Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Adam T Craig | + 1832 word(s) | 1832 | 2022-01-12 05:02:15 | | | |
| 2 | Amina Yu | + 1 word(s) | 1833 | 2022-01-17 02:49:16 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Craig, A. Arboviral Disease Outbreaks in the Pacific Islands Countries. Encyclopedia. Available online: https://encyclopedia.pub/entry/18303 (accessed on 07 February 2026).
Craig A. Arboviral Disease Outbreaks in the Pacific Islands Countries. Encyclopedia. Available at: https://encyclopedia.pub/entry/18303. Accessed February 07, 2026.
Craig, Adam. "Arboviral Disease Outbreaks in the Pacific Islands Countries" Encyclopedia, https://encyclopedia.pub/entry/18303 (accessed February 07, 2026).
Craig, A. (2022, January 17). Arboviral Disease Outbreaks in the Pacific Islands Countries. In Encyclopedia. https://encyclopedia.pub/entry/18303
Craig, Adam. "Arboviral Disease Outbreaks in the Pacific Islands Countries." Encyclopedia. Web. 17 January, 2022.
Copy Citation
Arthropod-borne viral (arboviral) diseases are a significant global health problem accounting for >17% of all infectious disease cases and 1 million deaths worldwide annually. Arboviral diseases are infections caused by over 100 viruses belonging to the Flaviviridae, Togaviridae, Reoviridae, Bunyaviridae, Rhabdoviridae and Orthomyxoviridae families. These viruses are spread to humans through arthropods, including mosquitos, ticks, and flies.
arboviral diseases
public health
surveillance
Pacific islands
dengue
Zika
chikungunya
outbreaks
low- and middle-income countries
1. Dengue Outbreaks
Dengue virus is a single-stranded positive-sense RNA virus of the family Flaviviridae, genus Flavivirus, primarily transmitted by Aedes mosquitoes. Four DENV serotypes have been identified; all can cause the full spectrum of disease [1]. WHO estimates there are 50–100 million cases and over 20,000 deaths due to dengue infection annually [2].
Dengue outbreaks of varying duration and serotype have been reported in the PICs since the late 1800s [3][4]. Historically, dengue outbreaks in PICs have had a cyclic pattern with one serotype dominating, being replaced every 3–5 years [4]; however, in 2014 Dupont-Rouzeyrol et al. reported this pattern had changed with co-circulation of serotypes becoming common [5]. Roth et al. (2014) identified 18 outbreaks of different serotypes occurring over the 2012–2014 period including, for the first time on record, all four DENV serotypes circulating at once [6][7]. Exposure to one serotype of dengue does not result in immunity to another serotype, leaving populations vulnerable to reinfection [4]. There is further evidence that those who have been infected with multiple serotypes are at increased risk of severe dengue infection [8][9].
During the study period, dengue was the most commonly reported arboviral disease; almost all (19 out of 22) PICs reported at least one dengue outbreak event. In total, our review identified 72 DENV outbreaks—15 DENV-1, 20 DENV-2, 14 DENV-3, four DENV-4, and an additional 19 outbreaks with undetermined serotype—suggesting the pattern identified by Dupont-Rouzeyrol et al. of co-circulation has continued (Figure 1).

Figure 1. Timeline of reported dengue outbreaks (confirmed/suspected and unspecified cases) in Pacific island countries and areas, October 2014–June 2020.
All four dengue serotypes were co-circulating across the Pacific Island region in 2016, 2017, and 2018; and DENV-1, DENV-2, DENV-3 and DENV-unspecified were co-circulating in 2014, 2015, 2019 and 2020 (Figure 2). In the study period, six PICs recorded co-circulation of two or more DENV serotypes (Cook Islands [10], Fiji [10][11], French Polynesia [10][12], New Caledonia [10][13][14], PNG [10][11][14][15][16][17], and Solomon Islands [10][11][14][16]; and two confirmed co-circulations of all four DENV serotypes (PNG 2016 [10][11][14][15][16][17], New Caledonia 2018 [10][11]).
