Respiratory syncytial virus (RSV) is the leading viral agent causing bronchiolitis and pneumonia in children under five years old worldwide. The RSV infection cycle starts with macropinocytosis-based entry into the host airway epithelial cell membrane, followed by virus transcription, replication, assembly, budding, and spread. It is not surprising that the host actin cytoskeleton contributes to different stages of the RSV replication cycle. RSV modulates actin-related protein 2/3 (ARP2/3) complex-driven actin polymerization for a robust filopodia induction on the infected lung epithelial A549 cells, which contributes to the virus’s budding, and cell-to-cell spread.
- cytoskeleton dynamics
- filopodia
- ARP2/3 complex
- actin polymerization
- cell-to-cell spread
- RSV
- bronchiolitis
- therapeutics
1. Introduction
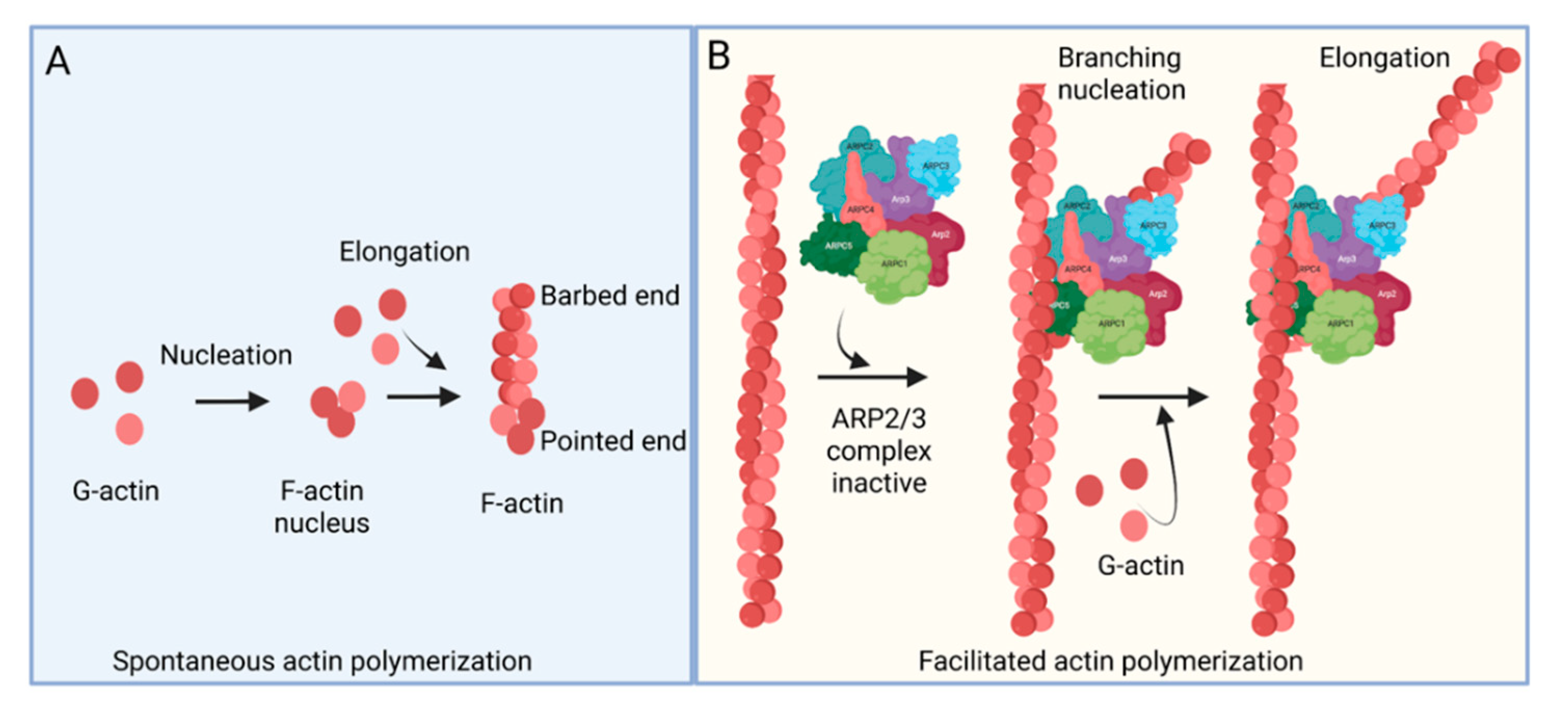
