Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Antonio Ruggiero | + 1945 word(s) | 1945 | 2022-01-07 06:50:44 | | | |
| 2 | Nora Tang | + 105 word(s) | 2050 | 2022-01-07 10:05:51 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Ruggiero, A. Peripheral Neuropathy Associated with Dinutuximab in Neuroblastoma Patients. Encyclopedia. Available online: https://encyclopedia.pub/entry/17862 (accessed on 08 February 2026).
Ruggiero A. Peripheral Neuropathy Associated with Dinutuximab in Neuroblastoma Patients. Encyclopedia. Available at: https://encyclopedia.pub/entry/17862. Accessed February 08, 2026.
Ruggiero, Antonio. "Peripheral Neuropathy Associated with Dinutuximab in Neuroblastoma Patients" Encyclopedia, https://encyclopedia.pub/entry/17862 (accessed February 08, 2026).
Ruggiero, A. (2022, January 07). Peripheral Neuropathy Associated with Dinutuximab in Neuroblastoma Patients. In Encyclopedia. https://encyclopedia.pub/entry/17862
Ruggiero, Antonio. "Peripheral Neuropathy Associated with Dinutuximab in Neuroblastoma Patients." Encyclopedia. Web. 07 January, 2022.
Copy Citation
Neuroblastoma is the most common extracranial solid tumor of childhood, with a median age at diagnosis of 17 months. Its incidence is 10.2 cases per million children aged <15 years. Neuroblastoma arises in tissues of the sympathetic nervous system, mostly in the adrenal medulla or paraspinal ganglia. It appears as a mass in the abdomen, pelvis, neck, or chest, with about half of the patients having metastatic disease at diagnosis. The presence of metastatic diseases over the age of 12 or 18 months and aggressive biological features (e.g., MYCN oncogene amplification) define high-risk neuroblastoma. The prognosis for such patients is poor, with a long-term survival rate of only 40%.
dinutuximab
neuropathic pain
peripheral neuropathy
neuroblastoma
pediatric cancer
molecular mechanisms
treatment
1. Dinutuximab Induced Neuropathic Pain
Neuropathic pain affects from 33% to 88% of pediatric patients undergoing dinutuximab infusion. Pain usually manifests as allodynia, which involves various regions of the body, especially the abdomen, extremities, back, and chest, and then spreads peripherally to the ankles and feet [1][2][3][4][5][6]. Anti-GD2 induced pain is characterized by mechanical allodynia without thermal hyperalgesia [7].
It usually begins within an hour from the start of dinutuximab beta infusion and is limited to the time of administration of this drug, ending shortly after the termination of the infusion; it usually occurs during the first infusion of the drug and decreases after each course [8]. A retrospective study on 26 patients affected by high-risk neuroblastoma who received dinutuximab based immunotherapy concluded that grade ≥3 (of a 1 to 5 scale) pain occurs in about 88% of patients during immunotherapy course 1, but in 42% of patients during course 5 [4].
GD2-induced neuropathic pain is mediated by the reactivity of the antibody with the GD2 antigen on the surface of peripheral nerve fibers, particularly C-fibers [7]. The inability of GD2 antibodies to cross the blood brain barrier implicates the involvement of the peripheral nervous system [7].
The pain-inducing mechanism is unclear. It probably involves the same immune response by ADCC and CDC elicited for treatment; the antigen–anti-GD2-antibody complex on the GD2-expressing nerve fibers is thought to be the first trigger for pain (Figure 1a–c).
