
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Nicolas MASURIER | + 2110 word(s) | 2110 | 2022-01-05 09:04:46 | | | |
| 2 | Catherine Yang | Meta information modification | 2110 | 2022-01-13 02:01:14 | | | | |
| 3 | Nicolas MASURIER | Meta information modification | 2110 | 2022-01-13 15:00:43 | | |
Video Upload Options
Thienopyrimidine emerges as an attractive scaffold in medicinal chemistry with a wide array of pharmacological properties, such as antibacterial, antifungal, antiparasitic and antiviral. Considering the fusion between pyrimidine and thiophene rings, three different thienopyrimidines can be obtained, namely thieno[2,3-d]pyrimidines, thieno[3,2-d]pyrimidines and thieno[3,4-d]pyrimidines. Different synthetic pathways involving the construction of the pyrimidine or the thiophene ring were reported in the literature to access polysubstituted thienopyrimidines. In these approaches, the synthetic strategies mostly involved the synthesis of a thienopyrimidin-4-one derivative, where position 4 could be modified via further functionalization.
1. Synthesis from Thiophene Derivatives
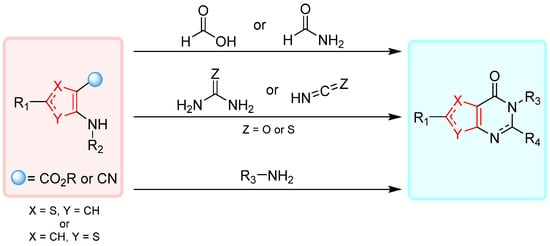
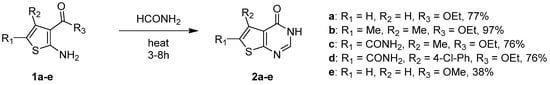
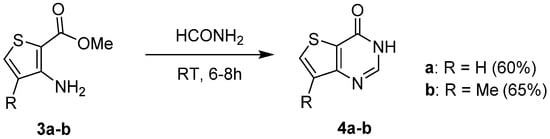
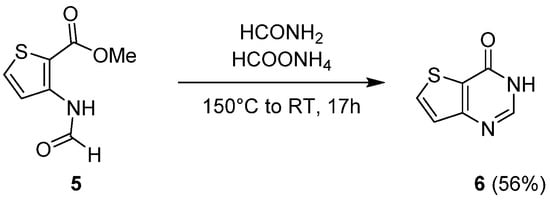
1.1. Cyclization with Carbonyl Reactants
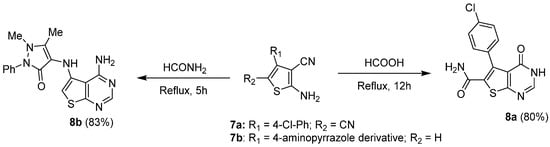
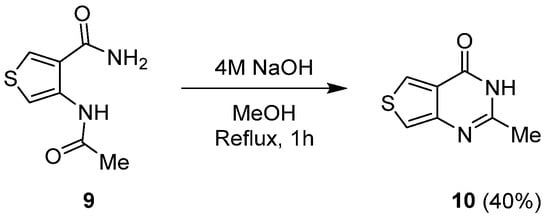
