
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Carine Le Goff | + 1809 word(s) | 1809 | 2021-12-28 07:17:22 | | | |
| 2 | Yvaine Wei | + 2 word(s) | 1811 | 2022-01-11 10:44:16 | | |
Video Upload Options
Extracellular matrix (ECM) undergoes remodeling processes to regulate vascular smooth muscle and endothelial cells’ proliferation, differentiation, and adhesion. Abnormalities affecting the ECM can lead to alteration in cellular behavior and from this, this can conduce to the development of pathologies. Metalloproteases play a key role in maintaining the homeostasis of ECM by mediating the cleavage of different ECM components. There are different types of metalloproteases: matrix metalloproteinases (MMPs), disintegrin and metalloproteinases (ADAMs), and ADAMs with thrombospondin motifs (ADAMTSs). ADAMTSs have been found to participate in cardiovascular physiology and diseases and specifically in aortic aneurysms. This entry aims to decipher the potential role of ADAMTS proteins in the physiopathologic development of Thoracic Aortic Aneurysms (TAA) and Abdominal Aortic Aneurysms (AAA).
1. Introduction
An aortic aneurysm (AA) is a dilatation that occurs in the aorta, a major blood vessel that comes out of the heart and carries blood throughout the body. Two types of aortic aneurysms can transpire: (1) abdominal aortic aneurysms (AAA), which occur in the descending aorta in the abdomen, and (2) thoracic aortic aneurysms (TAA) which occur in the aortic section of the chest cavity (ascending, cross, and early descending).
Beyond cellular components such as smooth muscle cells, fibroblasts, endothelial cells, and immune cells, the aortic extracellular matrix (ECM) has a crucial role in maintaining homeostasis and the physiopathological mechanisms of thoracic aortic aneurysms and dissections (TAAD). Almost all the thickness of the aorta is made up of ECM proteins, such as fibrillar proteins (e.g., collagens, fibrillins, and elastin), proteoglycans (e.g., heparan-sulfates glycoproteins and perlecan), and metalloproteases. ECM proteins, components of vessel walls, are in permanent replacement due to a consistent turnover of proteins with the help of metalloproteases, specifically matrix metalloproteases (MMP) and a disintegrin and metalloproteases with thrombospondin motifs (ADAMTS). The hallmarks of TAA are the progressive degradation of the media due to the decreased integrity of elastic fibers, increased elastolysis activity (through metalloproteases), and TGF-β signaling pathway overactivation [1].
ADAMTS Proteins
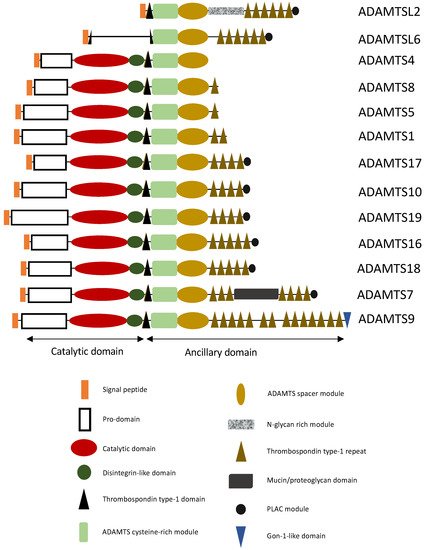
2. ADAMTS/ADAMTSL and TAA
2.1. THSD4/ADAMTSL6 Mutations Responsible for TAA
2.2. Proteoglycanases Involved in TAA
3. ADAMTS Linked to AAA
3.1. ADAMTS8
3.2. ADAMTS9
3.3. ADAMTS7
4. ADAMTSL2, ADAMTS10 and 17 Possibly Involved in Aorta Pathology
5. ADAMTS18 Involved in Aorta Development
6. ADAMTS19 Implicated in Valve Development
References
- Milewicz, D.M.; Ramirez, F. Therapies for Thoracic Aortic Aneurysms and Acute Aortic Dissections: Old Controversies and New Opportunities. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 126–136.
- Dubail, J.; Apte, S.S. Insights on ADAMTS Proteases and ADAMTS-like Proteins from Mammalian Genetics. Matrix Biol. 2015, 44–46, 24–37.
- Apte, S.S. A Disintegrin-like and Metalloprotease (Reprolysin-Type) with Thrombospondin Type 1 Motif (ADAMTS) Superfamily: Functions and Mechanisms. J. Biol. Chem. 2009, 284, 31493–31497.
- Majerus, E.M.; Zheng, X.; Tuley, E.A.; Sadler, J.E. Cleavage of the ADAMTS13 Propeptide Is Not Required for Protease Activity. J. Biol. Chem. 2003, 278, 46643–46648.