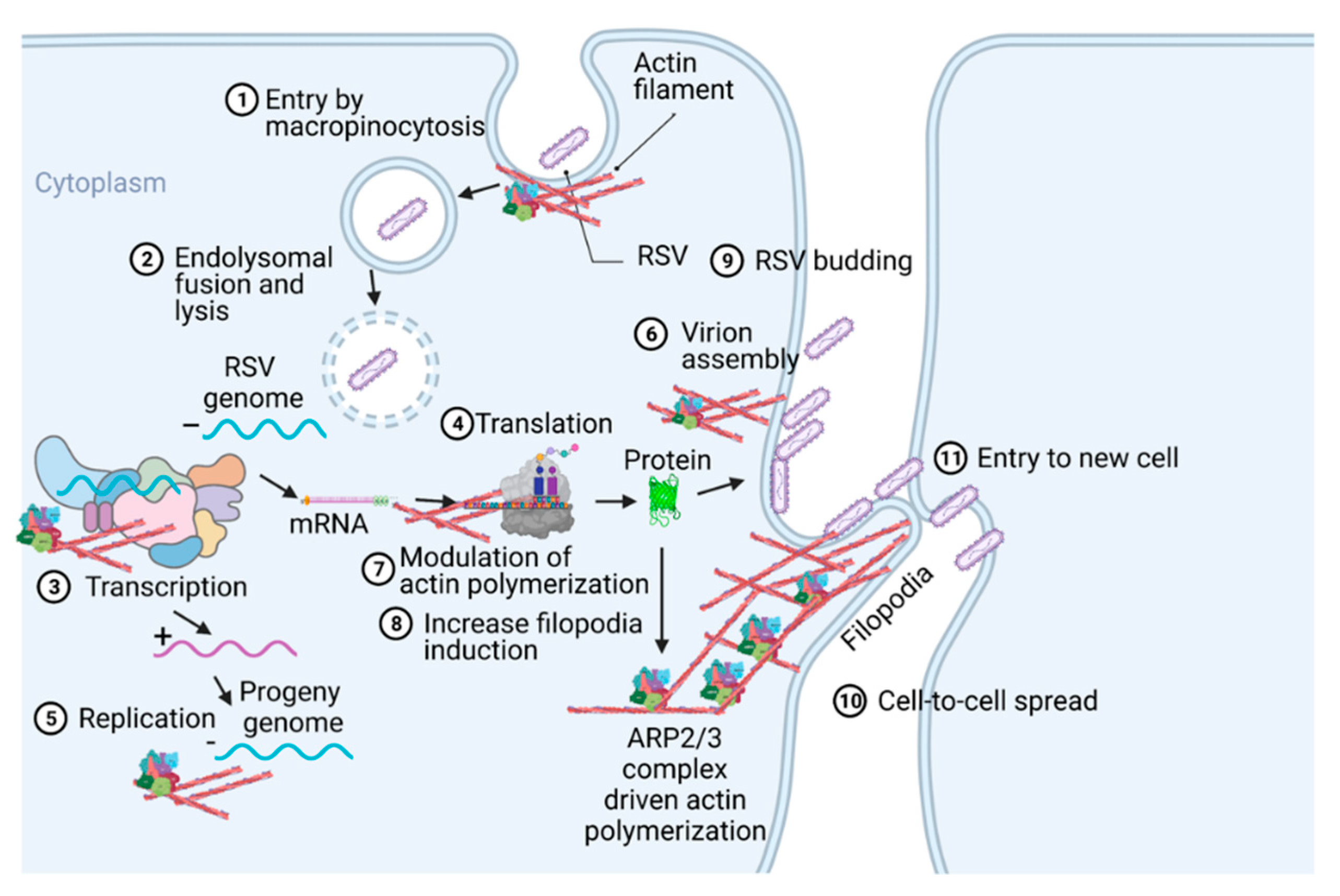
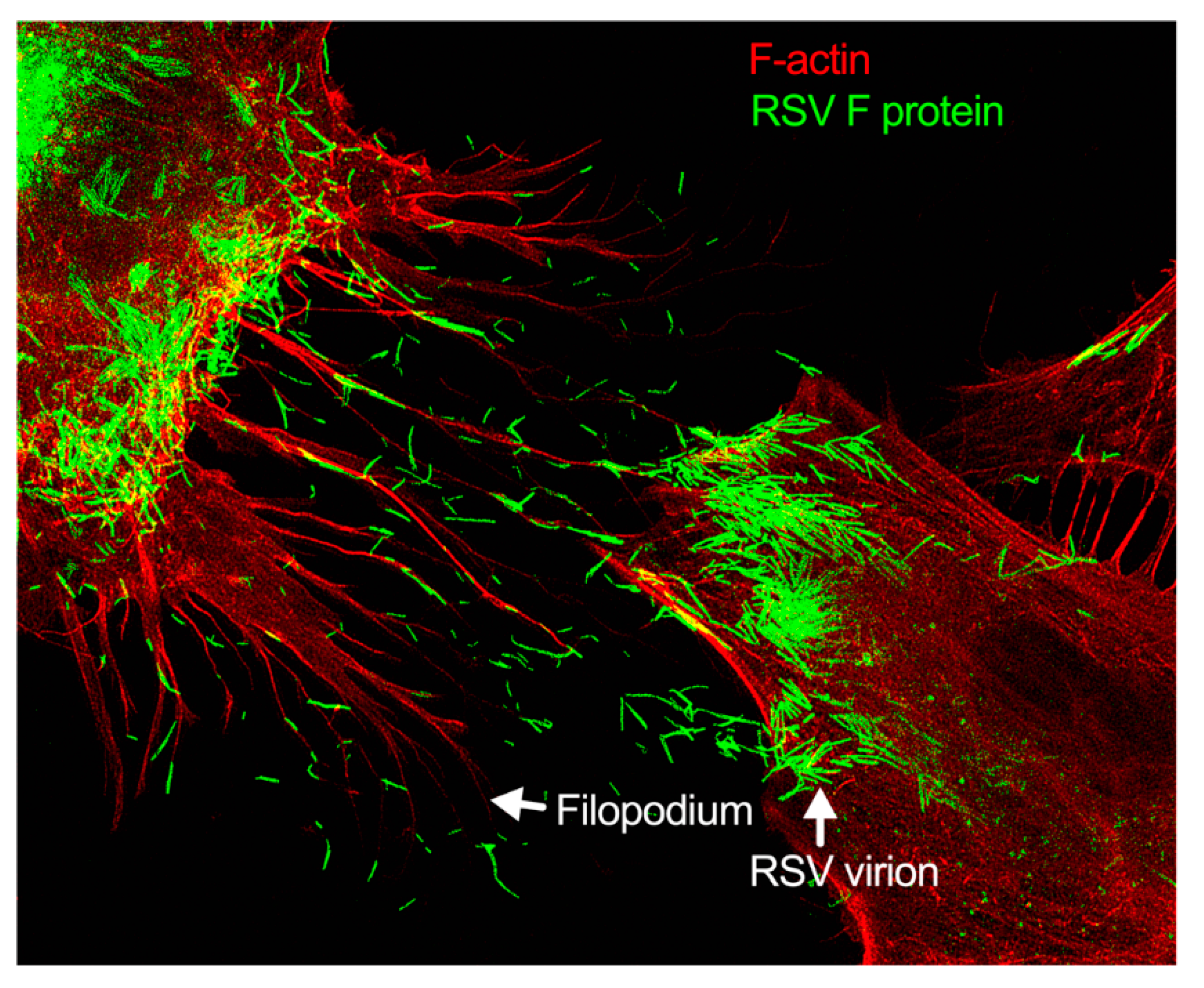
2. Pathogen-Induced Subversion of Cytoskeleton
3. Role of Cytoskeleton in RSV Infection Cycle
4. Role of Filopodia in RSV Cell-to-Cell Spread
This entry is adapted from the peer-reviewed paper 10.3390/pathogens11010026
References
- Nemerow, G.R.; Cheresh, D.A. Herpesvirus hijacks an integrin. Nat. Cell Biol. 2002, 4, E69.