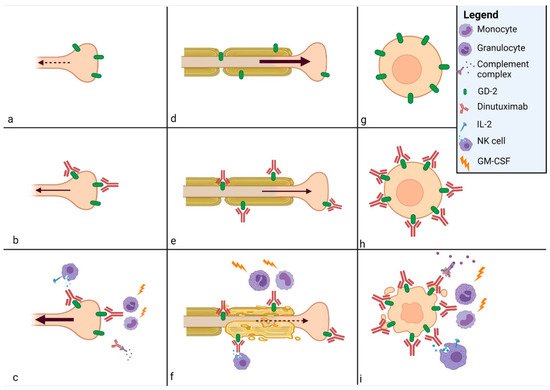
Figure 1. Mechanisms of action of dinutuximab beta. GD2 is expressed on (a) nociceptive fiber, (d) motor and sensory fibers, and (g) neuroblastoma cell membrane. The complex antigen–anti-GD2-antibody is formed on the surface of (b) nociceptive fiber, (e) motor and sensory fibers, and (h) neuroblastoma cell membrane. The complex antigen GD2-antibody activates the antibody-dependent cellular cytotoxicity and the complement-dependent cyto-toxicity, which cause (c) neuropathic pain on nociceptive fiber, (f) peripheral neuropathy on motor and sensory fibers, and (i) apoptosis of neuroblastoma cell. IL-2 acts on Natural Killer (NK) cells and granulocyte-macrophage colony-stimulating factor (GM-CSF) on monocytes and granulocytes to enhance the immunological response.
Antibodies and immune cell products from the antibody–antigen response generate ectopic activity in axons, which results in hyperalgesia and spontaneous pain [9].
It is possible that patients who benefit from dinutuximab treatment are those with a high percentage of neuroblastoma cells that are GD2 positive [10]. Nevertheless, not all patients with progressive neuroblastoma will have high expression of GD2 on tumor cells [11]. Thus, such patients may have toxicity with no benefit from dinutuximab. Furthermore, the selective pressure of dinutuximab therapy may result in decreased GD2 expression, which has already been observed with targeting CD20 on lymphoma with rituximab, targeting CD19 on leukemia with CAR-T cells, and with antibodies to EGF in breast cancer [12][13][14].
Prevention and Treatment
Because of the high frequency of neuropathic pain during dinutuximab beta infusion, various strategies of prevention and treatment have been evaluated.
First, a continuous 10-day infusion appeared more tolerable than the discontinuous 5-day infusions on consecutive days. Mueller et al. compared data from 53 patients with high-risk neuroblastoma who received continuous 10-day infusions of dinutuximab beta with those of 226 patients from the study by Yu et al. who received discontinuous once-daily infusions of dinutuximab on 4 consecutive days [1][15]. The incidence of treatment-related adverse events was lower with the continuous infusion than with the once-daily infusion; particularly, the incidence of neuropathic pain decreased from 51.8% of patients under once-daily infusion to 37.7% of patients under continuous infusion.
Furthermore, as one of the mechanisms of generation of neuropathic pain seems to be the complement activation at the GD2-expressing nerve fibers level [16], humanized anti-GD2 antibodies (hu14.18K322A) have been shown to resolve pain more rapidly, through reduction of complement activation, even though opioid requirements were not reduced [2][17]. Moreover, initial data show some complete responses in the treatment of recurrent or refractory neuroblastoma, but a randomized trial is needed to determine if the elimination of complement binding may maintain anti-GD2 activity in addition to decreasing neuropathic pain [18][19].
Aggressive pain control is such a priority that the U.S. Food and Drug Administration recommends permanent discontinuation of dinutuximab in patients with severe pain that is uncontrollable by analgesic therapy.
Because neuropathic pain usually occurs at the beginning of the treatment, its onset can be predicted and premedication with analgesics should be administered. Triple therapy with gabapentin, non-opioid analgesics, and opioids is recommended for pain treatment [8] (Figure 2). Three days prior to dinutuximab beta infusion, oral gabapentin administration should be started at the dose of 10 mg/kg/day. The next day, the dose is increased to 10 mg/kg for two daily administrations and the next day to 10 mg/kg for three daily administrations (the maximum single dose of gabapentin must not exceed 300 mg). Gabapentin reverses the tactile allodynia that follows anti-GD2 administration; its administration should be continued as long as required by the patient and be tapered off up to suspension after weaning off intravenous morphine infusion [9].
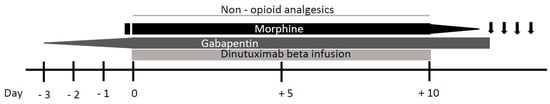
Figure 2. Neuropathic pain management during continuous dinutuximab beta infusion. Gabapentin administration is started three days prior to dinutuximab beta infusion and increased up to 10 mg/kg for three daily administrations. After a bolus, morphine is commenced just before dinutuximab beta infusion and continued as a 24 h intravenous infusion (0.03 mg/kg/h). Following reduction of its infusion rate, morphine is stopped 4 h after the end of dinutuximab beta infusion. If neuropathic pain persist after the intravenous morphine weaning off, oral morphine sulphate or tramadol can be administered on demand.