- Koo, B.-H.; Longpré, J.-M.; Somerville, R.P.T.; Alexander, J.P.; Leduc, R.; Apte, S.S. Regulation of ADAMTS9 Secretion and Enzymatic Activity by Its Propeptide. J. Biol. Chem. 2007, 282, 16146–16154.
- Somerville, R.P.T.; Jungers, K.A.; Apte, S.S. Discovery and Characterization of a Novel, Widely Expressed Metalloprotease, ADAMTS10, and Its Proteolytic Activation. J. Biol. Chem. 2004, 279, 51208–51217.
- Koo, B.-H.; Apte, S.S. Cell-Surface Processing of the Metalloprotease Pro-ADAMTS9 Is Influenced by the Chaperone GRP94/Gp96. J. Biol. Chem. 2010, 285, 197–205.
- Wang, W.-M.; Ge, G.; Lim, N.H.; Nagase, H.; Greenspan, D.S. TIMP-3 Inhibits the Procollagen N-Proteinase ADAMTS-2. Biochem. J. 2006, 398, 515–519.
- Kashiwagi, M.; Tortorella, M.; Nagase, H.; Brew, K. TIMP-3 Is a Potent Inhibitor of Aggrecanase 1 (ADAM-TS4) and Aggrecanase 2 (ADAM-TS5). J. Biol. Chem. 2001, 276, 12501–12504.
- Tortorella, M.D.; Arner, E.C.; Hills, R.; Easton, A.; Korte-Sarfaty, J.; Fok, K.; Wittwer, A.J.; Liu, R.-Q.; Malfait, A.-M. A2-Macroglobulin Is a Novel Substrate for ADAMTS-4 and ADAMTS-5 and Represents an Endogenous Inhibitor of These Enzymes. J. Biol. Chem. 2004, 279, 17554–17561.
- Yamamoto, K.; Owen, K.; Parker, A.E.; Scilabra, S.D.; Dudhia, J.; Strickland, D.K.; Troeberg, L.; Nagase, H. Low Density Lipoprotein Receptor-Related Protein 1 (LRP1)-Mediated Endocytic Clearance of a Disintegrin and Metalloproteinase with Thrombospondin Motifs-4 (ADAMTS-4). J. Biol. Chem. 2014, 289, 6462–6474.
- Tsutsui, K.; Manabe, R.; Yamada, T.; Nakano, I.; Oguri, Y.; Keene, D.R.; Sengle, G.; Sakai, L.Y.; Sekiguchi, K. ADAMTSL-6 Is a Novel Extracellular Matrix Protein That Binds to Fibrillin-1 and Promotes Fibrillin-1 Fibril Formation. J. Biol. Chem. 2010, 285, 4870–4882.
- Cikach, F.S.; Koch, C.D.; Mead, T.J.; Galatioto, J.; Willard, B.B.; Emerton, K.B.; Eagleton, M.J.; Blackstone, E.H.; Ramirez, F.; Roselli, E.E.; et al. Massive Aggrecan and Versican Accumulation in Thoracic Aortic Aneurysm and Dissection. JCI Insight 2018, 3, e97167.
- Santamaria, S.; Martin, D.R.; Dong, X.; Yamamoto, K.; Apte, S.S.; Ahnström, J. Post-Translational Regulation And Proteolytic Activity Of The Metalloproteinase Adamts8. J. Biol. Chem. 2021, 297, 101323.
- Omura, J.; Satoh, K.; Kikuchi, N.; Satoh, T.; Kurosawa, R.; Nogi, M.; Ohtsuki, T.; Al-Mamun, M.E.; Siddique, M.A.H.; Yaoita, N.; et al. ADAMTS8 Promotes the Development of Pulmonary Arterial Hypertension and Right Ventricular Failure: A Possible Novel Therapeutic Target. Circ. Res. 2019, 125, 884–906.
- Nandadasa, S.; Kraft, C.M.; Wang, L.W.; O’Donnell, A.; Patel, R.; Gee, H.Y.; Grobe, K.; Cox, T.C.; Hildebrandt, F.; Apte, S.S. Secreted Metalloproteases ADAMTS9 and ADAMTS20 Have a Non-Canonical Role in Ciliary Vesicle Growth during Ciliogenesis. Nat. Commun. 2019, 10, 953.
- Kern, C.B.; Wessels, A.; McGarity, J.; Dixon, L.J.; Alston, E.; Argraves, W.S.; Geeting, D.; Nelson, C.M.; Menick, D.R.; Apte, S.S. Reduced Versican Cleavage Due to Adamts9 Haploinsufficiency Is Associated with Cardiac and Aortic Anomalies. Matrix Biol. 2010, 29, 304–316.
