
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Taner Yonar | + 1110 word(s) | 1110 | 2022-01-13 04:30:53 | | | |
| 2 | Yvaine Wei | Meta information modification | 1110 | 2022-01-14 09:26:46 | | |
Video Upload Options
Electrochemical processes such as electrodialysis (ED) and electrodialysis bipolar membrane (EDBM) can contribute to soft-water production and the evaluation of waste fluxes. EDBM is a new technology that combines the separation function of electrodialysis with water separation at the bipolar membrane interface, which can convert salts into corresponding acids and bases without adding external components. In this system, anions and cations are separated from wastewater separately and combined with H+ and OH− ions via bipolar membranes to form acidic and alkaline solutions.
1. Introduction
For the time being, the widely known and used desalination technologies are reverse osmosis (RO), nanofiltration (NF), electrodialysis (ED), multistage flash distillation, and multiple-effect distillation. RO contributes about 65% of total installed desalination capacity worldwide [1]. The handling/treatment of the concentrated brine byproduct, on the other hand, is a key challenge in the use of RO desalination. The recovery rate of industrial RO desalination systems is normally about 50%. Therefore, over half of the feed water is released as saline into the sea or adjacent regions, with serious environmental consequences [2]. Membrane clogging is another issue with RO desalination. This problem occurs as a result of all strong acids (e.g., HCl or H2SO4) and bases (e.g., NaOH) utilized to adjust pH values or reagents used for cleaning [3]. Electrodialysis bipolar membrane (EDBM) is a technology that integrates bipolar membranes with traditional electrodialysis [4]. EDBM is a promising method for treating and valorizing desalination brines by producing acids and bases, which are valuable compounds in almost any desalination plant. EDBM has also been effectively utilized to produce or purify acids, as well as to adjust pH during fermentation or chemical synthesis in biochemical and food processing [5][6][7]. Furthermore, there are some applications in the removal of heavy metals and exhaust emissions [8][9][10].
Briefly, the advantages of the ED system are: a small and simple pre-treatment is sufficient before the process; low operating pressure; no need for antiscalant (membrane protector); long membrane life; low operating and maintenance costs; effective on many ion forms; efficacious in waters with high ion content (10,000 mg/L TDS); obtaining output product in the order of 90% of the input-product water; 10% concentrate formation (advantage over RO); it is approximately 5 times longer-lasting than reverse osmosis (reverse osmosis, 1–2 years; ED, 8–10 years); two separate collections of concentrated products and ease of recovery; high selectivity for charged compounds. In addition to these huge benefits of EDBM, it has some cons, such as: electricity consumption; the need for expertise, qualified and trained personnel; ineffectiveness on microorganisms and most anthropogenic organic pollutants [11].
Sustainable production of pure chemicals is one of the main goals of EDBM today. Based on this idea, Virag et al. demonstrated the development and optimization of a continuous and simultaneous isolation process for three biophenols based on temperature-shift adsorption. They concentrated the product and waste streams, recycled the solvent in-line, and investigated their impact on the E factor, carbon footprint, and economic sustainability of the process. As a result, they stated that the application of the hybrid process consisting of printing technology and nanofiltration can be extended from complex mixtures to the isolation of other natural compounds [12].
