The genus Aeromonas has been recognised as an important pathogenic species in aquaculture that causes motile Aeromonas septicaemia (MAS) or less severe, chronic infections. This study compares the pathogenicity of the different Aeromonas spp. that were previously isolated from freshwater fish with signs of MAS. A total of 124 isolates of Aeromonas spp. were initially screened for the ability to grow on M9 agar with myo-inositol as a sole carbon source, which is a discriminatory phenotype for the hypervirulent A. hydrophila (vAh) pathotype. Subsequently, LD50 of six selected Aeromonas spp. were determined by intraperitoneal injection of bacterial suspension containing 10^3, 10^5, and 10^7 CFU/mL of the respective Aeromonas sp. to red hybrid tilapias. The kidneys, livers and spleens of infected moribund fish were examined for histopathological changes. The screening revealed that only A. dhakensis 1P11S3 was able to grow using myo-inositol as a sole carbon source, and no vAh strains were identified. The LD50–240h of A. dhakensis 1P11S3 was 10^7 CFU/mL, while the non-myo-inositol utilizing A. dhakensis 4PS2 and A. hydrophila 8TK3 was lower at 10^5 CFU/mL. Similarly, tilapia challenged with the myo-inositol A. dhakensis 1P11S3 showed significantly (p < 0.05) less severe signs, gross and histopathological lesions, and a lower mortality rate than the non-myo-inositol A. dhakensis 4PS2 and A. hydrophila 8TK3. These findings suggested that myo-inositol utilizing A. dhakensis 1P11S3 was not a hypervirulent Aeromonas sp. under current experimental disease challenge conditions, and that diverse Aeromonas spp. are of concern in aquaculture farmed freshwater fish. Therefore, future study is warranted on genomic level to further elucidate the influence of myo-inositol utilizing ability on the pathogenesis of Aeromonas spp., since this ability correlates with hypervirulence in A. hydrophila strains.
1. Introduction
Aquaculture is one of the most important sectors that provides valuable sources of protein, aside from generating income for certain countries. FAO
[1] reported a new height in global aquaculture production in 2018, with 114.5 million tonnes in live weight, generating USD 263.6 billion of farmgate sale value. Malaysia has been relying on the aquaculture sector as an important source of revenue, and FAO
[2] also states that fish is an essential food in Southeast Asian nations, such as Malaysia, Indonesia, Myanmar, Thailand, Vietnam, Philippines, and Cambodia. In 2018, it was reported that the red hybrid tilapia (
Oreochrommis niloticus × O. mossambicus) and black tilapia (
Oreochromis spp.) accounted for 30.7% of total freshwater production in Malaysia, wherein red hybrid tilapia alone constituted about 97% of total tilapia production
[3]. Tilapia farming is preferred because of their resilience in many environmental conditions, disease resistance, marketability, and easier-to-produce market-sized fish using a varied range of feed, ranging from natural organisms to humanmade pellets
[4][5].
However, disease outbreak is one of the main problems which resulted in economic, environmental and social impacts on aquaculture. Fish farms are vulnerable to losses due to outbreaks of bacterial infections such as motile
Aeromonas septicemia (MAS)
[6]. This disease is caused by members of the genus
Aeromonas, such as
Aeromonas hydrophila,
A. veronii,
A. dhakensis,
A. jandaei,
A. sobria and
A. caviae [7][8][9][10][11][12]. The disease was described as having two forms, the acute hemorrhagic septicemia and the chronic ulcerative syndrome
[13][14][15]. The genus
Aeromonas plays an important role in diseases of aquaculture, with
Aeromonas spp. showing a ubiquitous distribution in aquatic habitats including freshwater, seawater, estuaries and even in chlorinated water
[16][17]. The ability of
Aeromonas spp. to thrive in a wide range of temperatures, from 4 °C to 42 °C, and tolerate up to 5.5 g/L NaCl and in a range of pH from 5 to 10 contributes to their widespread distribution
[18][19].
Aeromonas spp. are generally opportunistic pathogens that are normal residents of the fish gut microbiota
[20].