- Gäbel, G.; Northoff, B.H.; Weinzierl, I.; Ludwig, S.; Hinterseher, I.; Wilfert, W.; Teupser, D.; Doderer, S.A.; Bergert, H.; Schönleben, F.; et al. Molecular Fingerprint for Terminal Abdominal Aortic Aneurysm Disease. J. Am. Heart Assoc. 2017, 6, e006798.
- Liu, C.; Kong, W.; Ilalov, K.; Yu, S.; Xu, K.; Prazak, L.; Fajardo, M.; Sehgal, B.; Di Cesare, P.E.; Liu, C.; et al. ADAMTS-7: A Metalloproteinase That Directly Binds to and Degrades Cartilage Oligomeric Matrix Protein. FASEB J. 2006, 20, 988–990.
- Riessen, R.; Fenchel, M.; Chen, H.; Axel, D.I.; Karsch, K.R.; Lawler, J. Cartilage Oligomeric Matrix Protein (Thrombospondin-5) Is Expressed by Human Vascular Smooth Muscle Cells. Arterioscler. Thromb. Vasc. Biol. 2001, 21, 47–54.
- Wang, L.; Zheng, J.; Bai, X.; Liu, B.; Liu, C.; Xu, Q.; Zhu, Y.; Wang, N.; Kong, W.; Wang, X. ADAMTS-7 Mediates Vascular Smooth Muscle Cell Migration and Neointima Formation in Balloon-Injured Rat Arteries. Circ. Res. 2009, 104, 688–698.
- Bauer, R.C.; Tohyama, J.; Cui, J.; Cheng, L.; Yang, J.; Zhang, X.; Ou, K.; Paschos, G.K.; Zheng, X.L.; Parmacek, M.S.; et al. Knockout of Adamts7, a Novel Coronary Artery Disease Locus in Humans, Reduces Atherosclerosis in Mice. Circulation 2015, 131, 1202–1213.
- Kessler, T.; Zhang, L.; Liu, Z.; Yin, X.; Huang, Y.; Wang, Y.; Fu, Y.; Mayr, M.; Ge, Q.; Xu, Q.; et al. ADAMTS-7 Inhibits Re-Endothelialization of Injured Arteries and Promotes Vascular Remodeling Through Cleavage of Thrombospondin-1. Circulation 2015, 131, 1191–1201.
- Le Goff, C.; Cormier-Daire, V. The ADAMTS(L) Family and Human Genetic Disorders. Hum. Mol. Genet. 2011, 20, R163–R167.
- Spranger, J.; Gilbert, E.; Tuffli, G.; Rossiter, F.; Opitz, J. Geleophysic Dwarfism—A “Focal” Mucopolysaccharidosis ? Lancet 1971, 298, 97–98.
- Le Goff, C.; Morice-Picard, F.; Dagoneau, N.; Wang, L.W.; Perrot, C.; Crow, Y.J.; Bauer, F.; Flori, E.; Prost-Squarcioni, C.; Krakow, D.; et al. ADAMTSL2 Mutations in Geleophysic Dysplasia Demonstrate a Role for ADAMTS-like Proteins in TGF-β Bioavailability Regulation. Nat. Genet. 2008, 40, 1119–1123.
- Allali, S.; Le Goff, C.; Pressac-Diebold, I.; Pfennig, G.; Mahaut, C.; Dagoneau, N.; Alanay, Y.; Brady, A.F.; Crow, Y.J.; Devriendt, K.; et al. Molecular Screening of ADAMTSL2 Gene in 33 Patients Reveals the Genetic Heterogeneity of Geleophysic Dysplasia. J. Med. Genet. 2011, 48, 417–421.
- Ye, S.; Yang, N.; Lu, T.; Wu, T.; Wang, L.; Pan, Y.-H.; Cao, X.; Yuan, X.; Wisniewski, T.; Dang, S.; et al. Adamts18 Modulates the Development of the Aortic Arch and Common Carotid Artery. iScience 2021, 24, 102672.
- Lu, T.; Lin, X.; Pan, Y.-H.; Yang, N.; Ye, S.; Zhang, Q.; Wang, C.; Zhu, R.; Zhang, T.; Wisniewski, T.M.; et al. ADAMTS18 Deficiency Leads to Pulmonary Hypoplasia and Bronchial Microfibril Accumulation. iScience 2020, 23, 101472.
- Wünnemann, F.; MIBAVA Leducq Consortium Principal Investigators; Ta-Shma, A.; Preuss, C.; Leclerc, S.; van Vliet, P.P.; Oneglia, A.; Thibeault, M.; Nordquist, E.; Lincoln, J.; et al. Loss of ADAMTS19 Causes Progressive Non-Syndromic Heart Valve Disease. Nat. Genet. 2020, 52, 40–47.