2. Concentrate Disposal Studies and Analysis Results with EDBM System
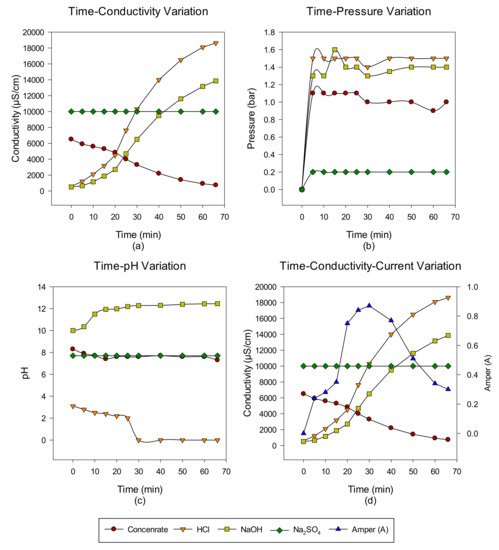
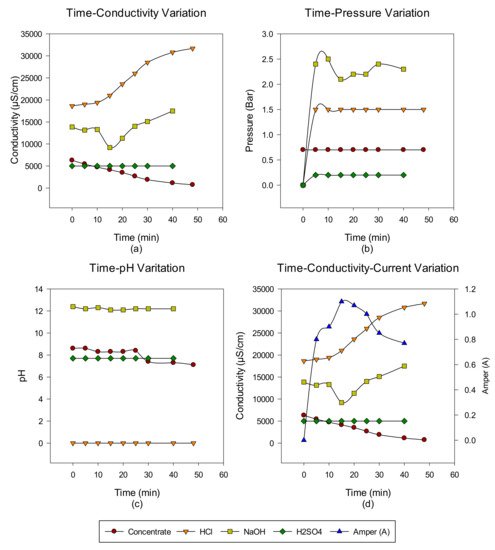
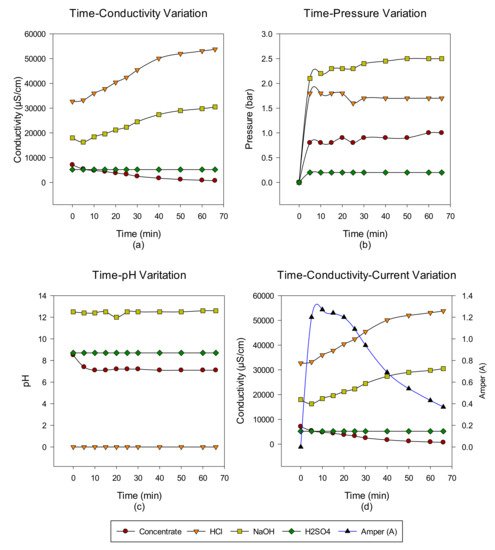
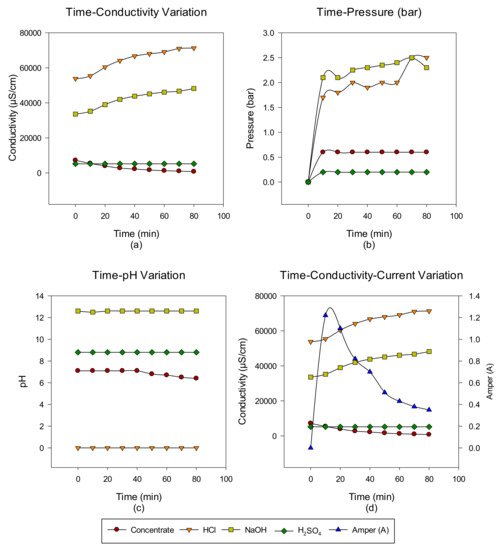
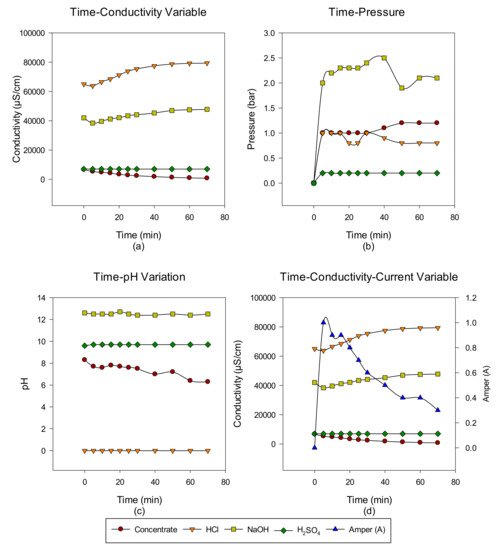
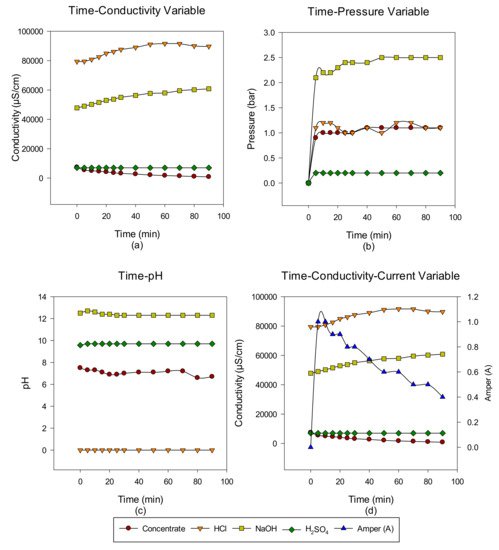
3. Conclusions
References
- Burn, S.; Hoang, M.; Zarzo, D.; Olewniak, F.; Campos, E.; Bolto, B.; Barron, O. Desalination techniques—A review of the opportunities for desalination in agriculture. Desalination 2015, 364, 2–16.