Dengue outbreaks ranged from less than 10 to more than 12,250 cases (due to limited testing capacity, suspected case numbers were often reported). New Caledonia was the PIC that reported the most outbreaks (n = 13) (Figure 1) [10][11][13][14].
Of the 72 dengue outbreaks reported, case numbers were available for 55. At least 1000 cases were reported in 18 outbreaks, and 16 outbreaks had less than 100 cases.
During the study period, the largest dengue outbreak on record in the PICs occurred in the Solomon Islands in 2016/17. This outbreak is reported to have affected at least 12,329 people, resulting in 877 hospitalizations and 16 deaths [10][14][16]. Another notable outbreak occurred in Guam in 2019, which although small (18 cases), is considered important as it was the first autochthonous outbreak of the disease in Guam in 75 years [10].
PNG reported two separate periods of co-circulation of four and then three DENV serotypes in 2016 and 2018, respectively. The first episode of co-circulating dengue was from January to June of 2016 [10][11][14][15][16][17], followed by cases of DENV-1, DENV-2, DENV-4, and DENV-unspecified in 2018 (Figure 2) [10]. Curiously, no outbreaks were reported in PNG outside of these times. This may suggest periods of no dengue circulation; however, this seems unlikely and contradicts more recent evidence suggesting the persistent circulation of DENV-1, DENV-2, and DENV-3 in PNG for over a decade [15][16]. This is significant as endemic PNG strains serve as a potential reservoir for the spread of the virus to other countries, as demonstrated by molecular epidemiology studies that have linked outbreaks in Australia, Solomon Islands, and Fiji [15][18]. Further research is needed to understand the circulation of dengue in PNG, including elucidating the genetic diversity of strains and the potential risk for their regional dissemination.
Where Information about Dengue Outbreaks Was Found
Seventy-one of the 72 dengue outbreaks were reported as situation reports and updates on PacNet, usually within days of their detection. Therefore, outbreak information was brief and limited, mostly reporting case numbers for that week for a particular disease. Twenty-five out of the seventy-two outbreaks are featured in the peer-reviewed literature. These articles were understandably published well after the events (three months to three years later), were usually published as ‘event reports’, and contained more comprehensive and richer data than PacNet reports. Less than half (n = 30) of the dengue outbreaks were featured on ProMed, and none were featured on WHO’s Disease Outbreak News (Figure 2).
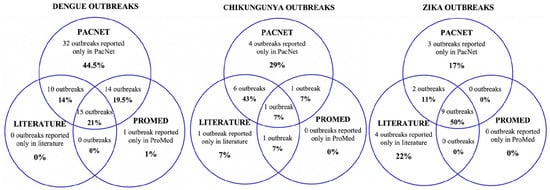
Figure 2. Sources of data reporting dengue, chikungunya and Zika outbreaks (confirmed/suspected and unspecified cases) in Pacific Island countries and areas, October 2014–June 2020. Note: In addition, one Zika outbreak was reported in WHO’s Disease Outbreak News [19]; this event was not reported on PacNet or ProMed but did feature in peer reviewed literature [20][21].
Data across the different sources were often conflicting. For example, information on PacNet about a dengue outbreak in American Samoa in 2016 reported 809 polymerase chain reaction (PCR) confirmed cases with no mention of any other syndromic cases. However, two years later, Cotter et al. (2018) reported 3240 outbreak-associated suspected cases based on syndromic surveillance data [22].
2. Chikungunya Outbreaks
Chikungunya virus is transmitted to humans by the bite of an infected Aedes mosquito, most commonly Ae. aegypti and Ae. albopictus, although Ae. polynesiensis can also transmit the virus [23][24]. Chikungunya infections usually manifest as a self-limiting dengue-like illness with high fever, severe arthralgias, myalgias, and maculopapular rash. Rare but severe complications may occur [25][24].
Roth et al. reported seven chikungunya outbreaks between 2012 and 2014 [6]. It was found 14 outbreaks in the study period affecting 11 PICs: three outbreaks in Fiji [26], two in New Caledonia [10], and one in each of America Samoa [10], Samoa [10], Tokelau [10], French Polynesia [10], Cook Islands [10][12], Kiribati [10], Marshall Islands [10], Nauru [10], and Tuvalu [10] (Figure 3).