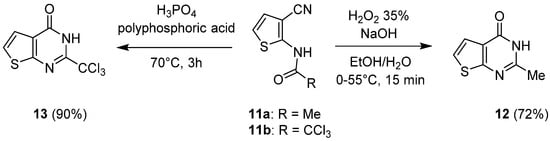
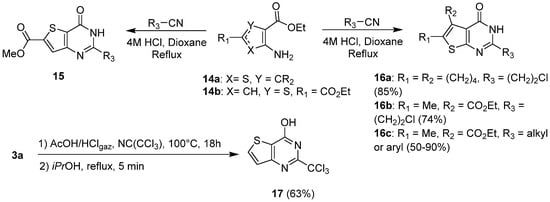
1.2. Cyclization with Nitrile Reactants
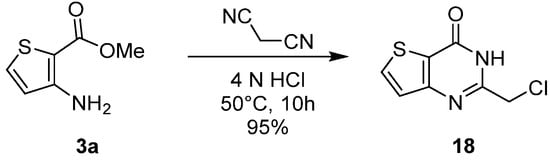
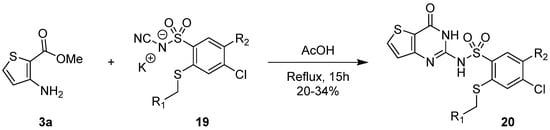
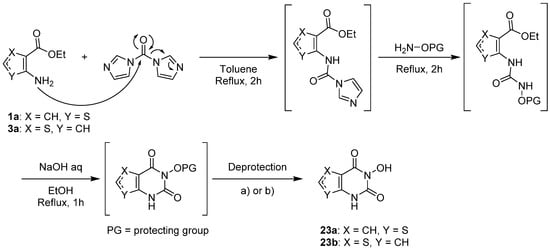
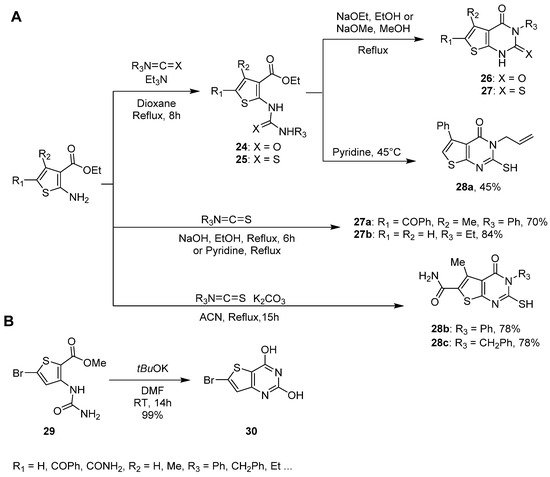
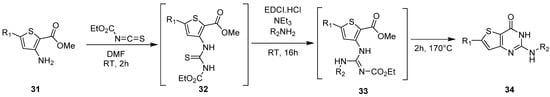
1.3. Synthesis from (Thio)urea Reagents, Iso(Thio)cyanate or (Thio)cyanate Derivatives
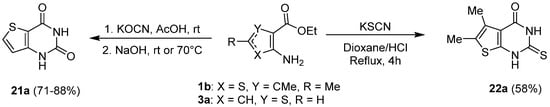
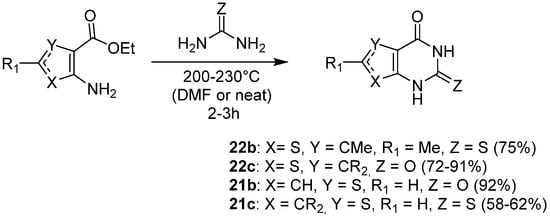
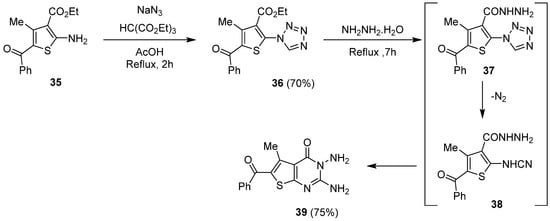
1.4. Synthesis via a Tetrazole Intermediate
1.5. Cyclization with Amine/Hydrazine Derivatives
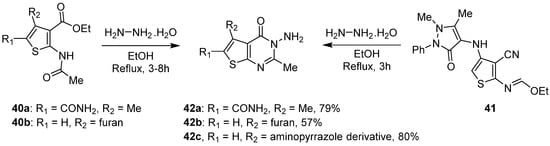
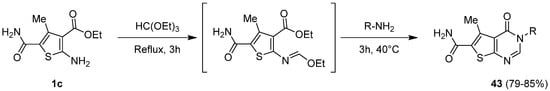
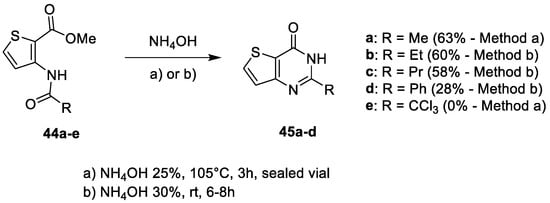
2. Synthesis of Thienopyrimidines from Pyrimidine Derivatives
2.1. Synthesis from the Thorpe-Ziegler Reaction
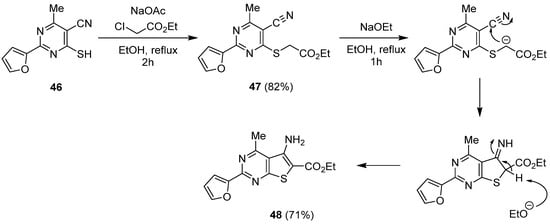
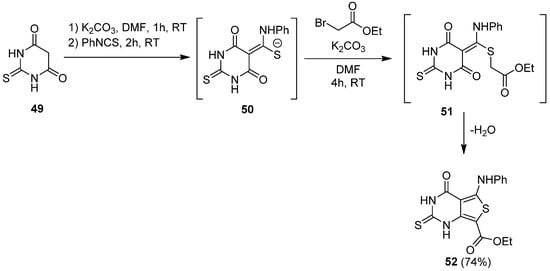
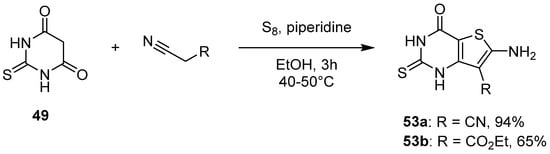
2.2. Synthesis from the Gewald Reaction
References
- Pullarao, B.; Sharif, S.D.K.; Kumar, D.R.; Ramachandran, D. Design, Synthesis and Biological Evaluation of Thiophene Based Pyrimidin-4-One Derivatives as New Type of Antimicrobial Agents. Asian J. Chem. 2016, 28, 1997–2000.
- Ortikov, I.S.; Turdibaev, Z.É.; Islamova, Z.I.; Élmuradov, B.Z.; Abdurazakov, A.S.; Bektemirov, A.M.; Osipova, S.O.; Khushbaktova, Z.A.; Syrov, V.N.; Shakhidoyatov, K.M. Search for Bactericides Among Derivatives of Deoxyvasicinone, Mackinazolinone, and Thienopyrimidinones. Pharm. Chem. J. 2017, 51, 456–464.
