia, psychosomatic disorders and depression due to sedative and calming effect [5][6].
Clopenthixol: Treatment of delusion, aggressiveness, destructiveness, impulsiveness, hallucination and paranoid schizophrenia [7][8].
Flupenthixol: Effective against hallucinations and delusions [2][9][10][11][12].
Thiothixene: Used for treatment of psychoses like schizophrenia and bipolar mania.
Zuclopenthixol: Used for schizophrenia, bipolar disorders, aggression and other psychotic disorders.
1.4. Other Potential Uses
Numerous in vitro and in vivo studies have shown the varying effects of widely used psychotropics on microorganisms [13][14]. Beside the therapeutic use of thioxanthenes as antipsychotics, they have also been shown to possess not only antibacterial effects but also to possess anti-mycobacterial, antiviral (anti-HIV and anti-SARS-CoV-2) as well as anti-parasitic properties [15][16][17][18]. The antimicrobial effect has been reported to not only be exerted by the drug itself but also in a symbiotic combination with antibiotics resulting in even stronger effects using lower doses of the drugs. In this context, the compounds are collectively known as ´non-antibiotics´ [14].
2. Introduction
| Class of Antipsychotics | Drugs and Their Chemical Structures |
|---|---|
1. Phenothiazines Phenothiazine basic ring structure |
|
| a. Amino alkyl compounds: (Low/medium potency agents that can antagonize α1-adenoreceptors, histamine H1 receptors and muscarinic cholinergic receptors) |
Chlorpromazine: |
| b. Piperidine compounds: (Low/medium potency agents and also muscarinic antagonist) |
Thioridazine: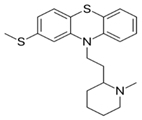 |
| c. Piperazine compounds: (Medium/high potency agents) |
Trifluoperazine: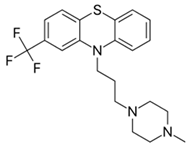 |
2. Butyrophenones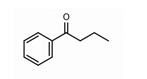 Butyrophenone basic ring structure (High potency agents) |
Haloperidol: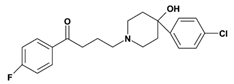 |
Droperidol: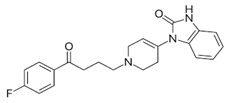 |
|
3. Thioxanthenes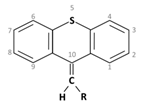 Thioxanthene basic ring structure (Medium potency agents) |
Chlorprothixene: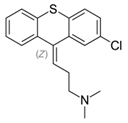 |
Flupenthixol: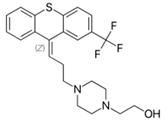 |
|
|
Clopenthixol:
|
4. Therapeutic Usage of Thioxanthenes
| Adverse Effects |
Drugs | ||||||
|---|---|---|---|---|---|---|---|
| (1) Phenothiazines | (2) Butyro- Phenones |
(3) Thioxanthenes | |||||
| Chlorpromazine | Thioridazine | Trifluoperazine | Haloperidol | Chlorprothixene | Flupenthixol | Clopenthixol | |
| Extra Pyramidal Side Effects: The muscle related side effects observed with antipsychotic medications are termed as ‘Extra -Pyramidal Side Effects’ or EPS [28] |
Low | Low | High | Very high | In a comparative study it was observed that Parkinsonian symptoms were more often found with chlorpromazine than chlorprothixene [29] |
Develops in high dosages, can be controlled by anti-parkinsonian drugs [30][31] |
High |
| Anti-cholinergic Effects: This includes symptoms like urinary difficulties, constipation, dry mouth, blurred visions and may lead to cognitive impairments. |
High | High | Low | Very low | Moderate | Low | Both clopenthixol and flupenthixol were found to have lower effect in comparison to chlorprothixene [32] |
| Sedation: This is common with antipsychotic medications and is dose dependent. |
High | High | Low | Produces much lesser sleepiness and calming effect than chlorpromazine [33] |
High | Low | Low |
| Hypotension: Antipsychotics commonly cause orthostatic hypotension, depending on the degree of α1 adrenoreceptor antagonism. |
High | High | Low | Very low | High | Moderate | Treatment with clopenthixol is often associated with orthostatic hypotension [37] |
| Other Effects: | Photosensitivity: Chlorpromazine is known to induce photosensitivity and skin pigmentation [34]. An intensive study with phenothiazines and thioxanthenes on schizophrenic patients [35] reported that patients receiving chlorpromazine showed statistically significant changes in the lens and cornea while patients treated with thioxanthenes did not. |
Hyperprolactinemia: Thioxanthenes cause high prolactin levels due to the blockade of prolactin inhibitory factors (PIF), that inhibits release of prolactin from the pituitary gland [36]. |
|||||
3. Pharmacological Properties of Thioxanthenes
References
- Meltzer, H. Y. Update on Typical and Atypical Antipsychotic Drugs. Annu. Rev. Med. 2013, 64, 393–406. https://doi.org/10.1146/annurev-med-050911-161504.
