Electrochemical processes such as electrodialysis (ED) and electrodialysis bipolar membrane (EDBM) can contribute to soft-water production and the evaluation of waste fluxes. EDBM is a new technology that combines the separation function of electrodialysis with water separation at the bipolar membrane interface, which can convert salts into corresponding acids and bases without adding external components. In this system, anions and cations are separated from wastewater separately and combined with H+ and OH− ions via bipolar membranes to form acidic and alkaline solutions.
- electrodialysis bipolar membrane
- concentrate
- membrane
1. Introduction
For the time being, the widely known and used desalination technologies are reverse osmosis (RO), nanofiltration (NF), electrodialysis (ED), multistage flash distillation, and multiple-effect distillation. RO contributes about 65% of total installed desalination capacity worldwide [1][4]. The handling/treatment of the concentrated brine byproduct, on the other hand, is a key challenge in the use of RO desalination. The recovery rate of industrial RO desalination systems is normally about 50%. Therefore, over half of the feed water is released as saline into the sea or adjacent regions, with serious environmental consequences [2][5]. Membrane clogging is another issue with RO desalination. This problem occurs as a result of all strong acids (e.g., HCl or H2SO4) and bases (e.g., NaOH) utilized to adjust pH values or reagents used for cleaning [3][6]. Electrodialysis bipolar membrane (EDBM) is a technology that integrates bipolar membranes with traditional electrodialysis [4][7]. EDBM is a promising method for treating and valorizing desalination brines by producing acids and bases, which are valuable compounds in almost any desalination plant. EDBM has also been effectively utilized to produce or purify acids, as well as to adjust pH during fermentation or chemical synthesis in biochemical and food processing [5][6][7][8,9,10]. Furthermore, there are some applications in the removal of heavy metals and exhaust emissions [8][9][10][11,12,13].
Briefly, the advantages of the ED system are: a small and simple pre-treatment is sufficient before the process; low operating pressure; no need for antiscalant (membrane protector); long membrane life; low operating and maintenance costs; effective on many ion forms; efficacious in waters with high ion content (10,000 mg/L TDS); obtaining output product in the order of 90% of the input-product water; 10% concentrate formation (advantage over RO); it is approximately 5 times longer-lasting than reverse osmosis (reverse osmosis, 1–2 years; ED, 8–10 years); two separate collections of concentrated products and ease of recovery; high selectivity for charged compounds. In addition to these huge benefits of EDBM, it has some cons, such as: electricity consumption; the need for expertise, qualified and trained personnel; ineffectiveness on microorganisms and most anthropogenic organic pollutants [11][17].
Sustainable production of pure chemicals is one of the main goals of EDBM today. Based on this idea, Virag et al. demonstrated the development and optimization of a continuous and simultaneous isolation process for three biophenols based on temperature-shift adsorption. They concentrated the product and waste streams, recycled the solvent in-line, and investigated their impact on the E factor, carbon footprint, and economic sustainability of the process. As a result, they stated that the application of the hybrid process consisting of printing technology and nanofiltration can be extended from complex mixtures to the isolation of other natural compounds [12][28].