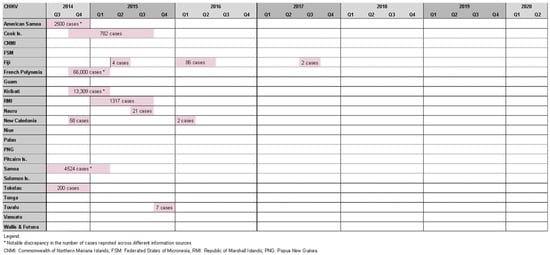
Figure 3. Timeline of reported chikungunya outbreaks (confirmed/suspected and unspecified cases) in the Pacific Island countries and areas, October2014–June 2020.
While half of these events resulted in relatively small outbreaks (affecting <100 people), there were also notable epidemics. The most striking occurred in French Polynesia from October 2014 to March 2015, affecting an estimated 66,000 people (estimated attack rate = ~25%) [27], and resulted in 64 admissions to intensive care and 18 deaths [28]. Health authorities observed a four- to nine-fold increase in Guillain–Barre syndrome (GBS) cases at the time, raising suspicion of a causal relationship with chikungunya infection. This was the second suspected arbovirus-triggered outbreak of GBS in French Polynesia, following Zika-associated cases in 2013/14 [29]. American Samoa (2500 cases) [30], Samoa (4524 cases) [10], the Cook islands (782 cases) [10], and Tokelau (200 cases) [31] experienced chikungunya outbreaks at a similar time raising suspicion of regional spread; however, this was disproven by genomic analysis that found the French Polynesian outbreak was epidemiologically linked to the Caribbean, suggesting the infection was imported by a traveler [27].
Other notable chikungunya outbreaks were reported in Kiribati (13,309 cases) [10], the Marshall Islands (1317 cases) [10], New Caledonia, (est. 58 cases) [10], and Fiji (86 cases) [26]. Cases were also identified in Queensland (Australia), and New Zealand among travelers recently returned from a PIC [10].
A serological survey conducted in Fiji in 2013 by Kama et al. soon after the first chikungunya cases were detected in the country found population antibody seroprevalence rates to be low (<1%), suggesting minimal exposure had occurred and that the population was immunologically naïve to CHIKV [32]. A follow-up serosurvey conducted in June 2017 (after two outbreaks in Fiji) found a seroconversion rate of 15.5% [26].
Where Information about Chikungunya Outbreaks Was Found
3. Zika Outbreaks
First isolated from a non-human primate in 1947 in Africa, human Zika infections had only been reported in 14 human cases (all in Africa) until 2007 [34] when the first documented large outbreak of the disease occurred in Yap State, Federated States of Micronesia [35]. Subsequently, Zika has spread to other PICs through international travelers and trade, with Roth et al. reporting three outbreaks (in Cook Islands, New Caledonia and French Polynesia) between 2012 and 2014 [6].
Our review identified 18 Zika outbreaks affecting 14 PICs, with four countries experiencing two outbreak events during the study period (American Samoa 2016/17 and 2018, RMI 2016 and 2017, New Caledonia 2014/15 and 2016, Solomon Islands 2015 and 2016). Zika outbreaks in Tonga 2016 [10][11][16][20][21], New Caledonia 2014/15 [10][36][37], and American Samoa 2016/17 [10][11][16][21][38][39] reported relatively high case counts of 2420, 1500, and 1004, respectively (Figure 4).
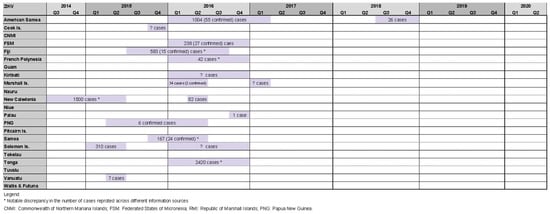
Figure 4. Timeline of reported Zika outbreaks (confirmed/suspected and unspecified cases) in the Pacific island countries and areas, October 2014–June 2020.
Zika-associated cases of microcephaly among newborn children in French Polynesia in 2013–2014, and subsequent Zika-associated GBS in South America in late 2015/early 2016 led World Health Organization of a ‘public health emergency of international health concern’ [40][41], triggering enhanced public health and clinical response measures globally.