- Nasrallah, H.; Tandon, R. Classic Antipsychotic Medications. In Essentials of Clinical Psychopharmacology; Schatzberg, A. F., Nemeroff, C. B., Eds.; American Psychiatric Publishing Inc.: Arlington, 2013; pp 219–236.
- Behere, P. B.; Das, A.; Behere, A. P. Antipsychotics. In Clinical Psychopharmacology; Springer Singapore: Singapore, 2019; pp 39–87. https://doi.org/10.1007/978-981-13-2092-7_2.
- Baumann, P.; Kirchherr, H.; Berney, P.; Hiemke, C. Flupentixol: Relevance of Stereoselective Therapeutic Drug Monitoring. Psychopharmacology (Berl). 2012, 221 (4), 719–720. https://doi.org/10.1007/s00213-012-2699-8.
- Li, P.; L. Snyder, G.; E. Vanover, K. Dopamine Targeting Drugs for the Treatment of Schizophrenia: Past, Present and Future. Curr. Top. Med. Chem. 2016, 16 (29), 3385–3403. https://doi.org/10.2174/1568026616666160608084834.
- Novir, S. B. A Theoretical Study of the Structural and Electronic Properties of Trans and Cis Structures of Chlorprothixene as a Nano-Drug. Curr. Appl. Phys. 2017, 17 (12), 1754–1764. https://doi.org/10.1016/j.cap.2017.08.020.
- Leucht, S.; Cipriani, A.; Spineli, L.; Mavridis, D.; Örey, D.; Richter, F.; Samara, M.; Barbui, C.; Engel, R. R.; Geddes, J. R.; Kissling, W.; Stapf, M. P.; Lässig, B.; Salanti, G.; Davis, J. M. Comparative Efficacy and Tolerability of 15 Antipsychotic Drugs in Schizophrenia: A Multiple-Treatments Meta-Analysis. Lancet 2013, 382 (9896), 951–962. https://doi.org/10.1016/S0140-6736(13)60733-3.
- Ravn, J.; Rud, C.; Wendelboe, J. 252 Mit Dem Neuen Psykofarmakon Clopenthixol (Sordinol, Ciatyl) Behandelte Psychiatrische Patienten. In Neuro-psychopharmacology; Bradley, P. B., Flugel, F., Hoch, P. ., Eds.; Elsevier Publishing Co., 1964; pp 285–289.
- Bostwick, J. R.; Guthrie, S. K.; Ellingrod, V. L. Antipsychotic-Induced Hyperprolactinemia. Pharmacotherapy 2009, 29 (1), 64–73. https://doi.org/10.1592/phco.29.1.64.
- Tardy, M.; Dold, M.; Engel, R. R.; Leucht, S. Flupenthixol versus Low-Potency First-Generation Antipsychotic Drugs for Schizophrenia. Cochrane Database Syst. Rev. 2014, 2014 (9), 1–26. https://doi.org/10.1002/14651858.CD009227.pub2.
- Van Coller, P. E. Flupenthixol (Fluanxol) in the Treatment of Psychosomatic Disorders in Medicine. Psychosomatics 1971, 12 (4), 256–259. https://doi.org/10.1016/S0033-3182(71)71516-3.
- Trueman, H. R.; Valentine, M. G. Flupenthixol Decanoate in Schizophrenia. Br. J. Psychiatry 1974, 124 (578), 58–59.
- Geiger, H.; Finkeistein, B. A. [Largactil in the Treatment of Tuberculosis]. Schweiz. Med. Wochenschr. 1954, 84 (37), 1063–1064.
- Kristiansen, J. E. The Antimicrobial Activity of Non-Antibiotics. Report from a Congress on the Antimicrobial Effect of Drugs Other than Antibiotics on Bacteria, Viruses, Protozoa, and Other Organisms. Acta Pathol. Microbiol. Immunol. Scand. Suppl. 1992, 100 (30), 7–14.