- Habib, N.S.; Soliman, R.; El-Tombary, A.A.; El-Hawash, S.A.; Shaaban, O.G. Synthesis and Biological Evaluation of Novel Series of ThienoPyrimidine Derivatives as Anticancer and Antimicrobial Agents. Med. Chem. Res. 2013, 22, 3289–3308.
- Kanawade, S.B.; Toche, R.B.; Rajani, D.P. Synthetic Tactics of New Class of 4-AminothienoPyrimidine-6-Carbonitrile Derivatives Acting as Antimicrobial Agents. Eur. J. Med. Chem. 2013, 64, 314–320.
- Woodring, J.L.; Patel, G.; Erath, J.; Behera, R.; Lee, P.J.; Leed, S.E.; Rodriguez, A.; Sciotti, R.J.; Mensa-Wilmot, K.; Pollastri, M.P. Evaluation of Aromatic 6-Substituted Thienopyrimidines as Scaffolds against Parasites That Cause Trypanosomiasis, Leishmaniasis, and Malaria. Med. Chem. Commun. 2015, 6, 339–346.
- Shao, X.; AbdelKhalek, A.; Abutaleb, N.S.; Velagapudi, U.K.; Yoganathan, S.; Seleem, M.N.; Talele, T.T. Chemical Space Exploration around ThienoPyrimidin-4(3H)-One Scaffold Led to a Novel Class of Highly Active Clostridium Difficile Inhibitors. J. Med. Chem. 2019, 62, 9772–9791.
- Aly, H.M.; Saleh, N.M.; Elhady, H.A. Design and Synthesis of Some New Thiophene, Thienopyrimidine and Thienothiadiazine Derivatives of Antipyrine as Potential Antimicrobial Agents. Eur. J. Med. Chem. 2011, 46, 4566–4572.