Recently, hypervirulent vAh strains were identified in diseased carp species in China and from diseased channel catfish from the USA, which were found to cause acute persistent MAS
[21]. It was reported as a primary pathogen
[22][23] among grass carp (
Ctenopharyngodon idella) and channel catfish (
Ictalurus punctatus) suffering from mass mortalities without concurrent infection
[21][24][25]. Most of the affected fish were of market size
[23][26], while another study reported the strain in juvenile fish
[27].
The vAh strains have an apparently unique ability among
A. hydrophila strains to utilize
myo-inositol as a sole carbon source and Hossain et al.
[26] further reported that
myo-inositol utilizing gene cluster was part of epidemic associated region in vAh strains. Therefore, this characteristic has been used to screen vAh isolates among
A. hydrophila [24][26][28]. Nevertheless, the only known vAh strains are among
A. hydrophila, while
A. dhakensis,
A. hydrophila,
A. jandaei,
A. veronii,
A. sobria and
A. caviae were believed to be unable to utilize inositol
[10][29][30][31]. However, with more recent data, there is a possibility that horizontal gene transfer may have happened between vAh and other notorious virulent
Aeromonas spp., such as
A. dhakensis.
2. Characterization of Aeromonas spp.
Out of the 124 Aeromonas isolates tested in this study, one isolate, the A. dhakensis 1P11S3 was found to utilize myo-inositol. The growth on M9 agar was observed as early as 12 h post-incubation and could clearly be seen after 24 h, while the M9 broth became turbid after 24 h of incubation with A. dhakensis 1P11S3 (Figure A1). All isolates (n = 124) produced complete haemolysis (β-haemolysis) on 7% blood agar (n = 124).
3. Clinicopathological Changes of Infected Fish
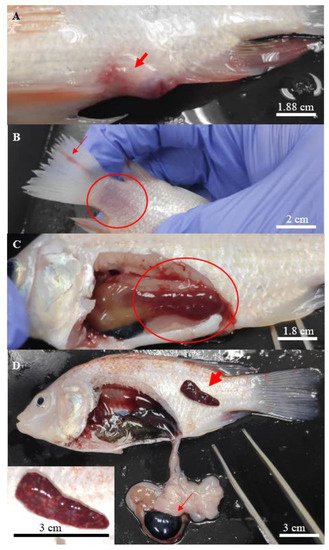
In general, all Aeromonas-infected fish showed various degrees of typical gross lesions and clinical signs of aeromoniasis. These symptoms included irregular breathing and lethargy, reduced feed consumption, displayed erratic movement with loss of balance, isolating and swimming to the surface of the water. The gross lesions included swollen and haemorrhagic site of injection (Figure 1A), scale loss that exposed the underlying skin at the caudal fin (Figure 1B) and necrosis of the fins (Figure 1B).
Figure 1. Clinical signs found on the hybrid red tilapias infected with Aeromonas. (A) focal haemorrhage on the site of injection, (B) scale loss (circle) at the caudal peduncle with rotting caudal fin (thin arrow), (C) liver with deep haemorrhage (circle), and (D) haemorrhage with multiple white spots observed on the enlarged spleen (thick arrow), enlarged gallbladder (thin arrow), with a close-up picture of the affected spleen at the bottom left.
Post-mortem examinations revealed hepatomegaly (Figure 1C) in a majority of the infected fish, while multifocal splenic infarction (Figure 1D) was observed in fish infected with A. hydrophila 8TK3. Some infected fish had a haemorrhagic spleen and swollen gall bladder (Figure 1D). Overall, fish infected by A. hydrophila 8TK3 and A. dhakensis 4PS2 showed more severe gross lesions than other isolates. All control fish exhibited neither clinical sign nor gross lesion.
4. Lethal Dose of Aeromonas spp. in Red Hybrid Tilapia
The rates of mortality following infection by different isolates of Aeromonas were summarized in Table 1. Mortalities were observed as early as 24 h post-challenge in all infected groups, except for A. jandaei 7KL3 and lasted for 216 h. By 240 h, the cumulative mortality of fish challenged with 103 CFU/mL of bacteria ranged between 0% and 20%, between 20% and 60% with 105 CFU/mL and between 35% and 95% with 107 CFU/mL. The two highest cumulative mortalities (95% and 90%) involved non-myo-inositol utilizing A. dhakensis 4PS2 (107 CFU/mL) and A. hydrophila 8TK3 (107 CFU/mL), which was higher than the myo-inositol utilizing A. dhakensis 1P11S3 (107 CFU/mL) with 55% cumulative mortality.