Non-opioid analgesics such as paracetamol or ibuprofen should be used during the treatment.
The use of opioids for the duration of antibody therapy given as continuous infusion over 10 days is essential for pain control, with higher opioid doses given in the first infusion day and course than in subsequent days and courses. Morphine should be commenced 2 h before dinutuximab beta infusion with a bolus of 0.02–0.05 mg/kg. Subsequently, continuous intravenous morphine infusion should be started at a dosing rate of 0.03 mg/kg/h and continued during all dinutuximab beta infusion. In response to patient’s pain perception, it may be necessary to increase the infusion rate or, on the other hand, it could be possible to wean off morphine over 5 days, progressively decreasing its dosing rate. If continuous morphine infusion is required for more than 5 days, treatment should be gradually reduced by 20% per day after the last day of dinutuximab beta infusion.
In the case of neuropathic pain persisting after the intravenous morphine is weaned off, oral morphine sulphate (0.2–0.4 mg/kg every 4–6 h) or tramadol (for moderate neuro-pathic pain) can be administered on demand.
For subsequent courses, analgesic therapy at the highest dose of opioid required for adequate pain control during the prior course should be considered at the start and then modulated according to the severity of pain [20].
Other analgesic regimens were experimented but, to our knowledge, the previous is the best strategy for prevention and treatment of dinutuximab beta-related neuropathic pain. Opioid transdermal delivery system has not been utilized as it is not adjustable on the basis of patients’ variable pain intensity [21][22].
The concurrent administration of low-dose lidocaine has been shown to reduce opioid consumption in neuroblastoma patients treated with immunotherapy, but it determined a very high incidence of vomiting and may not be appropriate in the ward setting [9]. Gorges and colleagues studied the use of dexmedetomidine and hydromorphone to manage the pain associated with anti-GD2 infusion. They reported adverse effects such as hypotension, hypoxemia, and bradycardia in 30%, 8%, and 4% of treatment days, respectively [23]. Bertolizio et al. retrospectively analyzed the efficacy of a multimodal regimen with gabapentin, ketamine, and morphine for preventing and treating neuropathic pain during dinutuximab therapy [24]. Even if the addition of ketamine to opioids is known to decrease morphine consumption and pain, this study showed higher morphine consumption and a lower incidence of moderate and severe pain with fewer adverse effects in comparison with data by Georges et al.; this may be due to avoidance of dexmedetomidine [23][25].
2. Dinutuximab Induced Peripheral Neuropathy
Peripheral neuropathy of any kind results from a partial loss of innervation and it can also lead to chronic pain. Neuropathy is due to a physical, metabolic, or chemical injury of axons that determines a disconnection of nerve branches from their terminals.
In a normal pathway, these injuries lead to Wallerian degeneration and apoptosis. Then, dedifferentiation of resident Schwann cells and the infiltration of systemic immune cells open the way for axonal regeneration and nerve repair. If the balance between degeneration and regeneration is interrupted, chronic pain may begin [26][27] (Figure 1d–f).
Peripheral neuropathy is a common side effect of chemotherapeutic agents, such as oxaliplatin and vincristine, through different mechanisms. Oxaliplatin accumulates in dorsal root ganglion neurons and causes functional changes in nerve excitability to undergo severe neuropathy [28]. Vincristine determines a compromission of microtubule function and axonal transport with mitochondrial toxicity [29][30]. Another pathway observed in the chemotherapeutic drugs neurotoxicity is the stimulation of NK cells by upregulation of stress related proteins in tumor and/or downregulation of inhibitory self-ligands on the target cell surface [31][32][33].
Peripheral neuropathy is a rare side effect of dinutuximab beta administration; it can be severe with a prevalence of between 2 and 6% of patients [4]. Ladenstein et al. reported seven patients with grade 3 and 4 toxicities, the most frequent being paresthesia or deficits in motor function; one patients with tetraparesis improved over time, but did not recover completely [34]. Mody et al. described one patient treated with irinotecan, temozolomide, and dinutuximab that presented with grade 3 peripheral motor neuropathy started on day 6 of cycle 6. He suffered a bilateral lower extremity weakness and was unable to walk independently for 4 weeks, but completely recovered after 2 weeks [35]. One patient with grade 3 peripheral motoneuropathy was reported by Mueller et al., while another patient with peripheral neurotoxicity, which did not resolve completely, was seen by Blom et al. [4][15].