In 2019, Sheel et al. linked the emergence of Zika in PICs to environmental stresses caused by climate change and associated pressures on fragile public health systems [42]. Craig et al. (2017) tested a hypothesis that in the absence of a dedicated surveillance system, routinely collected data on the incidence of acute flaccid paralysis (AFP), a cardinal sign of GBS, may provide a useful early warning indicator for Zika-related neurological complications. However, the authors found no conclusive evidence to support this hypothesis [21].
Where Information about Zika Outbreaks Was Found
A total of 14 out of 18 Zika outbreaks identified were reported on PacNet [10], 15 out of 18 outbreaks were reported in the literature [16][20][21][32][36][37][38][39][42][43][44][45], and 11 outbreaks were reported on both PacNet and in the literature. Nine outbreaks were reported on ProMed mail [11], and only one of the Zika outbreaks was reported in WHO’s Disease Outbreak News [19] (Figure 2).
Interestingly, six Zika event-related posts on PacNet reported the identification of the virus in international travelers arriving into Australia or New Zealand from a PIC where local transmission had not recently been reported [10], suggesting that ZIKV may have been circulating undetected in those countries. This highlights the value of regional disease intelligence sharing for the early detection of emergent pathogens of outbreak potential.
In 2016, Musso et al. reported the analysis of blood collected from French Polynesian blood donors. They found 42 out of 1505 asymptomatic blood donors (2.8%) were PCR positive for ZIKV [45]. This suggests that community transmission of Zika continued in French Polynesia well after communication about the outbreak ceased in mid-2014 [10].
4. Other Arboviral Disease Outbreaks
The review did not find definitive evidence of other arboviral outbreaks; however, the likely circulation of Ross River Virus (RRV) in French Polynesia [46] and Barmah Forest Virus in PNG [47] was mentioned in the literature.
The suspicion of RRV circulation was supported by Aubry et al. (2017), which presents serological evidence of circulation among the French Polynesian population before 2014 [48]. Lau et al. (2017) suggest that diagnosing RRV, if present, would be unlikely as most PICs lack the capacity to perform serology to confirm infection with the virus [49]. Further, symptomatic surveillance would likely lack sensitivity given the similarity in symptom profiles with other more expected arboviral infections. With 55% to 75% of RRV infections being asymptomatic, symptom-based surveillance methods are likely inadequate [46].
A report by Caly et al. (2019) found evidence that Barmah Forest virus, a virus that has never been detected outside Australia, was circulating in PNG [47].
References
- Hasan, S.; Jamdar, S.F.; Alalowi, M.; Al Ageel Al Beaiji, S.M. Dengue virus: A global human threat: Review of literature. J. Int. Soc. Prev. Community Dent. 2016, 6, 1–6.
- Fredericks, A.C.; Fernandez-Sesma, A. The burden of dengue and chikungunya worldwide: Implications for the southern United States and California. Ann. Glob. Health 2014, 80, 466–475.
- Perry, W.J. The mosquitoes and mosquito-borne diseases on New Caledonia, an historic account: 1885–1946. Am. J. Trop. Med. 1950, s1-30, 103–114.
- Calvez, E.; Guillaumot, L.; Millet, L.; Marie, J.; Bossin, H.; Rama, V.; Faamoe, A.; Kilama, S.; Teurlai, M.; Mathieu-Daudé, F.; et al. Genetic diversity and phylogeny of aedes aegypti, the main arbovirus vector in the Pacific. PLoS Negl. Trop. Dis. 2016, 10, e0004374.
- Dupont-Rouzeyrol, M.; Aubry, M.; O’Connor, O.; Roche, C.; Gourinat, A.-C.; Guigon, A.; Pyke, A.; Grangeon, J.-P.; Nilles, E.; Chanteau, S.; et al. Epidemiological and molecular features of dengue virus type-1 in New Caledonia, South Pacific, 2001–2013. Virol. J. 2014, 11, 61.