Table 1. The number of mortalities of red hybrid tilapias infected with six strains of Aeromonas spp.
| Species |
Bacterial Concentration |
Number of Deaths at Specific Time (Hour Post Infection) |
Sample Number |
Cumulative Mortality (%) |
LD50–240h
(CFU/mL) |
| (CFU/mL) |
24 |
48 |
72 |
96 |
120 |
144 |
168 |
192 |
216 |
240 |
| A. dhakensis 1P11S3 * |
103 |
2 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
20 |
2 (10) |
1 × 107 |
| |
105 |
2 |
1 |
1 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
20 |
4 (20) |
|
| |
107 |
2 |
4 |
1 |
4 |
0 |
0 |
0 |
0 |
0 |
0 |
20 |
11 (55) |
|
| A. dhakensis 4PS2 |
103 |
0 |
0 |
0 |
1 |
0 |
0 |
0 |
0 |
0 |
0 |
20 |
1 (5) |
1 × 105 |
| |
105 |
1 |
5 |
6 |
8 |
0 |
0 |
0 |
0 |
0 |
0 |
20 |
8 (40) |
|
| |
107 |
11 |
0 |
3 |
4 |
1 |
0 |
0 |
0 |
0 |
0 |
20 |
19 (95) |
|
| A. hydrophila 8TK3 |
103 |
0 |
3 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
20 |
3 (15) |
1 × 105 |
| |
105 |
0 |
2 |
6 |
2 |
1 |
0 |
1 |
0 |
0 |
0 |
20 |
12 (60) |
|
| |
107 |
7 |
2 |
6 |
1 |
1 |
0 |
1 |
0 |
0 |
0 |
20 |
18 (90) |
|
| A. veronii 6TS5 |
103 |
0 |
1 |
0 |
0 |
1 |
0 |
0 |
0 |
0 |
0 |
20 |
2 (10) |
1 × 107 |
| |
105 |
2 |
3 |
1 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
20 |
6 (30) |
|
| |
107 |
3 |
0 |
1 |
3 |
0 |
0 |
1 |
0 |
1 |
0 |
20 |
9 (45) |
|
| A. caviae 7X11 |
103 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
20 |
0 (0) |
1 × 107 |
| |
105 |
2 |
0 |
0 |
1 |
0 |
2 |
0 |
1 |
0 |
0 |
20 |
6 (30) |
|
| |
107 |
1 |
3 |
3 |
1 |
1 |
1 |
0 |
0 |
0 |
0 |
20 |
10 (50) |
|
| A. jandaei 7KL3 |
103 |
0 |
2 |
0 |
1 |
1 |
0 |
0 |
0 |
0 |
0 |
20 |
4 (20) |
1 × 1011 |
| |
105 |
0 |
3 |
0 |
0 |
0 |
2 |
0 |
0 |
0 |
0 |
20 |
5 (25) |
|
| |
107 |
0 |
3 |
2 |
0 |
0 |
0 |
1 |
1 |
0 |
0 |
20 |
7 (35) |
|
| Control (sterile TSB) |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
20 |
0 (0) |
|
The LD50–240h for all Aeromonas spp. were presented in Table 1. The lowest LD50–240h of 105 CFU/mL was for A. hydrophila 8TK3 and A. dhakensis 4PS2, lower than the 107 CFU/mL of A. dhakensis 1P11S3, A. veronii 6TS5 and A. caviae 7X11, while the highest LD50–240h of 1011 CFU/mL was calculated for A. jandaei 7KL3.