- Desroches, J.; Kieffer, C.; Primas, N.; Hutter, S.; Gellis, A.; El-Kashef, H.; Rathelot, P.; Verhaeghe, P.; Azas, N.; Vanelle, P. Discovery of New Hit-Molecules Targeting Plasmodium Falciparum through a Global SAR Study of the 4-Substituted-2-Trichloromethylquinazoline Antiplasmodial Scaffold. Eur. J. Med. Chem. 2017, 125, 68–86.
- De Schutter, J.W.; Morrison, J.P.; Morrison, M.J.; Ciulli, A.; Imperiali, B. Targeting Bacillosamine Biosynthesis in Bacterial Pathogens: Development of Inhibitors to a Bacterial Amino-Sugar Acetyltransferase from Campylobacter Jejuni. J. Med. Chem. 2017, 60, 2099–2118.
- Mavrova, A.T.; Vuchev, D.; Anichina, K.; Vassilev, N. Synthesis, Antitrichinnellosis and Antiprotozoal Activity of Some Novel ThienoPyrimidin-4(3H)-Ones Containing Benzimidazole Ring. Eur. J. Med. Chem. 2010, 45, 5856–5861.
- Kim, J.; Kwon, J.; Lee, D.; Jo, S.; Park, D.-S.; Choi, J.; Park, E.; Hwang, J.Y.; Ko, Y.; Choi, I.; et al. Serendipitous Discovery of 2-((Phenylsulfonyl)Methyl)-ThienoPyrimidine Derivatives as Novel HIV-1 Replication Inhibitors. Bioorganic Med. Chem. Lett. 2014, 24, 5473–5477.
- Sławiński, J.; Żołnowska, B.; Pirska, D.; Kędzia, A.; Kwapisz, E. Synthesis and Antibacterial Activity of Novel 4-Chloro-2-Mercaptobenzenesulfonamide Derivatives. J. Enzym. Inhib. Med. Chem. 2013, 28, 41–51.
- Patel, A. Modi Synthesis and Biological Evaluation of Schiff Base Involving Thieno Pyrimidine Moiety as Antimicrobial Agents. RJLBPCS 2019, 5, 31–41.
- Giri, T.; Sailaja, G.; Laxminarayana, E.; Thirumala Chary, M.; Ramesh, M. Synthesis and Antibacterial Activity of Novel 4--Benzohydrazide Derivatives. Russ. J. Gen. Chem. 2017, 87, 1275–1280.
- Tharikoppula, G.; Eppakayala, L.; Maringanti, T.C.; Kamalapuram, C.; Kudle, K.R. Synthesis and Antibacterial Activity of Thienopyrimidine Amide Derivatives. Asian J. Chem. 2017, 29, 1515–1521.
- Temburnikar, K.W.; Zimmermann, S.C.; Kim, N.T.; Ross, C.R.; Gelbmann, C.; Salomon, C.E.; Wilson, G.M.; Balzarini, J.; Seley-Radtke, K.L. Antiproliferative Activities of Halogenated Thienopyrimidines. Bioorganic Med. Chem. 2014, 22, 2113–2122.
- Prabhakar, V.; Babu Kondra, S.; Maddula, S.R. Synthesis, Structural Elucidation of Novel ThienoPyrimidine Core Unit Containing 1,2,4-Triazoles and Thiophenes as Potent Antimicrobial Activity. Org. Chem. Curr. Res. 2016, 5, 1000169.
- Prabhakar, V.; Babu, S.J.; Siva Jyothi, S.V.L. Synthesis, Structural Elucidation and Anti-Bacterial Evaluation of Some Novel Heterocyclic Molecules Derived from Thienopyrimidine as a Core Unit. Org. Chem. Curr. Res. 2016, 5, 1000172.
- Sang, Y.; Han, S.; Han, S.; Pannecouque, C.; De Clercq, E.; Zhuang, C.; Chen, F. Follow On-Based Optimization of the Biphenyl-DAPYs as HIV-1 Nonnucleoside Reverse Transcriptase Inhibitors against the Wild-Type and Mutant Strains. Bioorganic Chem. 2019, 89, 102974.