- Roth, A.; Mercier, A.; Lepers, C.; Hoy, D.; Duituturaga, S.; Benyon, E.; Guillaumot, L.; Souares, Y. Concurrent outbreaks of dengue, chikungunya and Zika virus infections—An unprecedented epidemic wave of mosquito-borne viruses in the Pacific 2012–2014. Eurosurveillance 2014, 19, 20929.
- Singh, N.; Kiedrzynski, T.; Lepers, C.; Benyon, E.K. Dengue in the Pacific—An update of the current situation. Pac. Health Surveill. Response 2005, 12, 111–119.
- Soo, K.M.; Khalid, B.; Ching, S.M.; Chee, H.Y. Meta-analysis of dengue severity during infection by different dengue virus serotypes in primary and secondary infections. PLoS ONE 2016, 11, e0154760.
- Guzman, M.G.; Vazquez, S. The complexity of antibody-dependent enhancement of dengue virus infection. Viruses 2010, 2, 2649–2662.
- Pacific Public Health Surveillance Network. PacNet listserv. Various Posts, May 2014–October 2020. Available online: https://www.pphsn.net/Services/PacNet/intro.htm (accessed on 22 July 2020).
- International Society for Infectious Diseases. ProMed-Mail. Various Posts, May 2014–October 2020. Available online: www.promedmail.org (accessed on 22 July 2020).
- Aubry, M.; Cao-Lormeau, V.M. History of arthropod-borne virus infections in French Polynesia. New Microbes New Infect. 2019, 29, 100513.
- Serie, M.; Clausse, D.; Noel, M.; Abraham, P.; Forfait, C.; Gaimard, S.; Betton, D. HepatoDengue: Severity and outcome of acute hepatitis in Dengue Fever in New Caledonia. Ann. Intensive Care 2020, 10, 16.
- Craig, A.T.; Joshua, C.A.; Sio, A.R.; Teobasi, B.; Dofai, A.; Dalipanda, T.; Hardie, K.; Kaldor, J.; Kolbe, A. Enhanced surveillance during a public health emergency in a resource-limited setting: Experience from a large dengue outbreak in Solomon Islands, 2016–2017. PLoS ONE 2018, 13, e0198487.
- Moore, P.R.; van den Hurk, A.F.; Mackenzie, J.S.; Pyke, A.T. Dengue viruses in Papua New Guinea: Evidence of endemicity and phylogenetic variation, including the evolution of new genetic lineages. Emerg. Microbes Infect. 2017, 6, e114.
- Mavian, C.; Dulcey, M.; Munoz, O.; Salemi, M.; Vittor, A.Y.; Capua, I. Islands as hotspots for emerging mosquito-borne viruses: A one-health perspective. Viruses 2018, 11, 11.
- Pulsan, F.; Sobi, K.; Anga, G.; Vince, J.; Duke, T. An outbreak of dengue fever in children in the National Capital District of Papua New Guinea in 2016. Paediatr. Int. Child Health 2020, 40, 177–180.
- Luang-Suarkia, D.; Mitja, O.; Ernst, T.; Bennett, S.; Tay, A.; Hays, R.; Smith, D.W.; Imrie, A. Hyperendemic dengue transmission and identification of a locally evolved DENV-3 lineage, Papua New Guinea 2007–2010. PLoS Negl. Trop. Dis. 2018, 12, e0006254.
- World Health Organization. Zika Virus Infection—Papua New Guinea—Disease Outbreak News, 22 April 2016. 2016. Available online: https://www.who.int/emergencies/disease-outbreak-news/item/22-april-2016-zika-png-en (accessed on 22 July 2020).
- Hills, S.L.; Fischer, M.; Petersen, L.R. Epidemiology of Zika Virus Infection. J. Infect. Dis. 2017, 216, S868–S874.
- Craig, A.T.; Butler, M.T.; Pastore, R.; Paterson, B.J.; Durrheim, D.N. Acute flaccid paralysis incidence and Zika virus surveillance, Pacific Islands. Bull. World Health Organ. 2017, 95, 69–75.