- Mortensen, I.; Kristiansen, J. E.; Christensen, A. V; Hvidberg, E. F. The Antibacterial Effect of Some Neuroleptics on Strains Isolated from Patients with Meningitis. Pharmacol. Toxicol. 1992, 71 (6), 449–451.
- Jeyaseeli, L.; Gupta, A. Das; Asok Kumar, K.; Mazumdar, K.; Dutta, N. K.; Dastidar, S. G. Antimicrobial Potentiality of the Thioxanthene Flupenthixol through Extensive in Vitro and in Vivo Experiments. Int. J. Antimicrob. Agents 2006, 27 (1), 58–62. https://doi.org/10.1016/j.ijantimicag.2005.08.014.
- Kristiansen, J. E.; Andersen, L. P.; Vestergaard, B. F.; Hvidberg, E. F. Effect of Selected Neuroleptic Agents and Stereo‐Isomeric Analogues on Virus and Eukaryotic Cells. Pharmacol. Toxicol. 1991, 69 (5), 399–403. https://doi.org/10.1111/j.1600-0773.1991.tb01260.x.
- Kristiansen, J. E.; Jepsen, S. The Susceptibility of Plasmodium Falciparum in Vitro to Chlorpromazine and the Stereo-Isomeric Compounds Cis(Z)- and Trans(E)-Clopenthixol. Acta Pathol. Microbiol. Immunol. Scand. B. 1985, 93 (3), 249–251.
- Ahmed, U.; Jones, H.; Adams, C.E. Chlorpromazine for psychosis induced aggression or agitation. Cochrane Database Syst. Rev. 2010, 4.
- Baumeister, A.A. The Chlorpromazine Enigma. J. Hist. Neurosci. 2013, 22, 14–29.
- Tomida, K.; Takahashi, N.; Saito, S.; Maeno, N.; Iwamoto, K.; Yoshida, K.; Kimura, H.; Iidaka, T.; Ozaki, N. Relationship of psychopathological symptoms and cognitive function to subjective quality of life in patients with chronic schizophrenia. Psychiatry Clin. Neurosci. 2010, 64, 62–69.
- Renard, J.; Norris, C.; Rushlow, W.; Laviolette, S.R. Neuronal and molecular effects of cannabidiol on the mesolimbic dopamine system: Implications for novel schizophrenia treatments. Neurosci. Biobehav. Rev. 2017, 75, 157–165.
- Newman, W.J.; Newman, B.M. Rediscovering clozapine: After a turbulent history, current guidance on initiating and monitoring. Curr. Psychiatr. 2016, 15, 42–49.
- Meltzer, H.Y. Update on typical and atypical antipsychotic drugs. Annu. Rev. Med. 2013, 64, 393–406.
- Baumann, P.; Kirchherr, H.; Berney, P.; Hiemke, C. Flupentixol: Relevance of stereoselective therapeutic drug monitoring. Psychopharmacology 2012, 221, 719–720.
- Behere, P.B.; Das, A.; Behere, A.P. Antipsychotics. In Clinical Psychopharmacology; Springer: Singapore, 2019; pp. 39–87.
- Nasrallah, H.; Tandon, R. Classic Antipsychotic Medications. In Essentials of Clinical Psychopharmacology; Schatzberg, A.F., Nemeroff, C.B., Eds.; American Psychiatric Publishing Inc.: Arlington, TX, USA, 2013; pp. 219–236.
- Kendall, T. The rise and fall of the atypical antipsychotics. Br. J. Psychiatry 2011, 199, 266–268.
- Remvig, J.; Sonne, L.M. Chlorprothixene (“Truxal”) compared to chlorpromazine. Psychopharmacologia 1961, 2, 203–208.
- Madhusoodanan, S.; Alexeenko, L.; Sanders, R.; Brenner, R. Extrapyramidal symptoms associated with antidepressants—A review of the literature and an analysis of spontaneous reports. Ann. Clin. Psychiatry 2010, 22, 148–156.
- Correll, C.U. Mechanism of Action of Antipsychotic Medications. J. Clin. Psychiatry 2014, 75, e23.
- Taylor, D. Psychopharmacology and adverse effects of antipsychotic long-acting injections: A review. Br. J. Psychiatry 2009, 195, s13–s19.