5. Histopathological Analysis
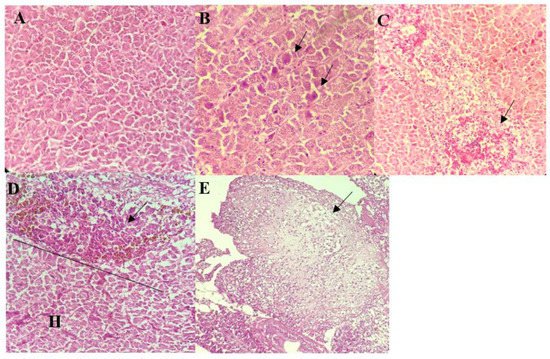
The livers of all infected fish appeared congested with necrosis of hepatocytes. These were frequently accompanied by moderate to severe hydropic degeneration of the hepatocytes (Figure 2B,C). Individual necrosis of the hepatocytes was most severe in fish infected by A. hydrophila 8TK3 (p < 0.05), followed by A. dhakensis 4PS2, A. dhakensis 1P11S3, A. veronii 6TS5, A. caviae 7X11, and A. jandaei 7KL3. Infiltration of inflammatory cells, haemorrhages and hemosiderosis were also observed in the liver of all groups (Figure 2D). In addition, liver of fish infected with A. hydrophila 8TK3 showed hepatic infarction (Figure 2E).
Figure 2. Histological findings of the liver of control and infected red hybrid tilapias by Aeromonas spp. (A) Liver of control fish showed normal liver architecture and hepatocytes, 400×. (B) Individual hepatocyte necrosis exhibiting intense cytoplasmic eosinophilia (arrows), 400×. (C) Extensive hydropic degeneration and necrosis of cells surrounding a central vein (arrow), 400×. (D) Infiltration of inflammatory cells. Note that some inflammatory cells are showing brownish cytoplasmic pigmentation suggestive of hemosiderosis (arrow) ((H): healthy cells), 400×. (E) Hepatic infarction showing complete loss of liver architecture (arrow), 400×.
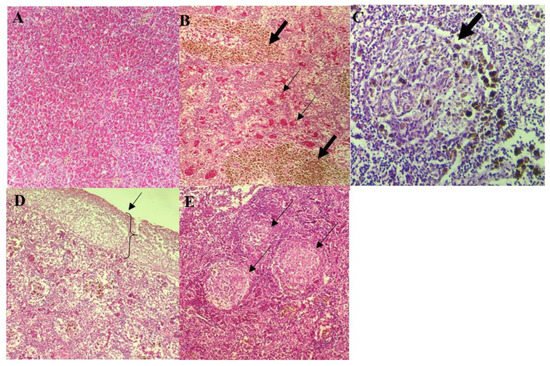
Normal splenic architecture was observed in control group with presence of numerous erythrocytes in the red pulp and mildly scattered MMC (Figure 3A). On the other hand, the spleens of infected fish appeared congested involving multiple blood vessels with numerous MMC (Figure 3B), and infiltration of inflammatory cells associated with multifocal areas of splenic necrosis (Figure 3C). Different Aeromonas spp. were observed to produce different severity of lesions, ranging from mild to severe. Spleens of fish infected by A. hydrophila 8TK3 showed complete loss of splenic architecture (Figure 3D). Fish infected with A. jandaei 7KL3 showed white pulp hypoplasia, affecting small multifocal areas (Figure 3E). In general, fish infected with A. hydrophila 8TK3 showed most severe splenic lesion (p < 0.05), followed by A. dhakensis 4PS2, A. dhakensis 1P11S3, A. veronii 6TS5, A. caviae 7X11 and A. jandaei 7KL3.
Figure 3. Histological findings of the spleen of control and infected red hybrid tilapias by Aeromonas spp. (A) Normal spleen of control fish showing normal splenic architecture, 200×. (B) Presence of abundant MMC (thick arrow) and multiple areas of splenic vascular congestion (thin arrow), 400×. (C) Severe, focal area of necrosis (arrow), with the presence of MMC, 400×. (D) Severe necrosis observed mainly at the splenic surface. 200×. (E) Multifocal areas of splenic white pulp hypoplasia (arrow), 200×.