- Cotter, C.J.; Tufa, A.J.; Johnson, S.; Matai’a, M.; Sciulli, R.; Ryff, K.; Hancock, T.; Whelen, C.; Sharp, T.; Anesi, M. Outbreak of Dengue Virus Type 2—American Samoa, November 2016–October 2018. MMWR Morb. Mortal. Wkly. Rep. 2018, 67, 1319–1322.
- Gilotra, S.K.; Shah, K.V. Laboratory studies on transmission of Chikungunya virus by mosquitoes. Am. J. Epidemiol. 1967, 86, 379–385.
- Caglioti, C.; Lalle, E.; Castilletti, C.; Carletti, F.; Capobianchi, M.R.; Bordi, L. Chikungunya virus infection: An overview. New Microbiol. 2013, 36, 211–227.
- Heymann, D.L. Control of Communicable Diseases Manual: An Official Report of the American Public Health Association, 20th ed.; American Public Health Association: Washington, DC, USA, 2015.
- Aubry, M.; Kama, M.; Henderson, A.D.; Teissier, A.; Vanhomwegen, J.; Mariteragi-Helle, T.; Paoaafaite, T.; Manuguerra, J.C.; Christi, K.; Watson, C.H.; et al. Low chikungunya virus seroprevalence two years after emergence in Fiji. Int. J. Infect. Dis. 2020, 90, 223–225.
- Aubry, M.; Teissier, A.; Roche, C.; Richard, V.; Yan, A.S.; Zisou, K.; Rouault, E.; Maria, V.; Lastère, S.; Cao-Lormeau, V.M.; et al. Chikungunya outbreak, French Polynesia, 2014. Emerg. Infect. Dis. 2015, 21, 724–726.
- Koeltz, A.; Lastere, S.; Jean-Baptiste, S. Intensive care admissions for severe Chikungunya virus infection, French Polynesia. Emerg. Infect. Dis. 2018, 24, 794–796.
- Oehler, E.; Fournier, E.; Leparc-Goffart, I.; Larre, P.; Cubizolle, S.; Sookhareea, C.; Lastère, S.; Ghawche, F. Increase in cases of Guillain-Barré syndrome during a Chikungunya outbreak, French Polynesia, 2014 to 2015. Eurosurveillance 2015, 20, 30079.
- United States Centers for Disease Control and Prevention. Chikunguya Virus: 2014 Data. Available online: https://www.cdc.gov/chikungunya/geo/united-states-2014.html (accessed on 30 July 2021).
- Wahid, B.; Ali, A.; Rafique, S.; Idrees, M. Global expansion of chikungunya virus: Mapping the 64-year history. Int. J. Infect. Dis. 2017, 58, 69–76.
- Kama, M.; Aubry, M.; Naivalu, T.; Vanhomwegen, J.; Mariteragi-Helle, T.; Teissier, A.; Paoaafaite, T.; Hué, S.; Hibberd, M.L.; Manuguerra, J.C.; et al. Sustained low-level transmission of Zika and Chikungunya viruses after emergence in the Fiji Islands. Emerg. Infect. Dis. 2019, 25, 1535–1538.
- Nhan, T.X.; Musso, D. The burden of chikungunya in the Pacific. Clin. Microbiol. Infect. 2015, 21, e47–e48.
- Dick, G.W.; Kitchen, S.F.; Haddow, A.J. Zika virus. I. Isolations and serological specificity. Trans. R. Soc. Trop. Med. Hyg. 1952, 46, 509–520.
- Duffy, M.R.; Chen, T.H.; Hancock, W.T.; Powers, A.M.; Kool, J.L.; Lanciotti, R.S.; Pretrick, M.; Marfel, M.; Holzbauer, S.; Dubray, C.; et al. Zika virus outbreak on Yap Island, Federated States of Micronesia. N. Engl. J. Med. 2009, 360, 2536–2543.
- Simon, O.; Acket, B.; Forfait, C.; Girault, D.; Gourinat, A.C.; Millon, P.; Daures, M.; Vanhomwegen, J.; Billot, S.; Biron, A.; et al. Zika virus outbreak in New Caledonia and Guillain-Barré syndrome: A case-control study. J. Neurovirol. 2018, 24, 362–368.
