Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Masfique Mehedi | + 1885 word(s) | 1885 | 2021-12-27 02:50:57 | | | |
| 2 | Peter Tang | Meta information modification | 1885 | 2022-01-05 02:45:10 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Mehedi, M. ARP2/3 Complex-Driven Actin Polymerization in RSV Infection. Encyclopedia. Available online: https://encyclopedia.pub/entry/17746 (accessed on 07 February 2026).
Mehedi M. ARP2/3 Complex-Driven Actin Polymerization in RSV Infection. Encyclopedia. Available at: https://encyclopedia.pub/entry/17746. Accessed February 07, 2026.
Mehedi, Masfique. "ARP2/3 Complex-Driven Actin Polymerization in RSV Infection" Encyclopedia, https://encyclopedia.pub/entry/17746 (accessed February 07, 2026).
Mehedi, M. (2022, January 04). ARP2/3 Complex-Driven Actin Polymerization in RSV Infection. In Encyclopedia. https://encyclopedia.pub/entry/17746
Mehedi, Masfique. "ARP2/3 Complex-Driven Actin Polymerization in RSV Infection." Encyclopedia. Web. 04 January, 2022.
Copy Citation
Respiratory syncytial virus (RSV) is the leading viral agent causing bronchiolitis and pneumonia in children under five years old worldwide. The RSV infection cycle starts with macropinocytosis-based entry into the host airway epithelial cell membrane, followed by virus transcription, replication, assembly, budding, and spread. It is not surprising that the host actin cytoskeleton contributes to different stages of the RSV replication cycle. RSV modulates actin-related protein 2/3 (ARP2/3) complex-driven actin polymerization for a robust filopodia induction on the infected lung epithelial A549 cells, which contributes to the virus’s budding, and cell-to-cell spread.
cytoskeleton dynamics
filopodia
ARP2/3 complex
actin polymerization
cell-to-cell spread
RSV
bronchiolitis
therapeutics
1. Introduction
Microorganisms, including viruses, use the host cell’s cytoskeleton to destabilize the host cell’s physiological mechanisms to allow for the virus’s survival and aid its pathogenesis. Most bacteria and viruses utilize the host cytoskeleton for multiple activities, including attachment, invasion, movement within and between cells, and replication, resulting in disease progression [1][2][3]. Actin microfilaments are unique among the cellular cytoskeletons, as they are composed of a highly dynamic network of actin polymers. Host cells contain actin-associated proteins that modulate cell migration, contraction, and shape changes during the cell cycle and in response to extracellular stimuli [4][5][6]. During a microbial attack, the induction of macropinocytosis, phagocytosis, membrane ruffling, vacuole formation, and vacuole remodeling depends on signaling the actin cytoskeleton [4][5][6]. Pathogens, like viruses and bacteria, have different strategies to hijack the host cell machinery to promote their replicative cycles; specifically, the host cytoskeleton is their common target [3][4].
The ARP2/3 complex, an actin filament nucleating and regulating factor, plays a central role in cellular actin assembly. The complex is an assembly of seven proteins, including actin-related proteins ARP2, ARP3, and five additional subunits called actin-related protein 2/3 complex (ARPC), including p41, p34, p21, p20, and p16 (noted as ARPC 1–5, respectively) (Figure 1) [7].
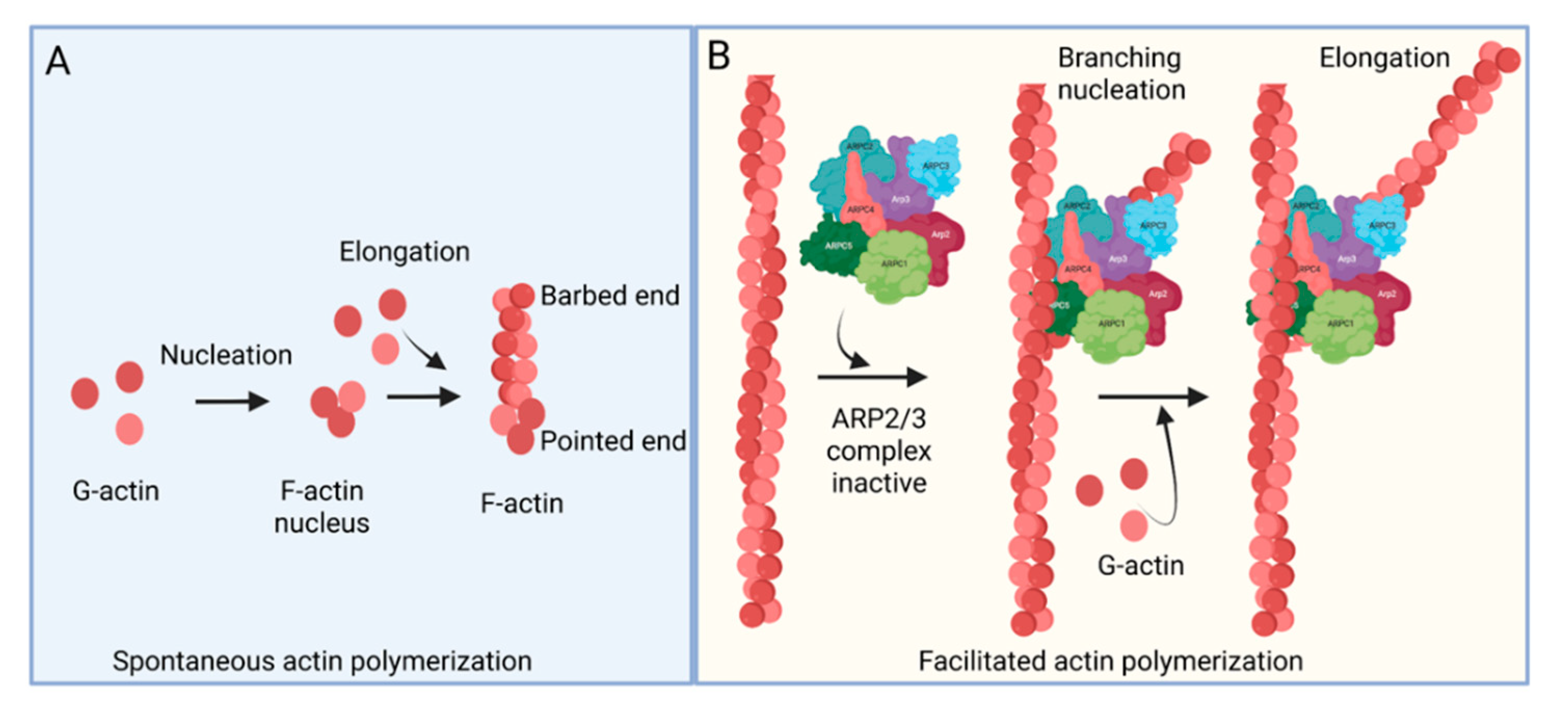