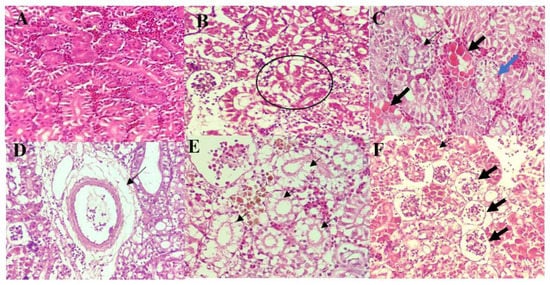
The kidneys of control fish showed normal architecture with intact, healthy tubular epithelia (Figure 4A). However, all groups of infected fish showed varying degree of tubular epithelial and glomerular degeneration and necrosis (Figure 4B–D). Necrosis of tubular epithelial cells was seen as either desquamation from the basement membrane or karyolysis of the nucleus (Figure 4C). The latter was only observed in fish infected by A. hyrophila 8TK3, A. dhakensis 4PS2, and A. caviae 7X11. Fish infected by A. jandaei 7KL3 showed perivascular oedema, accompanied by necrotic tubular epithelium and infiltration of inflammatory cells (Figure 4D). These lesions were not seen in fish infected by other species of Aeromonas. Additionally, the tubular epithelial changes were more severe among these groups (A. dhakensis 4PS2 and A. hydrophila 8TK3), where complete lysis of tubular epithelial cells was frequently observed (Figure 4E). All fish infected with Aeromonas spp. showed desquamation (Figure 4F) and atrophied glomerulus. Cumulatively, histopathological changes in the kidney were most severe in fish infected with A. dhakensis 4PS2 and A. hydrophila 8TK3 (p < 0.05), followed by A. jandaei 7KL3, A. dhakensis 1P11S3, A. veronii 6TS5, and A. caviae 7X11.
Figure 4. Histological findings of the kidney of control and infected red hybrid tilapias by Aeromonas spp. (A) Kidney of control fish showing normal and intact renal epithelial cells, 400×. (B) Hydropic degeneration (circle) of tubular epithelium, 400×. (C) Karyolysis and severe cytoplasmic eosinophilia of the tubular epithelial cells (thick arrow), with detachment of tubular epithelium from the basement membrane (thin arrow), and severe vacuolation (blue arrow), 400×. (D) Dilution of tissue surrounding a blood vessel suggestive of perivascular oedema, 400×. (E) Severe lysis of tubular epithelium (thin arrow), 400×. (F) Necrosis of glomerulus (thick arrow) and karyolysis of tubular epithelial cells (thin arrow), 400×.
The result of all histopathological scoring of liver, spleen and kidney was presented in Table 2. In summary, the most severe histopathological changes were observed in liver (A. hydrophila 8TK3), spleen (A. hydrophila 8TK3) and kidney (A. hydrophila 8TK3 and A. dhakensis 4PS2) of the infected fish. The myo-inositol utilizing A. dhakensis 1P11S3 caused mild to moderate histopathological changes in liver, spleen and kidney of the red hybrid tilapia.
Table 2. Summary of histopathological changes scoring in liver, spleen and kidney of red hybrid tilapias following experimental infection by Aeromonas spp.
| Species |
Organ |
| Liver |
Spleen |
Kidney |
| A. dhakensis 1P11S3 (107 CFU/mL) * |
1.0 ± 0.0 a |
2.0 ± 0.1 a |
1.0 ± 0.0 a |
| A. dhakensis 4PS2 (107 CFU/mL) |
2.0 ± 0.1 c |
2.0 ± 0.0 a |
3.0 ± 0.0 c |
| A. hydrophila 8TK3 (107 CFU/mL) |
3.0 ± 0.0 b |
3.0 ± 0.0 b |
3.0 ± 0.0 b |
| A. veronii 6TS5 (107 CFU/mL) |
1.0 ± 0.0 a |
2.0 ± 0.0 a |
1.0 ± 0.1 a |
| A. caviae 7X11 (107 CFU/mL) |
1.0 ± 0.0 a |
2.0 ± 0.0 a |
1.0 ± 0.0 a |
| A. jandaei 7KL3 (107 CFU/mL) |
1.0 ± 0.0 a |
1.1 ± 0.1 c |
2.1 ± 0.1 d |
| Control (sterile TSB) |
0.0 ± 0.0 d |
0.0 ± 0.0 d |
0.0 ± 0.0 e |