- Smith, G.A.; Enquist, L.W. Break ins and break outs: Viral interactions with the cytoskeleton of mammalian cells. Annu. Rev. Cell Dev. Biol. 2002, 18, 135–161.
- Jimenez, A.; Chen, D.; Alto, N.M. How bacteria subvert animal cell structure and function. Annu. Rev. Cell Dev. Biol. 2016, 32, 373–397.
- Gruenheid, S.; Finlay, B.B. Microbial pathogenesis and cytoskeletal function. Nature 2003, 422, 775.
- Sibley, L.D.; Andrews, N.W. Cell invasion by un-palatable parasites. Traffic 2000, 1, 100–106.
- Terebiznik, M.R.; Vieira, O.V.; Marcus, S.L.; Slade, A.; Yip, C.M.; Trimble, W.S.; Meyer, T.; Finlay, B.B.; Grinstein, S. Elimination of host cell PtdIns (4, 5) P 2 by bacterial SigD promotes membrane fission during invasion by Salmonella. Nat. Cell Biol. 2002, 4, 766.
- Welch, M.D. The world according to Arp: Regulation of actin nucleation by the Arp2/3 complex. Trends Cell Biol. 1999, 9, 423–427.
- Krause, M.; Gautreau, A. Steering cell migration: Lamellipodium dynamics and the regulation of directional persistence. Nat. Rev. Mol. Cell Biol. 2014, 15, 577–590.
- Krause, C.I. The ABCs of RSV. Nurse Pract. 2018, 43, 20–26.
- Collins, P.L.; Fearns, R.; Graham, B.S. Respiratory syncytial virus: Virology, reverse genetics, and pathogenesis of disease. Curr. Top. Microbiol. Immunol. 2013, 372, 3–38.
- Levine, S.; Klaiber-Franco, R.; Paradiso, P.R. Demonstration that glycoprotein G is the attachment protein of respiratory syncytial virus. J. Gen. Virol. 1987, 68, 2521–2524.
- McLellan, J.S.; Ray, W.C.; Peeples, M.E. Structure and function of respiratory syncytial virus surface glycoproteins. Curr. Top. Microbiol. Immunol. 2013, 372, 83–104.
- Collins, P.L.; Wertz, G.W. cDNA cloning and transcriptional mapping of nine polyadenylylated RNAs encoded by the genome of human respiratory syncytial virus. Proc. Natl. Acad. Sci. USA 1983, 80, 3208–3212.
- Krempl, C.; Murphy, B.R.; Collins, P.L. Recombinant respiratory syncytial virus with the G and F genes shifted to the promoter-proximal positions. J. Virol. 2002, 76, 11931–11942.
- Ghildyal, R.; Ho, A.; Jans, D.A. Central role of the respiratory syncytial virus matrix protein in infection. FEMS Microbiol. Rev. 2006, 30, 692–705.