Figure 1. Actin polymerization. (A) Spontaneous actin polymerization. Globular actins (G-actin) form follicular actin (F-actin) nucleus (shown as pointed end). Spontaneous addition of G-actin elongates F-actin at the barbed end. (B) Facilitated actin polymerization. ARP2/3 complex, a seven-protein complex, consists of ARP2, ARP3, ARPC1, ARPC2, ARPC3, ARPC4, and ARPC5. ARP2/3 complex involves branched actin polymerization [8].
Respiratory syncytial virus (RSV) causes severe lower respiratory illnesses, such as bronchiolitis and pneumonia, in children under the age of five. Elderly adults, as well as adults with chronic diseases, are at an increased risk for contracting a severe illness from an RSV infection. Importantly, almost everyone has been infected by RSV by the time they reach two years of age. RSV infection primarily causes common cold-like symptoms that progress to lower respiratory tract disease in 40 percent of infected infants [9]. RSV belongs to the Pneumoviridae virus family. It is a negative-sense, single-stranded, non-segmented RNA virus. Its genome consists of 10 genes that encode 11 proteins. The proteins encoded by RSV are nonstructural protein 1(NS1), NS2, nucleoprotein (N), phosphoprotein (P), matrix (M), short hydrophobic (SH), glycoprotein (G), fusion (F), M2-1 and M2-2, and RNA-dependent RNA polymerase (L) [10]. The two main proteins that are widely studied for anti-viral drug discovery are F and G protein. F protein is a surface glycoprotein that is involved in the RSV infection. G protein attaches to the host cell receptor [11]. F protein enables the virion membrane to fuse with the host cell membrane [12]. Upon entering the host cell, RSV undergoes transcription, translation, and replication in the host cytoplasm [10]. During transcription, the viral polymerase starts mRNA synthesis for all genes from 3′ to 5′ end of the genome [13]. Importantly, RSV 3′ to 5′ end genes undergo a higher to lower transcription gradient [13][14]. All protein-specific mRNAs are translated by host cell translational machinery [10]. N protein is involved in creating a template for RNA synthesis and P protein is a polymerase co-factor [10]. M protein is involved in the inhibition of host transcription and is associated with viral inclusion bodies [15]. M2-1 is a transcription processivity factor and M2-2 regulates RNA synthesis [16][17]. NS1 and NS2 proteins are non-structural proteins that are involved in various processes including interfering with the innate immune response and inhibiting apoptosis [18][19]. SH protein is a transmembrane protein near the N-terminus involved in various processes including the inhibition of TNF signaling and reducing apoptosis [20]. The virus is assembled at the cell surface with viral proteins and genomic RNA. The assembled new virion budded out from the surface of the cell [10]. RSV attachment to cell membrane activated various signaling cascades like Epidermal growth factor (EGF), cell division protein 42 (Cdc42) which led to actin rearrangement and increased fluid uptake which results in RSV uptake by macropinocytosis, as shown in Figure 2. Actin rearrangement plays an important role in RSV entry, as treatment with cytochalasin D and latrunculin A disrupt actin filament and reduce RSV infection in HeLa cells [21]. Previously, it has also shown that the cytoskeleton protein actin is involved in RSV endocytosis, replication, gene expression, and morphogenesis (Figure 2) [22][23][24][25]. It has recently been shown that ARP2/3 and virus-induced filopodia contribute to RSV cell-to-cell spread (Figure 2 and Figure 3) [26][27][28].