The mechanisms of anti-GD2 neurotoxicity remain unclear. It is certain that anti-GD2 antibodies bind the GD2 antigen localized on the cell membrane of axons of peripheral nerves and affect its function in vivo [36][37]. Subsequently, activated by anti-GD2 antibodies via ADCC, NK cells seem to have a potential pathogenic role by forming an immunological synapse with the axon of a sensory neuron and thus releasing ions and proteases into the target neuron; this would lead to axon microtubule destabilization and axon degeneration [38].
A sensorimotor polyneuropathy associated with demyelination and a mononuclear infiltrate in both endoneurial and perivascular space of sural nerve was documented in adults after treatment of metastatic melanomas with an anti-GD2 monoclonal antibody [39].
A study of Yuki et al. tried to clarify what causes the neurotoxicity of anti-GD2 monoclonal antibody. They showed that anti-GD2 reacts with the peripheral nerve myelin and assumed that this binding causes antibody-dependent cell mediated and complement-dependent demyelination, resulting in the development of sensorimotor demyelinating polyneuropathy [39].
As anti-GD2 modified to reduce complement activation determined the reduction but not the elimination of side effects on peripheral nervous system in a rat model, other mechanisms are probably involved, such as an immune-mediated inflammation [40][16][18][19]. However, the complete mechanism needs to be clarified.
Treatment
If dinutuximab beta infusion determines motor or sensory peripheral neuropathy that lasts for more than 4 days, a non-inflammatory cause should be considered and excluded, as it could be expression of neuroblastoma progression, infection, metabolic syndrome, and concomitant medication.
Exams like magnetic resonance and motor and sensory electromyography studies should be conducted.
To the best of our knowledge, there are no studies that specifically address the treatment of dinutuximab induced peripheral neuropathy. In patients with grade 2 neuropathy, dinutuximab beta infusion should be interrupted, to be resumed only after neurologic symptoms resolve. Infusion must be discontinued if subjects present any objective prolonged weakness possibly as a result of dinutuximab beta administration [8][41]. If symptoms do not improve after a few days, steroid therapy should be considered.
References
- Yu, A.L.; Gilman, A.L.; Ozkaynak, M.F.; London, W.B.; Kreissman, S.G.; Chen, H.X.; Smith, M.; Anderson, B.; Villablanca, J.G.; Matthay, K.K.; et al. Anti-GD2 Antibody with GM-CSF, Interleukin-2, and Isotretinoin for Neuroblastoma. N. Engl. J. Med. 2010, 363, 1324–1334.
- Anghelescu, D.L.; Bs, J.L.G.; Faughnan, L.; Wu, J.; Mao, S.; Furman, W.L.; Santana, V.M.; Navid, F. Comparison of pain outcomes between two anti-GD2 antibodies in patients with neuroblastoma. Pediatr. Blood Cancer 2015, 62, 224–228.
- Gilman, A.L.; Ozkaynak, M.F.; Matthay, K.K.; Krailo, M.; Yu, A.L.; Gan, J.; Sternberg, A.; Hank, J.A.; Seeger, R.; Reaman, G.H.; et al. Phase I Study of Ch14.18 with Granulocyte-Macrophage Colony-Stimulating Factor and Interleukin-2 in Children with Neuroblastoma after Autologous Bone Marrow Transplantation or Stem-Cell Rescue: A Report from the Children’s Oncology Group. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2009, 27, 85–91.
- Blom, T.; Lurvink, R.; Aleven, L.; Mensink, M.; Wolfs, T.; Dierselhuis, M.; van Eijkelenburg, N.; Kraal, K.; van Noesel, M.; van Grotel, M.; et al. Treatment-Related Toxicities During Anti-GD2 Immunotherapy in High-Risk Neuroblastoma Patients. Front. Oncol. 2021, 10, 601076.