- Bermingham, A.; Collins, P.L. The M2-2 protein of human respiratory syncytial virus is a regulatory factor involved in the balance between RNA replication and transcription. Proc. Natl. Acad. Sci. USA 1999, 96, 11259–11264.
- Fearns, R.; Collins, P.L. Role of the M2-1 transcription antitermination protein of respiratory syncytial virus in sequential transcription. J. Virol. 1999, 73, 5852–5864.
- Spann, K.M.; Tran, K.C.; Collins, P.L. Effects of nonstructural proteins NS1 and NS2 of human respiratory syncytial virus on interferon regulatory factor 3, NF-kappaB, and proinflammatory cytokines. J. Virol. 2005, 79, 5353–5362.
- Bitko, V.; Shulyayeva, O.; Mazumder, B.; Musiyenko, A.; Ramaswamy, M.; Look, D.C.; Barik, S. Nonstructural proteins of respiratory syncytial virus suppress premature apoptosis by an NF-kappaB-dependent, interferon-independent mechanism and facilitate virus growth. J. Virol. 2007, 81, 1786–1795.
- Fuentes, S.; Tran, K.C.; Luthra, P.; Teng, M.N.; He, B. Function of the respiratory syncytial virus small hydrophobic protein. J. Virol. 2007, 81, 8361–8366.
- Krzyzaniak, M.A.; Zumstein, M.T.; Gerez, J.A.; Picotti, P.; Helenius, A. Host cell entry of respiratory syncytial virus involves macropinocytosis followed by proteolytic activation of the F protein. PLoS Pathog. 2013, 9, e1003309.
- Ulloa, L.; Serra, R.; Asenjo, A.; Villanueva, N. Interactions between cellular actin and human respiratory syncytial virus (HRSV). Virus Res. 1998, 53, 13–25.
- Burke, E.; Dupuy, L.; Wall, C.; Barik, S. Role of cellular actin in the gene expression and morphogenesis of human respiratory syncytial virus. Virology 1998, 252, 137–148.
- Taylor, M.P.; Koyuncu, O.O.; Enquist, L.W. Subversion of the actin cytoskeleton during viral infection. Nat. Rev. Microbiol. 2011, 9, 427–439.
- Kallewaard, N.L.; Bowen, A.L.; Crowe, J.E., Jr. Cooperativity of actin and microtubule elements during replication of respiratory syncytial virus. Virology 2005, 331, 73–81.
- Mehedi, M.; Collins, P.L.; Buchholz, U.J. A novel host factor for human respiratory syncytial virus. Commun. Integr. Biol. 2017, 10, e1006062.
- Mehedi, M.; McCarty, T.; Martin, S.E.; Le Nouen, C.; Buehler, E.; Chen, Y.C.; Smelkinson, M.; Ganesan, S.; Fischer, E.R.; Brock, L.G.; et al. Actin-Related Protein 2 (ARP2) and Virus-Induced Filopodia Facilitate Human Respiratory Syncytial Virus Spread. PLoS Pathog. 2016, 12, e1006062.
- Mehedi, M.; Smelkinson, M.; Kabat, J.; Ganesan, S.; Collins, P.L.; Buchholz, U.J. Multicolor stimulated emission depletion (STED) microscopy to generate high-resolution images of respiratory syncytial virus particles and infected cells. Bio-Protocol 2017, 7, e2543.
- Dramsi, S.; Cossart, P. Intracellular pathogens and the actin cytoskeleton. Annu. Rev. Cell Dev. Biol. 1998, 14, 137–166.
- Alto, N.M.; Orth, K. Subversion of cell signaling by pathogens. Cold Spring Harb. Perspect. Biol. 2012, 4, a006114.
- Colonne, P.M.; Winchell, C.G.; Voth, D.E. Hijacking host cell highways: Manipulation of the host actin cytoskeleton by obligate intracellular bacterial pathogens. Front. Cell. Infect. Microbiol. 2016, 6, 107.