- Al-Harbi, R.A.K.; Abdel-Rahman, A.A.H. Synthesis and Anti-HBV Activity of 2-(Methylthio)ThienoPyrimidin- 4(1H)-One Analogues of ACV. Der Pharma Chem. 2013, 5, 1–7.
- Kankanala, J.; Kirby, K.A.; Huber, A.D.; Casey, M.C.; Wilson, D.J.; Sarafianos, S.G.; Wang, Z. Design, Synthesis and Biological Evaluations of N-Hydroxy Thienopyrimidine-2,4-Diones as Inhibitors of HIV Reverse Transcriptase-Associated RNase H. Eur. J. Med. Chem. 2017, 141, 149–161.
- Abu-Hashem, A.A.; Abu-Zied, K.M.; El-Shehry, M.F. Synthetic Utility of Bifunctional Thiophene Derivatives and Antimicrobial Evaluation of the Newly Synthesized Agents. Mon. Chem. 2011, 142, 539–545.
- Dewal, M.B.; Wani, A.S.; Vidaillac, C.; Oupický, D.; Rybak, M.J.; Firestine, S.M. ThienoPyrimidinedione Derivatives as Antibacterial Agents. Eur. J. Med. Chem. 2012, 51, 145–153.
- Shaaban, O.G.; Issa, D.A.E.; El-Tombary, A.A.; Abd El Wahab, S.M.; Abdel Wahab, A.E.; Abdelwahab, I.A. Synthesis and Molecular Docking Study of Some 3,4-DihydrothienoPyrimidine Derivatives as Potential Antimicrobial Agents. Bioorganic Chem. 2019, 88, 102934.
- Endo, Y.; Kawai, K.; Asano, T.; Amano, S.; Asanuma, Y.; Sawada, K.; Onodera, Y.; Ueo, N.; Takahashi, N.; Sonoda, Y.; et al. 2-(Isopropylamino)ThienoPyrimidin-4(3H)-One Derivatives as Selective Phosphodiesterase 7 Inhibitors with Potent in Vivo Efficacy. Bioorganic Med. Chem. Lett. 2015, 25, 1910–1914.
- González Cabrera, D.; Le Manach, C.; Douelle, F.; Younis, Y.; Feng, T.-S.; Paquet, T.; Nchinda, A.T.; Street, L.J.; Taylor, D.; de Kock, C.; et al. 2,4-Diaminothienopyrimidines as Orally Active Antimalarial Agents. J. Med. Chem. 2014, 57, 1014–1022.
- Cohen, A.; Suzanne, P.; Lancelot, J.-C.; Verhaeghe, P.; Lesnard, A.; Basmaciyan, L.; Hutter, S.; Laget, M.; Dumètre, A.; Paloque, L.; et al. Discovery of New Thienopyrimidinone Derivatives Displaying Antimalarial Properties toward Both Erythrocytic and Hepatic Stages of Plasmodium. Eur. J. Med. Chem. 2015, 95, 16–28.
- Chambhare, R.V.; Khadse, B.G.; Bobde, A.S.; Bahekar, R.H. Synthesis and Preliminary Evaluation of Some N-Pyrimidin-3-Yl]-Carboxamide and 3-Substituted-5-(2-Furanyl)-2-Methyl-3H-ThienoPyrimidin-4-Ones as Antimicrobial Agents. Eur. J. Med. Chem. 2003, 38, 89–100.
- Abdel Hamid, A.M.; Shehta, W. Synthesis of Some Novel Furan-Tagged Thienopyrimidine Derivatives as Antibacterial Agents: Synthesis of Some Novel Furan-Tagged Thienopyrimidine Derivatives as Antibacterial Agents. J. Heterocycl. Chem. 2019, 56, 485–492.
- Aly, H.M.; Saleh, N.M. Utility of a Pyrimidine Thione Derivative in the Synthesis of New Fused PyrimidoPyrimidine, PyridoPyrimidine and Different Types of Thienopyrimidine Derivatives. Int. J. Adv. Res. 2014, 2, 694–702.