- European Centre for Disease Prevention and Control. Rapid Risk Assessment: Zika Virus Infection Outbreak, Brazil and the Pacific Region, 26 May 2015; ECDC: Solna, Sweden, 2015.
- Healy, J.M.; Burgess, M.C.; Chen, T.-H.; Hancock, W.T.; Toews, K.-A.E.; Anesi, M.S.; Tulafono, R.T., Jr.; Mataia, M.A.; Sili, B.; Solaita, J.; et al. Notes from the Field: Outbreak of Zika Virus Disease—American Samoa, 2016. Morb. Mortal. Wkly. Rep. 2016, 65, 1146–1147.
- Samarasekera, U.; Triunfol, M. Concern over Zika virus grips the world. Lancet 2016, 387, 521–524.
- Heymann, D.L.; Hodgson, A.; Sall, A.A.; Freedman, D.O.; Staples, J.E.; Althabe, F.; Baruah, K.; Mahmud, G.; Kandun, N.; Vasconcelos, P.F.; et al. Zika virus and microcephaly: Why is this situation a PHEIC? Lancet 2016, 387, 719–721.
- World Health Organization. WHO statement on the first meeting of the International Health Regulations (2005) (IHR 2005) Emergency Committee on Zika Virus and Observed Increase in Neurological Disorders and Neonatal Malformations. Available online: https://www.who.int/news/item/01-02-2016-who-statement-on-the-first-meeting-of-the-international-health-regulations-(2005)-(ihr-2005)-emergency-committee-on-zika-virus-and-observed-increase-in-neurological-disorders-and-neonatal-malformations (accessed on 30 May 2021).
- Sheel, M.; Collins, J.; Kama, M.; Nand, D.; Faktaufon, D.; Samuela, J.; Biaukula, V.; Haskew, C.; Flint, J.; Roper, K.; et al. Evaluation of the early warning, alert and response system after Cyclone Winston, Fiji, 2016. Bull. World Health Organ. 2019, 97, 178–189.
- Musso, D.; Gubler, D.J. Zika Virus. Clin. Microbiol. Rev. 2016, 29, 487–524.
- Paixão, E.S.; Barreto, F.; Teixeira, M.d.G.; Costa, M.d.C.; Rodrigues, L.C. History, epidemiology, and clinical manifestations of Zika: A systematic review. Am. J. Public Health 2016, 106, 606–612.
- Bierlaire, D.; Mauguin, S.; Broult, J.; Musso, D. Zika virus and blood transfusion: The experience of French Polynesia. Transfusion 2017, 57, 729–733.
- Aubry, M.; Finke, J.; Teissier, A.; Roche, C.; Broult, J.; Paulous, S.; Desprès, P.; Cao-Lormeau, V.M.; Musso, D. Silent circulation of Ross River virus in French Polynesia. Int. J. Infect. Dis. 2015, 37, 19–24.
- Caly, L.; Horwood, P.F.; Vijaykrishna, D.; Lynch, S.; Greenhill, A.R.; Pomat, W.; Rai, G.; Kisa, D.; Bande, G.; Druce, J.; et al. Divergent Barmah Forest virus from Papua New Guinea. Emerg. Infect. Dis. 2019, 25, 2266–2269.
- Aubry, M.; Teissier, A.; Huart, M.; Merceron, S.; Vanhomwegen, J.; Roche, C.; Vial, A.-L.; Teururai, S.; Sicard, S.; Paulous, S.; et al. Ross River virus seroprevalence, French Polynesia, 2014–2015. Emerg. Infect. Dis. 2017, 23, 1751.
- Lau, C.; Aubry, M.; Musso, D.; Teissier, A.; Paulous, S.; Desprès, P.; de-Lamballerie, X.; Pastorino, B.; Cao-Lormeau, V.M.; Weinstein, P. New evidence for endemic circulation of Ross River virus in the Pacific Islands and the potential for emergence. Int. J. Infect. Dis. 2017, 57, 73–76.
More
Information
Subjects:
Public, Environmental & Occupational Health; Health Policy & Services; Health Care Sciences & Services
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.2K
Entry Collection:
Environmental Sciences
Revisions:
2 times
(View History)
Update Date:
17 Jan 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