- Ozkaynak, M.F.; Sondel, P.M.; Krailo, M.D.; Gan, J.; Javorsky, B.; Reisfeld, R.A.; Matthay, K.K.; Reaman, G.H.; Seeger, R.C. Phase I Study of Chimeric Human/Murine Anti-Ganglioside G(D2) Monoclonal Antibody (Ch14.18) with Granulocyte-Macrophage Colony-Stimulating Factor in Children with Neuroblastoma Immediately after Hematopoietic Stem-Cell Transplantation: A Children’s Cancer Group Study. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2000, 18, 4077–4085.
- Simon, T.; Hero, B.; Faldum, A.; Handgretinger, R.; Schrappe, M.; Niethammer, D.; Berthold, F. Consolidation Treatment with Chimeric Anti-GD2-Antibody ch14.18 in Children Older Than 1 Year with Metastatic Neuroblastoma. J. Clin. Oncol. 2004, 22, 3549–3557.
- Sorkin, L.S.; Yu, A.L.; Junger, H.; Doom, C.M. Antibody Directed against GD(2) Produces Mechanical Allodynia, but Not Thermal Hyperalgesia When Administered Systemically or Intrathecally despite Its Dependence on Capsaicin Sensitive Afferents. Brain Res. 2002, 930, 67–74.
- Qarziba (Dinutuximab Beta)—Summary of Product Characteristics (SmPC)—(Emc). Available online: https://www.medicines.org.uk/emc/product/9441/smpc (accessed on 28 September 2021).
- Sorkin, L.S. Antibody Activation and Immune Reactions: Potential Linkage to Pain and Neuropathy. Pain Med. 2000, 1, 296–302.
- Terzic, T.; Cordeau, M.; Herblot, S.; Teira, P.; Cournoyer, S.; Beaunoyer, M.; Peuchmaur, M.; Duval, M.; Sartelet, H. Expression of Disialoganglioside (GD2) in Neuroblastic Tumors: A Prognostic Value for Patients Treated with Anti-GD2 Immunotherapy. Pediatr. Dev. Pathol. 2018, 21, 355–362.
- Schumacher-Kuckelkorn, R.; Volland, R.; Gradehandt, A.; Hero, B.; Simon, T.; Berthold, F. Lack of immunocytological GD2 expression on neuroblastoma cells in bone marrow at diagnosis, during treatment, and at recurrence. Pediatr. Blood Cancer 2016, 64, 46–56.
- Duman, B.B.; Sahin, B.; Ergin, M.; Güvenç, B. Loss of CD20 antigen expression after rituximab therapy of CD20 positive B cell lymphoma (diffuse large B cell extranodal marginal zone lymphoma combination): A case report and review of the literature. Med. Oncol. 2011, 29, 1223–1226.
- Sotillo, E.; Barrett, D.M.; Black, K.L.; Bagashev, A.; Oldridge, D.A.; Wu, G.; Sussman, R.T.; LaNauze, C.; Ruella, M.; Gazzara, M.R.; et al. Convergence of Acquired Mutations and Alternative Splicing of CD19 Enables Resistance to CART-19 Immunotherapy. Cancer Discov. 2015, 5, 1282–1295.
- Mukohara, T. Mechanisms of resistance to anti-human epidermal growth factor receptor 2 agents in breast cancer. Cancer Sci. 2010, 102, 1–8.
- Mueller, I.; Ehlert, K.; Endres, S.; Pill, L.; Siebert, N.; Kietz, S.; Brock, P.; Garaventa, A.; Valteau-Couanet, D.; Janzek, E.; et al. Tolerability, response and outcome of high-risk neuroblastoma patients treated with long-term infusion of anti-GD2 antibody ch14.18/CHO. mAbs 2018, 10, 55–61.
- Sorkin, L.S.; Otto, M.; Baldwin, W.M.; Vail, E.; Gillies, S.D.; Handgretinger, R.; Barfield, R.C.; Yu, H.M.; Yu, A.L. Anti-GD2 with an FC point mutation reduces complement fixation and decreases antibody-induced allodynia. Pain 2010, 149, 135–142.
- Ahmed, M.; Cheung, N.-K.V. Engineering anti-GD2 monoclonal antibodies for cancer immunotherapy. FEBS Lett. 2014, 588, 288–297.