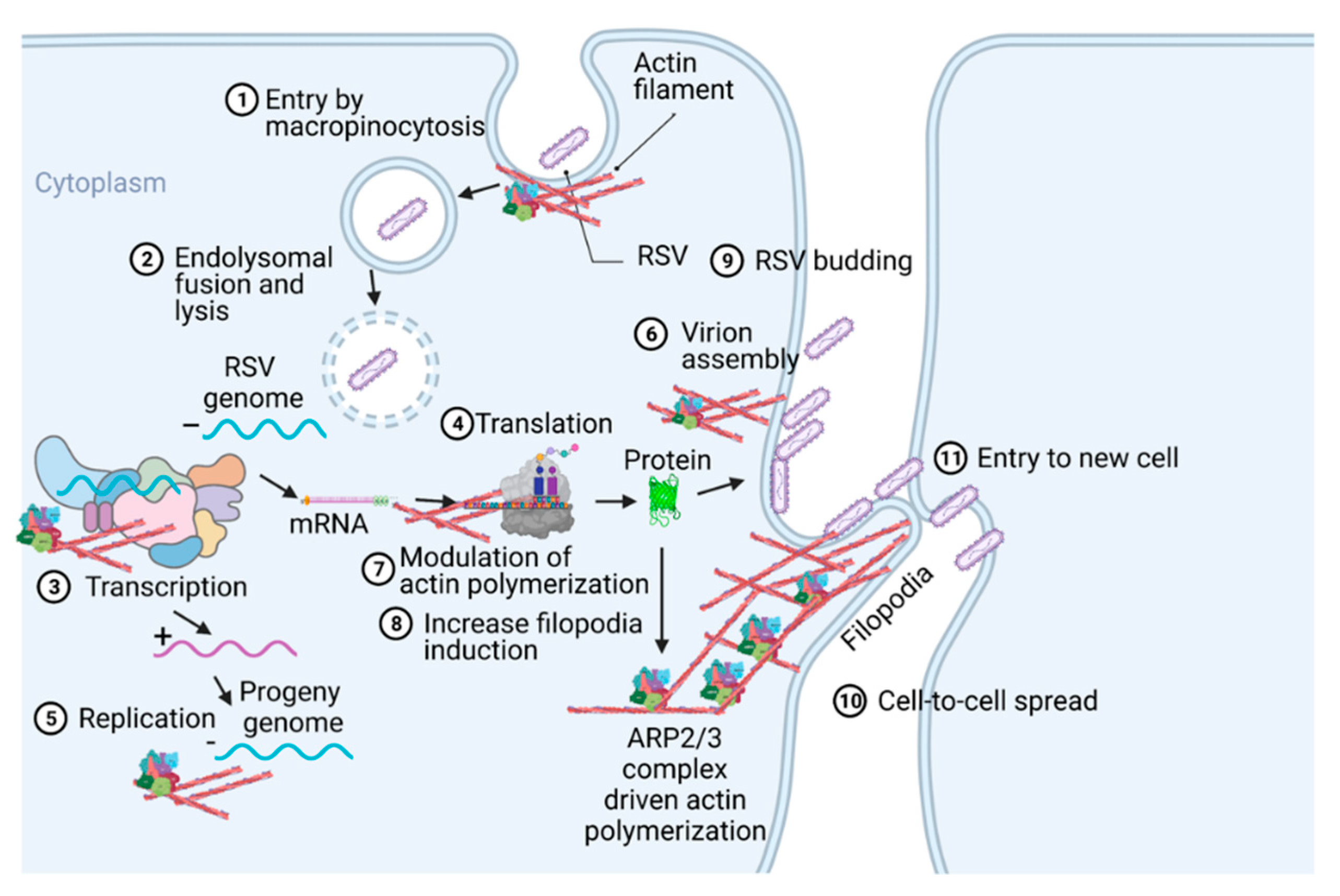
Figure 2. A pictogram of a replicative cycle of RSV. Different steps of RSV replicative cycle (including potential actin involvement) are indicated chronologically.