- Lattemann, S.; Höpner, T. Environmental impact and impact assessment of seawater desalination. Desalination 2008, 220, 1–15.
- Birnhack, L.; Voutchkov, N.; Lahav, O. Fundamental chemistry and engineering aspects of post-treatment processes for desalinated water—A review. Desalination 2011, 273, 6–22.
- Badruzzaman, M.; Oppenheimer, J.; Adham, S.; Kumar, M. Innovative beneficial reuse of reverse osmosis concentrate using bipolar membrane electrodialysis and electrochlorination processes. J. Membr. Sci. 2009, 326, 392–399.
- Rozoy, E.; Boudesocque, L.; Bazinet, L. Deacidification of Cranberry Juice by Electrodialysis with Bipolar Membranes. J. Agric. Food Chem. 2015, 63, 642–651.
- Chen, Q.; Xue, C.; Zhang, W.-M.; Song, W.-G.; Wan, L.-J.; Ma, K.-S. Green Production of Ultrahigh-Basicity Polyaluminum Salts with Maximum Atomic Economy by Ultrafiltration and Electrodialysis with Bipolar Membranes. Ind. Eng. Chem. Res. 2014, 53, 13467–13474.
- Zhang, X.; Wang, X.; Wang, Y.; Li, C.; Feng, H.; Xu, T. Production of Yellow Iron Oxide Pigments by Integration of the Air Oxidation Process with Bipolar Membrane Electrodialysis. Ind. Eng. Chem. Res. 2014, 53, 1580–1587.
- Ye, W.; Huang, J.; Lin, J.; Zhang, X.; Shen, J.; Luis, P.; Van der Bruggen, B. Environmental evaluation of bipolar membrane electrodialysis for NaOH production from wastewater: Conditioning NaOH as a CO2 absorbent. Sep. Purif. Technol. 2015, 144, 206–214.
- Jiang, C.; Chen, H.; Zhang, Y.; Feng, H.; Shehzad, M.A.; Wang, Y.; Xu, T. Complexation Electrodialysis as a general method to simultaneously treat wastewaters with metal and organic matter. Chem. Eng. J. 2018, 348, 952–959.
- Liu, Y.; Chen, J.; Cai, Z.; Chen, R.; Sun, Q.; Sun, M. Removal of copper and nickel from municipal sludge using an improved electrokinetic process. Chem. Eng. J. 2017, 307, 1008–1016.
- Scelsi, L.; Hodzic, A.; Soutis, C.; Hayes, S.A.; Rajendran, S.; AlMa’Adeed, M.A.; Kahraman, R. A review on composite materials based on recycled thermoplastics and glass fibres. Plast. Rubber Compos. 2011, 40, 1–10.
- Voros, V.; Drioli, E.; Fonte, C.; Szekely, G. Process Intensification via Continuous and Simultaneous Isolation of Antioxidants: An Upcycling Approach for Olive Leaf Waste. ACS Sustain. Chem. Eng. 2019, 7, 18444–18452.