- Suzuki, H.; Gen, K.; Inoue, Y. Comparison of the anti-dopamine D2 and anti-serotonin 5-HT 2A activities of chlorpromazine, bromperidol, haloperidol and second-generation antipsychotics parent compounds and metabolites thereof. J. Psychopharmacol. 2013, 27, 396–400.
- Dhanasekaran, S.; Kar, S.; Yadav, S. Chlorpromazine-induced severe exfoliative photoallergic reaction. Int. J. Nutr. Pharm. Neurol. Dis. 2015, 5, 34–36.
- Edler, K.; Gottfries, C.G.; Haslund, J.; Ravn, J. Eye changes in connection with neuroleptic treatment especially concerning phenothiazines and thioxanthenes. Acta Psychiatr. Scand. 1971, 47, 377–384.
- Ayano, G. First Generation Antipsychotics: Pharmacokinetics, Pharmacodynamics, Therapeutic Effects and Side effects: A review. RRJChem 2016, 4, 90–94.
- Lambert, T.; Taylor, D. Pharmacology of antipsychotic long-acting injections. In Antipsychotic Long-Acting Injections; Haddad, P., Lambert, T., Lauriello, J., Eds.; Oxford University Press Inc.: New York, NY, USA, 2011; pp. 23–47. ISBN 978-0-19-958604-2.
- Li, P.L.; Snyder, G.E.; Vanover, K. Dopamine Targeting Drugs for the Treatment of Schizophrenia: Past, Present and Future. Curr. Top. Med. Chem. 2016, 16, 3385–3403.
- Novir, S.B. A theoretical study of the structural and electronic properties of trans and cis structures of chlorprothixene as a nano-drug. Curr. Appl. Phys. 2017, 17, 1754–1764.
- de Wit, H. Flupenthixol. In Encyclopedia of Psychopharmacology; Stolerman, I.P., Ed.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 538–539.
- Bostwick, J.R.; Guthrie, S.K.; Ellingrod, V.L. Antipsychotic-induced hyperprolactinemia. Pharmacotherapy 2009, 29, 64–73.
- Tardy, M.; Dold, M.; Engel, R.R.; Leucht, S. Flupenthixol versus low-potency first-generation antipsychotic drugs for schizophrenia. Cochrane Database Syst. Rev. 2014, 2014, 1–26.
- Van Coller, P.E. Flupenthixol (fluanxol) in the treatment of psychosomatic disorders in medicine. Psychosomatics 1971, 12, 256–259.
- Trueman, H.R.; Valentine, M.G. Flupenthixol decanoate in schizophrenia. Br. J. Psychiatry 1974, 124, 58–59.
- Leucht, S.; Cipriani, A.; Spineli, L.; Mavridis, D.; Örey, D.; Richter, F.; Samara, M.; Barbui, C.; Engel, R.R.; Geddes, J.R.; et al. Comparative efficacy and tolerability of 15 antipsychotic drugs in schizophrenia: A multiple-treatments meta-analysis. Lancet 2013, 382, 951–962.
- Ravn, J.; Rud, C.; Wendelboe, J. 252 mit dem neuen Psykofarmakon Clopenthixol (Sordinol, Ciatyl) behandelte psychiatrische Patienten. In Neuro-Psychopharmacology; Bradley, P.B., Flugel, F., Hoch, P., Eds.; Elsevier Publishing Co.: Amsterdam, The Netherlands, 1964; pp. 285–289.
- Gordon, M. (Ed.) Psychopharmacological Agents; Elsevier: Amsterdam, The Netherlands, 2017; ISBN 1483274462/9781483274461.
- Schatzberg, A.F.; DeBattista, C. Manual of Clinical Psychopharmacology; American Psychiatric Publishing: Washington, DC, USA, 2015; ISBN 1-58562-481-0.
- Iversen, L.L.; Miller, R.J.; Horn, A.S. Effects of dopaminergic agonist and antagonist drugs on cyclic 3′,5′ –adenosine Monophosphate (cyclic AMP) production in rat brain homogenates. J. Pharmacol. 1974, 5, 117–118.
- Moller Nielsen, I.; Fjalland, B.; Pedersen, V.; Nymark, M. Pharmacology of neuroleptics upon repeated administration. Psychopharmacologia 1974, 34, 95–104.
- Casey, D.; Christensen, A. (Eds.) Psychopharmacology: Current Trends: Current Trends; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012; ISBN 3642732801/9783642732805.