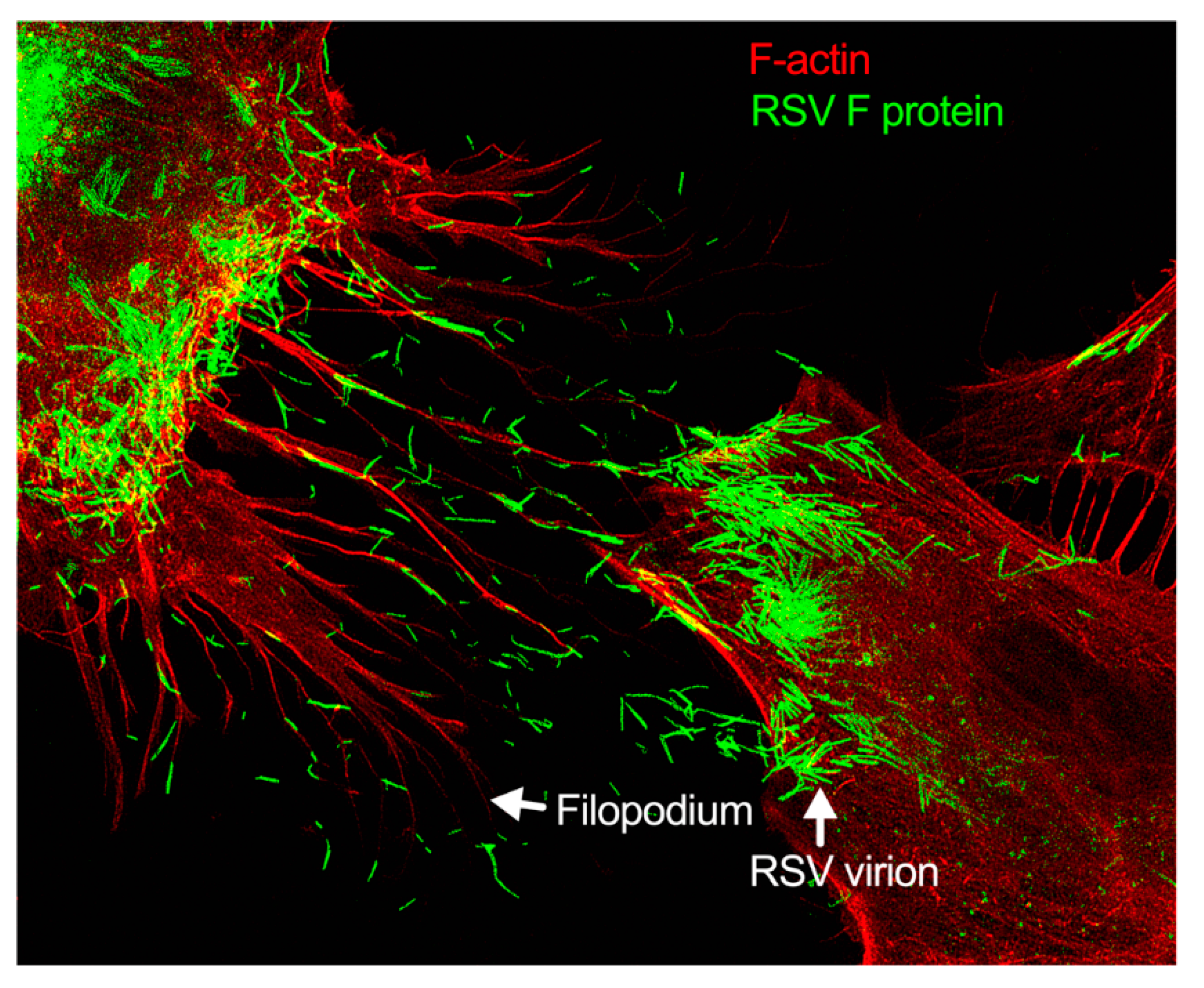
Figure 3. Filopodia-driven RSV cell-to-cell spread. Human lung airway epithelial cell line, A549 cells, were infected with RSV wild type (RSV-WT) (Strain A) at a multiplicity of infection (MOI) of 1 for 24 h. The infected cells were then fixed, permeabilized, and stained for RSV fusion (F) protein using antibody specific to F. F-actin and the nucleus were stained with rhodamine phalloidin and DAPI, respectively. The image was taken under a stimulated emission depletion (STED) microscope (Leica Microsystem) [27][28].