- Navid, F.; Sondel, P.M.; Barfield, R.; Shulkin, B.L.; Kaufman, R.A.; Allay, J.A.; Gan, J.; Hutson, P.; Seo, S.; Kim, K.; et al. Phase I Trial of a Novel Anti-GD2 Monoclonal Antibody, Hu14.18K322A, Designed to Decrease Toxicity in Children with Refractory or Recurrent Neuroblastoma. J. Clin. Oncol. 2014, 32, 1445–1452.
- Federico, S.M.; McCarville, M.B.; Shulkin, B.L.; Sondel, P.M.; Hank, J.A.; Hutson, P.; Meagher, M.; Shafer, A.; Ng, C.Y.; Leung, W.; et al. A Pilot Trial of Hu-manized Anti-GD2 Monoclonal Antibody (Hu14.18K322A) with Chemotherapy and Natural Killer Cells in Children with Re-current/Refractory Neuroblastoma. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2017, 23, 6441–6449.
- Ozkaynak, M.F.; Gilman, A.L.; London, W.B.; Naranjo, A.; Diccianni, M.B.; Tenney, S.C.; Smith, M.; Messer, K.S.; Seeger, R.; Reynolds, C.P.; et al. A Comprehensive Safety Trial of Chimeric Antibody 14.18 With GM-CSF, IL-2, and Isotretinoin in High-Risk Neuroblastoma Patients Following Myeloablative Therapy: Children’s Oncology Group Study ANBL0931. Front. Immunol. 2018, 9, 1355.
- Mastrangelo, S.; Capozza, M.A.; Triarico, S.; Attinà, G.; Maurizi, P.; Romano, A.; Ruggiero, A. Opioid transdermal delivery system: A useful method for pain management in children. Ann. Transl. Med. 2021, 9, 185.
- Attinà, G.; Romano, A.; Triarico, S.; Mastrangelo, S.; Maurizi, P.; Ruggiero, A. Transdermal buprenorphine for pain management in children. Drugs Context 2021, 10, 1–8.
- Görges, M.; West, N.; Deyell, R.; Winton, P.; Cheung, W.; Lauder, G. Dexmedetomidine and Hydromorphone: A Novel Pain Management Strategy for the Oncology Ward Setting during Anti-GD2 Immunotherapy for High-Risk Neuroblastoma in Children. Pediatr. Blood Cancer 2015, 62, 29–34.
- Bertolizio, G.; Otis, A.; Tam, K.; Aswar, S.; Garbin, M.; Ingelmo, P. Multimodal Analgesic Plan for Children Undergoing Chimeric 14.18 Immunotherapy. J. Pediatr. Hematol. Oncol. 2021, 43, e169–e172.
- Sheehy, K.A.; Muller, E.A.; Lippold, C.; Nouraie, M.; Finkel, J.C.; Quezado, Z.M.N. Subanesthetic ketamine infusions for the treatment of children and adolescents with chronic pain: A longitudinal study. BMC Pediatr. 2015, 15, 198.
- Garrovillo, K.; Garrett, J.; Bollin, K.; Nasraty, F.; Sikand, H. Dinutuximab in adult-onset chemotherapy refractory high-risk neuroblastoma. J. Oncol. Pharm. Pract. 2020, 26, 2058–2065.
- Davies, A.J.; Kim, H.W.; Gonzalez-Cano, R.; Choi, J.; Back, S.K.; Roh, S.E.; Johnson, E.; Gabriac, M.; Kim, M.-S.; Lee, J.; et al. Natural Killer Cells Degenerate Intact Sensory Afferents following Nerve Injury. Cell 2019, 176, 716–728.
- Park, S.B.; Lin, C.S.-Y.; Krishnan, A.; Goldstein, D.; Friedlander, M.L.; Kiernan, M.C. Oxaliplatin-induced neurotoxicity: Changes in axonal excitability precede development of neuropathy. Brain 2009, 132, 2712–2723.
- Triarico, S.; Romano, A.; Attinà, G.; Capozza, M.A.; Maurizi, P.; Mastrangelo, S.; Ruggiero, A. Vincristine-Induced Peripheral Neuropathy (VIPN) in Pediatric Tumors: Mechanisms, Risk Factors, Strategies of Prevention and Treatment. Int. J. Mol. Sci. 2021, 22, 4112.