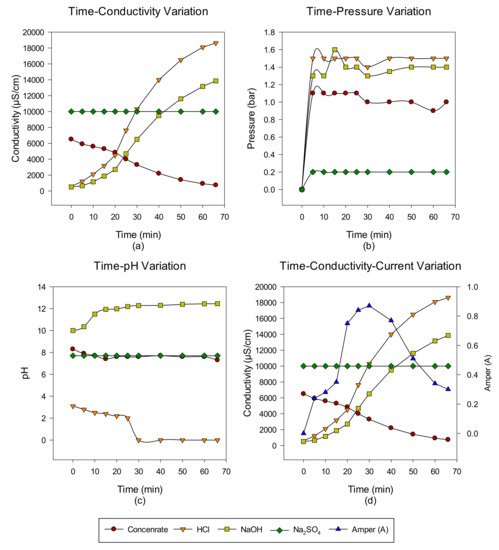
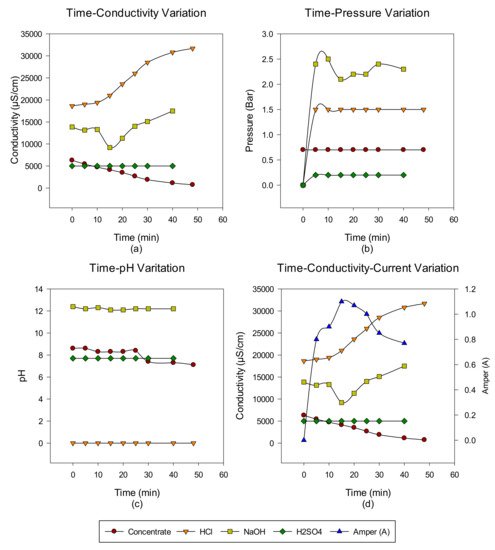
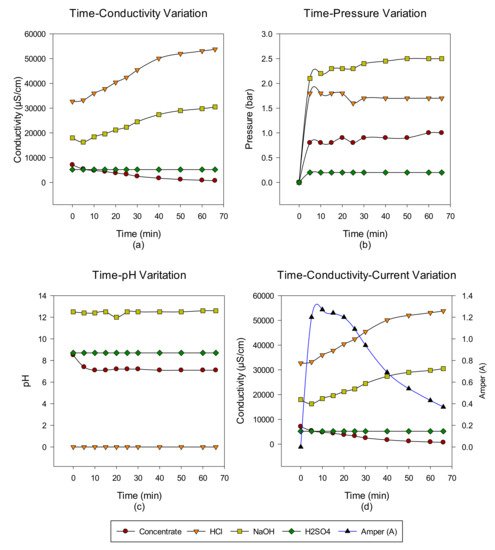
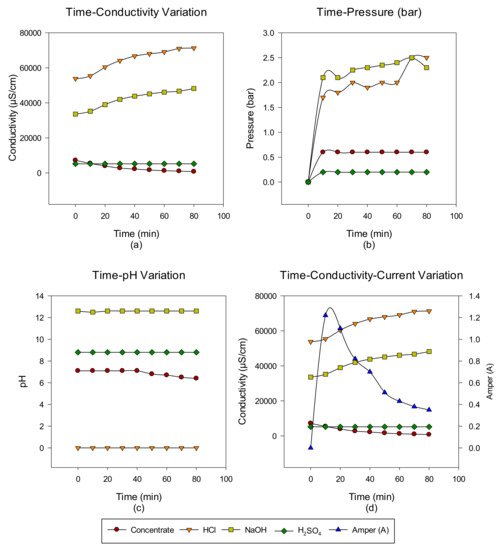
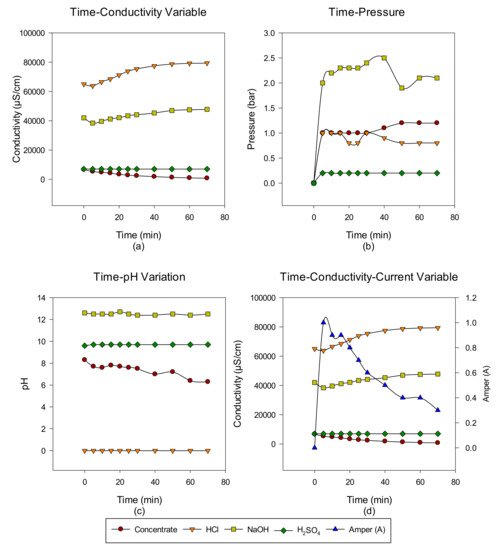
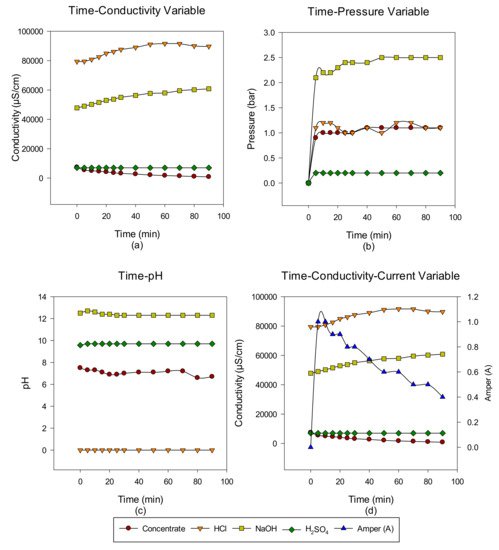
2. Concentrate Disposal Studies and Analysis Results with EDBM System