2. Pathogen-Induced Subversion of Cytoskeleton
The actin cytoskeleton is critically important to maintaining cellular morphology and motility. Pathogens co-opt the actin restructuring machinery of the host cell to access or create a favorable environment for their replication. Viruses are known to stimulate a rearrangement of the host cell actin cytoskeleton during the infection of the cell [29]. The actin cytoskeleton can control endocytosis and phagocytosis, as well as cell contraction, motility, and division [30]. The cytoskeleton’s capacity to continuously assemble and disassemble allows it to take on many roles [29]. Pathogens manipulate the cytoskeleton to drive cellular infection due to its role of being a major host structural component [31]. A pathogen will utilize effector proteins to hijack the cytoskeleton to successfully infect and replicate [31]. For example, actin reorganization is stimulated during microbial entry by binding with CR3 receptors in macrophage [32]. In contrast, translocated actin recruiting phosphoprotein (TARP) in non-phagocytic cell facilitates entry by promoting actin nucleation [33]. Cortactin, which is an actin regulatory protein, is also important for the entry in non-phagocytic cells [34]. ARP2/3 complex regulation by Src and PI-3 kinase were shown in bacterial internalization [35]. Actin-mediated propulsion is also necessary for cell-to-cell spread but not always dependent on N-WASP and ARP2/3 complex [36]. The ARP2/3 complex, as well as pathogen-induced filopodia, are used as mechanisms to subvert the cytoskeleton by stimulating actin assembly in the host cell [37]. The mechanism of the ARP2/3 complex is utilized in the formation of branched actin, while the activation of Cdc42 plays a role in the development and formation of filopodium [38]. Thus, various pathogens exploit the host cell actin cytoskeleton by implementing mechanisms to subvert it [37].
3. Role of Cytoskeleton in RSV Infection Cycle
The host cytoskeleton is involved in various stages of the RSV infectious cycle (Figure 2 and Figure 3). RSV interacts with multiple cellular and cytoskeletal proteins during infection. Nucleolin located in the apical cell surface interacts with RSV glycoproteins which is important for RSV entry [39]. The cytoskeletal proteins, e.g., actin and microtubules, are implicated in the RSV transcription, assembly, and budding [40]. F protein cytoplasmic tail and M protein are responsible for viral assembly, specifically, F protein interacts with inclusion bodies which in turn facilitate the release of M protein-ribonucleoprotein complex from inclusion bodies [41]. RSV M protein interacts with actin which is important for budding and responsible for the virion particle transport [42]. Cytochalasin D inhibits actin polymerization, which reduces the production of viral particles [23]. RSV also increases the polymerization of actin, which results in cytoplasmic extension in the infected cells [22]. Profilin, an actin modulatory protein, is also essential for RSV transcription [43]. Apart from actin and profilin, actin-associated signaling pathways like RhoA, PI3K, and Rac GTPase are involved in the production of virus filaments [44][45][46]. Microtubules play a role in both assembly and release of the virus but have a greater impact on the assembly [25]. Higher expression of RhoA and pMLC2 were observed during RSV infection which induced stress fiber formation and ROCK inhibitor Y-27632 showed the inhibition of RhoA activity [47].
Generally, respiratory viruses enter the human via the lung epithelia. This can prove challenging, however, as most of the lung epithelia are lined with a protective coating of mucus. The few cells that are not lined with mucus are guarded by macrophages. This has made particle release, the common method of viral spread, disadvantageous for these types of viruses. To avoid these pitfalls, respiratory viruses have most likely evolved novel mechanisms of spreading cell-to-cell. RSV modulates cytoskeletal rearrangement and actin remodeling for its replicative cycle in vitro [25], particularly ARP2/3 complex-regulated filopodia formation for its cell-to-cell spread [26][27]. When A549 cells are infected with RSV, the cell forms an actin-based projection called filopodia (Figure 2 and Figure 3) [26][27]. Filopodia are finger-like projections comprised of polymerized actin. The free barbed ends of the actin filament can add additional actin monomers, which leads to actin polymerization [48]. The formation of the filopodia is activated by Cdc42, a protein involved in the regulation of proliferation in the cell division cycle [49][50][51][52].
4. Role of Filopodia in RSV Cell-to-Cell Spread
Filopodia are typically involved in activities such as migration and wound healing. Sites of filopodia formation tend to have increased amounts of the protein katanin [53]. Katanin, in these increased amounts, has been linked to more aggressive migratory behaviors in prostate cancer cells [54]. Cells using directional migration also use their filopodia to establish a single polarity axis. The cell’s filopodium serve as the leading process for directional migration [55][56][57]. Filopodia can form multiple protrusions branching out from the filopodia (or the lamellipodia), and these branches are often able to be used by the cell to make directional decisions in chemotaxis and pathfinding [58][59]. However, herein lies the difference between the cell’s filopodium and the cell’s lamellipodium: the role of the lamellipodium tends to be more reserved for migratory purposes. The thin sheet protrusion pulls the cell forward. In contrast, filopodia play a role that is less focused on pulling the cell forward but more so on exploring the extracellular environment. The filopodia are less sheet-like and more finger-like [60][61]. In certain cells, filopodia may also play a role in phagocytosis. For example, in certain non-polarized cell types, such as primary human corneal fibroblasts, or a cancerous cell line Chinese hamster ovary (CHO) cells stably expressing nectin-1, HSV-1 entry occurs through a mechanism that seems to be “phagocytosis-like”. It includes filopodia-like actin rearrangements and RhoA GTPase activation [62]. Conversely, Clement et al. also showed that two actin-depolymerizing agents—CytoD and latrunculin B (LatB)—are potent inhibitors of this HSV-1 entry in these cell types. Further studies in polarized retinal pigment epithelial (RPE) cells, which have a natural tropism for HSV-1 infection, also revealed the role of actin in facilitating virus entry [62].