- Fukuda, Y.; Li, Y.; Segal, R.A. A Mechanistic Understanding of Axon Degeneration in Chemotherapy-Induced Peripheral Neuropathy. Front. Neurosci. 2017, 11, 481.
- Zingoni, A.; Fionda, C.; Borrelli, C.; Cippitelli, M.; Santoni, A.; Soriani, A. Natural Killer Cell Response to Chemotherapy-Stressed Cancer Cells: Role in Tumor Immunosurveillance. Front. Immunol. 2017, 8, 1194.
- Fine, J.H.; Chen, P.; Mesci, A.; Allan, D.S.; Gasser, S.; Raulet, D.; Carlyle, J.R. Chemotherapy-Induced Genotoxic Stress Promotes Sensitivity to Natural Killer Cell Cytotoxicity by Enabling Missing-Self Recognition. Cancer Res. 2010, 70, 7102–7113.
- Xiao, W.-H.; Yu, A.L.; Sorkin, L.S. Electrophysiological characteristics of primary afferent fibers after systemic administration of anti-GD2 ganglioside antibody. Pain 1997, 69, 145–151.
- Ladenstein, R.; Pötschger, U.; Valteau-Couanet, D.; Luksch, R.; Castel, V.; Yaniv, I.; Laureys, G.; Brock, P.; Michon, J.M.; Owens, C.; et al. Interleukin 2 with Anti-GD2 Antibody Ch14.18/CHO (Dinutuximab Beta) in Patients with High-Risk Neuroblastoma (HR-NBL1/SIOPEN): A Multicentre, Randomised, Phase 3 Trial. Lancet Oncol. 2018, 19, 1617–1629.
- Mody, R.; Naranjo, A.; Van Ryn, C.; Yu, A.; London, W.B.; Shulkin, B.; Parisi, M.T.; Servaes, S.-E.-N.; Diccianni, M.B.; Sondel, P.M.; et al. Irinotecan–temozolomide with temsirolimus or dinutuximab in children with refractory or relapsed neuroblastoma (COG ANBL1221): An open-label, randomised, phase 2 trial. Lancet Oncol. 2017, 18, 946–957.
- Slart, R.; Yu, A.L.; Yaksh, T.L.; Sorkin, L.S. An Animal Model of Pain Produced by Systemic Administration of an Immuno-therapeutic Anti-Ganglioside Antibody. Pain 1997, 69, 119–125.
- Saleh, M.N.; Khazaeli, M.B.; Wheeler, R.H.; Dropcho, E.; Liu, T.; Urist, M.; Miller, D.M.; Lawson, S.; Dixon, P.; Russell, C.H. Phase I trial of the murine monoclonal anti-GD2 antibody 14G2a in metastatic melanoma. Cancer Res. 1992, 52, 4342–4347.
- Davies, A.J.; Rinaldi, S.; Costigan, M.; Oh, S.B. Cytotoxic Immunity in Peripheral Nerve Injury and Pain. Front. Neurosci. 2020, 14, 142.
- Yuki, N.; Yamada, M.; Tagawa, Y.; Takahashi, H.; Handa, S. Pathogenesis of the neurotoxicity caused by anti-GD2 antibody therapy. J. Neurol. Sci. 1997, 149, 127–130.
- Ohmi, Y.; Ohkawa, Y.; Yamauchi, Y.; Tajima, O.; Furukawa, K.; Furukawa, K. Essential Roles of Gangliosides in the Formation and Maintenance of Membrane Microdomains in Brain Tissues. Neurochem. Res. 2012, 37, 1185–1191.
- De Bernardi, B.; Quaglietta, L.; Haupt, R.; Castellano, A.; Tirtei, E.; Luksch, R.; Mastrangelo, S.; Viscardi, E.; Indolfi, P.; Cellini, M.; et al. Neuroblastoma with symptomatic epidural compression in the infant: The AIEOP experience. Pediatr. Blood Cancer 2014, 61, 1369–1375.
More
Information
Subjects:
Oncology
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.6K
Revisions:
2 times
(View History)
Update Date:
07 Jan 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