During viral infection, viral proteins increase cell migration by disrupting and modulating actin dynamics to form long cellular extensions, like filopodia, and tunneling nanotubes in in vitro models [24].
References
- Nemerow, G.R.; Cheresh, D.A. Herpesvirus hijacks an integrin. Nat. Cell Biol. 2002, 4, E69.
- Smith, G.A.; Enquist, L.W. Break ins and break outs: Viral interactions with the cytoskeleton of mammalian cells. Annu. Rev. Cell Dev. Biol. 2002, 18, 135–161.
- Jimenez, A.; Chen, D.; Alto, N.M. How bacteria subvert animal cell structure and function. Annu. Rev. Cell Dev. Biol. 2016, 32, 373–397.
- Gruenheid, S.; Finlay, B.B. Microbial pathogenesis and cytoskeletal function. Nature 2003, 422, 775.
- Sibley, L.D.; Andrews, N.W. Cell invasion by un-palatable parasites. Traffic 2000, 1, 100–106.
- Terebiznik, M.R.; Vieira, O.V.; Marcus, S.L.; Slade, A.; Yip, C.M.; Trimble, W.S.; Meyer, T.; Finlay, B.B.; Grinstein, S. Elimination of host cell PtdIns (4, 5) P 2 by bacterial SigD promotes membrane fission during invasion by Salmonella. Nat. Cell Biol. 2002, 4, 766.
- Welch, M.D. The world according to Arp: Regulation of actin nucleation by the Arp2/3 complex. Trends Cell Biol. 1999, 9, 423–427.
- Krause, M.; Gautreau, A. Steering cell migration: Lamellipodium dynamics and the regulation of directional persistence. Nat. Rev. Mol. Cell Biol. 2014, 15, 577–590.
- Krause, C.I. The ABCs of RSV. Nurse Pract. 2018, 43, 20–26.
- Collins, P.L.; Fearns, R.; Graham, B.S. Respiratory syncytial virus: Virology, reverse genetics, and pathogenesis of disease. Curr. Top. Microbiol. Immunol. 2013, 372, 3–38.
- Levine, S.; Klaiber-Franco, R.; Paradiso, P.R. Demonstration that glycoprotein G is the attachment protein of respiratory syncytial virus. J. Gen. Virol. 1987, 68, 2521–2524.
- McLellan, J.S.; Ray, W.C.; Peeples, M.E. Structure and function of respiratory syncytial virus surface glycoproteins. Curr. Top. Microbiol. Immunol. 2013, 372, 83–104.
- Collins, P.L.; Wertz, G.W. cDNA cloning and transcriptional mapping of nine polyadenylylated RNAs encoded by the genome of human respiratory syncytial virus. Proc. Natl. Acad. Sci. USA 1983, 80, 3208–3212.
- Krempl, C.; Murphy, B.R.; Collins, P.L. Recombinant respiratory syncytial virus with the G and F genes shifted to the promoter-proximal positions. J. Virol. 2002, 76, 11931–11942.
- Ghildyal, R.; Ho, A.; Jans, D.A. Central role of the respiratory syncytial virus matrix protein in infection. FEMS Microbiol. Rev. 2006, 30, 692–705.
- Bermingham, A.; Collins, P.L. The M2-2 protein of human respiratory syncytial virus is a regulatory factor involved in the balance between RNA replication and transcription. Proc. Natl. Acad. Sci. USA 1999, 96, 11259–11264.
- Fearns, R.; Collins, P.L. Role of the M2-1 transcription antitermination protein of respiratory syncytial virus in sequential transcription. J. Virol. 1999, 73, 5852–5864.
- Spann, K.M.; Tran, K.C.; Collins, P.L. Effects of nonstructural proteins NS1 and NS2 of human respiratory syncytial virus on interferon regulatory factor 3, NF-kappaB, and proinflammatory cytokines. J. Virol. 2005, 79, 5353–5362.
- Bitko, V.; Shulyayeva, O.; Mazumder, B.; Musiyenko, A.; Ramaswamy, M.; Look, D.C.; Barik, S. Nonstructural proteins of respiratory syncytial virus suppress premature apoptosis by an NF-kappaB-dependent, interferon-independent mechanism and facilitate virus growth. J. Virol. 2007, 81, 1786–1795.
- Fuentes, S.; Tran, K.C.; Luthra, P.; Teng, M.N.; He, B. Function of the respiratory syncytial virus small hydrophobic protein. J. Virol. 2007, 81, 8361–8366.
- Krzyzaniak, M.A.; Zumstein, M.T.; Gerez, J.A.; Picotti, P.; Helenius, A. Host cell entry of respiratory syncytial virus involves macropinocytosis followed by proteolytic activation of the F protein. PLoS Pathog. 2013, 9, e1003309.
- Ulloa, L.; Serra, R.; Asenjo, A.; Villanueva, N. Interactions between cellular actin and human respiratory syncytial virus (HRSV). Virus Res. 1998, 53, 13–25.
- Burke, E.; Dupuy, L.; Wall, C.; Barik, S. Role of cellular actin in the gene expression and morphogenesis of human respiratory syncytial virus. Virology 1998, 252, 137–148.
- Taylor, M.P.; Koyuncu, O.O.; Enquist, L.W. Subversion of the actin cytoskeleton during viral infection. Nat. Rev. Microbiol. 2011, 9, 427–439.
- Kallewaard, N.L.; Bowen, A.L.; Crowe, J.E., Jr. Cooperativity of actin and microtubule elements during replication of respiratory syncytial virus. Virology 2005, 331, 73–81.
- Mehedi, M.; Collins, P.L.; Buchholz, U.J. A novel host factor for human respiratory syncytial virus. Commun. Integr. Biol. 2017, 10, e1006062.
- Mehedi, M.; McCarty, T.; Martin, S.E.; Le Nouen, C.; Buehler, E.; Chen, Y.C.; Smelkinson, M.; Ganesan, S.; Fischer, E.R.; Brock, L.G.; et al. Actin-Related Protein 2 (ARP2) and Virus-Induced Filopodia Facilitate Human Respiratory Syncytial Virus Spread. PLoS Pathog. 2016, 12, e1006062.
- Mehedi, M.; Smelkinson, M.; Kabat, J.; Ganesan, S.; Collins, P.L.; Buchholz, U.J. Multicolor stimulated emission depletion (STED) microscopy to generate high-resolution images of respiratory syncytial virus particles and infected cells. Bio-Protocol 2017, 7, e2543.
- Dramsi, S.; Cossart, P. Intracellular pathogens and the actin cytoskeleton. Annu. Rev. Cell Dev. Biol. 1998, 14, 137–166.
- Alto, N.M.; Orth, K. Subversion of cell signaling by pathogens. Cold Spring Harb. Perspect. Biol. 2012, 4, a006114.
- Colonne, P.M.; Winchell, C.G.; Voth, D.E. Hijacking host cell highways: Manipulation of the host actin cytoskeleton by obligate intracellular bacterial pathogens. Front. Cell. Infect. Microbiol. 2016, 6, 107.
- Meconi, S.; Jacomo, V.; Boquet, P.; Raoult, D.; Mege, J.L.; Capo, C. Coxiella burnetii induces reorganization of the actin cytoskeleton in human monocytes. Infect. Immun. 1998, 66, 5527–5533.
- Jewett, T.J.; Fischer, E.R.; Mead, D.J.; Hackstadt, T. Chlamydial TARP is a bacterial nucleator of actin. Proc. Natl. Acad. Sci. USA 2006, 103, 15599–15604.
- Rosales, E.M.; Aguilera, M.O.; Salinas, R.P.; Carminati, S.A.; Colombo, M.I.; Martinez-Quiles, N.; Beron, W. Cortactin is involved in the entry of Coxiella burnetii into non-phagocytic cells. PLoS ONE 2012, 7, e39348.
- Meconi, S.; Capo, C.; Remacle-Bonnet, M.; Pommier, G.; Raoult, D.; Mege, J.L. Activation of protein tyrosine kinases by Coxiella burnetii: Role in actin cytoskeleton reorganization and bacterial phagocytosis. Infect. Immun. 2001, 69, 2520–2526.
- Heinzen, R.A. Rickettsial actin-based motility: Behavior and involvement of cytoskeletal regulators. Ann. N. Y. Acad. Sci. 2003, 990, 535–547.
- Rottner, K.; Stradal, T.E.; Wehland, J. Bacteria-host-cell interactions at the plasma membrane: Stories on actin cytoskeleton subversion. Dev. Cell 2005, 9, 3–17.
- Carabeo, R. Bacterial subversion of host actin dynamics at the plasma membrane. Cell. Microbiol. 2011, 13, 1460–1469.
- Tayyari, F.; Marchant, D.; Moraes, T.J.; Duan, W.; Mastrangelo, P.; Hegele, R.G. Identification of nucleolin as a cellular receptor for human respiratory syncytial virus. Nat. Med. 2011, 17, 1132–1135.
- Shahriari, S.; Gordon, J.; Ghildyal, R. Host cytoskeleton in respiratory syncytial virus assembly and budding. Virol. J. 2016, 13, 161.
- Baviskar, P.S.; Hotard, A.L.; Moore, M.L.; Oomens, A.G. The respiratory syncytial virus fusion protein targets to the perimeter of inclusion bodies and facilitates filament formation by a cytoplasmic tail-dependent mechanism. J. Virol. 2013, 87, 10730–10741.
- Shahriari, S.; Wei, K.J.; Ghildyal, R. Respiratory Syncytial Virus Matrix (M) Protein Interacts with Actin In Vitro and in Cell Culture. Viruses 2018, 10, 535.
- Burke, E.; Mahoney, N.M.; Almo, S.C.; Barik, S. Profilin is required for optimal actin-dependent transcription of respiratory syncytial virus genome RNA. J. Virol. 2000, 74, 669–675.
- Jeffree, C.E.; Brown, G.; Aitken, J.; Su-Yin, D.Y.; Tan, B.H.; Sugrue, R.J. Ultrastructural analysis of the interaction between F-actin and respiratory syncytial virus during virus assembly. Virology 2007, 369, 309–323.
- Gower, T.L.; Peeples, M.E.; Collins, P.L.; Graham, B.S. RhoA is activated during respiratory syncytial virus infection. Virology 2001, 283, 188–196.
- Gower, T.L.; Pastey, M.K.; Peeples, M.E.; Collins, P.L.; McCurdy, L.H.; Hart, T.K.; Guth, A.; Johnson, T.R.; Graham, B.S. RhoA signaling is required for respiratory syncytial virus-induced syncytium formation and filamentous virion morphology. J. Virol. 2005, 79, 5326–5336.
- Linfield, D.T.; Gao, N.; Raduka, A.; Harford, T.J.; Piedimonte, G.; Rezaee, F. RSV attenuates epithelial cell restitution by inhibiting actin cytoskeleton-dependent cell migration. Am. J. Physiol. Lung Cell. Mol. Physiol. 2021, 321, L189–L203.
- Delorme-Axford, E.; Coyne, C.B. The actin cytoskeleton as a barrier to virus infection of polarized epithelial cells. Viruses 2011, 3, 2462–2477.
- Ridley, A.J. Life at the leading edge. Cell 2011, 145, 1012–1022.
- Heasman, S.J.; Ridley, A.J. Mammalian Rho GTPases: New insights into their functions from in vivo studies. Nat. Rev. Mol. Cell Biol. 2008, 9, 690–701.
- Parsons, J.T.; Horwitz, A.R.; Schwartz, M.A. Cell adhesion: Integrating cytoskeletal dynamics and cellular tension. Nat. Rev. Mol. Cell Biol. 2010, 11, 633–643.
- Spiering, D.; Hodgson, L. Dynamics of the Rho-family small GTPases in actin regulation and motility. Cell Adh. Migr. 2011, 5, 170–180.
- Liu, R.; Woolner, S.; Johndrow, J.E.; Metzger, D.; Flores, A.; Parkhurst, S.M. Sisyphus, the Drosophila myosin XV homolog, traffics within filopodia transporting key sensory and adhesion cargos. Development 2008, 135, 53–63.
- Ye, X.; Lee, Y.C.; Choueiri, M.; Chu, K.; Huang, C.F.; Tsai, W.W.; Kobayashi, R.; Logothetis, C.J.; Yu-Lee, L.Y.; Lin, S.H. Aberrant expression of katanin p60 in prostate cancer bone metastasis. Prostate 2012, 72, 291–300.
- Keren, K.; Pincus, Z.; Allen, G.M.; Barnhart, E.L.; Marriott, G.; Mogilner, A.; Theriot, J.A. Mechanism of shape determination in motile cells. Nature 2008, 453, 475–480.
- Ratner, S.; Sherrod, W.S.; Lichlyter, D. Microtubule retraction into the uropod and its role in T cell polarization and motility. J. Immunol. 1997, 159, 1063–1067.
- Theisen, U.; Straube, E.; Straube, A. Directional persistence of migrating cells requires Kif1C-mediated stabilization of trailing adhesions. Dev. Cell 2012, 23, 1153–1166.
- Andrew, N.; Insall, R.H. Chemotaxis in shallow gradients is mediated independently of PtdIns 3-kinase by biased choices between random protrusions. Nat. Cell Biol. 2007, 9, 193–200.
- Cooper, J.A. Cell biology in neuroscience: Mechanisms of cell migration in the nervous system. J. Cell Biol. 2013, 202, 725–734.
- Eilken, H.M.; Adams, R.H. Dynamics of endothelial cell behavior in sprouting angiogenesis. Curr. Opin. Cell Biol. 2010, 22, 617–625.
- Gupton, S.L.; Gertler, F.B. Filopodia: The fingers that do the walking. Sci. STKE 2007, 2007, re5.
- Clement, C.; Tiwari, V.; Scanlan, P.M.; Valyi-Nagy, T.; Yue, B.Y.; Shukla, D. A novel role for phagocytosis-like uptake in herpes simplex virus entry. J. Cell Biol. 2006, 174, 1009–1021.
More
Information
Subjects:
Virology
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
2.7K
Revisions:
2 times
(View History)
Update Date:
05 Jan 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