- Meconi, S.; Jacomo, V.; Boquet, P.; Raoult, D.; Mege, J.L.; Capo, C. Coxiella burnetii induces reorganization of the actin cytoskeleton in human monocytes. Infect. Immun. 1998, 66, 5527–5533.
- Jewett, T.J.; Fischer, E.R.; Mead, D.J.; Hackstadt, T. Chlamydial TARP is a bacterial nucleator of actin. Proc. Natl. Acad. Sci. USA 2006, 103, 15599–15604.
- Rosales, E.M.; Aguilera, M.O.; Salinas, R.P.; Carminati, S.A.; Colombo, M.I.; Martinez-Quiles, N.; Beron, W. Cortactin is involved in the entry of Coxiella burnetii into non-phagocytic cells. PLoS ONE 2012, 7, e39348.
- Meconi, S.; Capo, C.; Remacle-Bonnet, M.; Pommier, G.; Raoult, D.; Mege, J.L. Activation of protein tyrosine kinases by Coxiella burnetii: Role in actin cytoskeleton reorganization and bacterial phagocytosis. Infect. Immun. 2001, 69, 2520–2526.
- Heinzen, R.A. Rickettsial actin-based motility: Behavior and involvement of cytoskeletal regulators. Ann. N. Y. Acad. Sci. 2003, 990, 535–547.
- Rottner, K.; Stradal, T.E.; Wehland, J. Bacteria-host-cell interactions at the plasma membrane: Stories on actin cytoskeleton subversion. Dev. Cell 2005, 9, 3–17.
- Carabeo, R. Bacterial subversion of host actin dynamics at the plasma membrane. Cell. Microbiol. 2011, 13, 1460–1469.
- Tayyari, F.; Marchant, D.; Moraes, T.J.; Duan, W.; Mastrangelo, P.; Hegele, R.G. Identification of nucleolin as a cellular receptor for human respiratory syncytial virus. Nat. Med. 2011, 17, 1132–1135.
- Shahriari, S.; Gordon, J.; Ghildyal, R. Host cytoskeleton in respiratory syncytial virus assembly and budding. Virol. J. 2016, 13, 161.
- Baviskar, P.S.; Hotard, A.L.; Moore, M.L.; Oomens, A.G. The respiratory syncytial virus fusion protein targets to the perimeter of inclusion bodies and facilitates filament formation by a cytoplasmic tail-dependent mechanism. J. Virol. 2013, 87, 10730–10741.
- Shahriari, S.; Wei, K.J.; Ghildyal, R. Respiratory Syncytial Virus Matrix (M) Protein Interacts with Actin In Vitro and in Cell Culture. Viruses 2018, 10, 535.
- Burke, E.; Mahoney, N.M.; Almo, S.C.; Barik, S. Profilin is required for optimal actin-dependent transcription of respiratory syncytial virus genome RNA. J. Virol. 2000, 74, 669–675.
- Jeffree, C.E.; Brown, G.; Aitken, J.; Su-Yin, D.Y.; Tan, B.H.; Sugrue, R.J. Ultrastructural analysis of the interaction between F-actin and respiratory syncytial virus during virus assembly. Virology 2007, 369, 309–323.
- Gower, T.L.; Peeples, M.E.; Collins, P.L.; Graham, B.S. RhoA is activated during respiratory syncytial virus infection. Virology 2001, 283, 188–196.
- Gower, T.L.; Pastey, M.K.; Peeples, M.E.; Collins, P.L.; McCurdy, L.H.; Hart, T.K.; Guth, A.; Johnson, T.R.; Graham, B.S. RhoA signaling is required for respiratory syncytial virus-induced syncytium formation and filamentous virion morphology. J. Virol. 2005, 79, 5326–5336.
- Linfield, D.T.; Gao, N.; Raduka, A.; Harford, T.J.; Piedimonte, G.; Rezaee, F. RSV attenuates epithelial cell restitution by inhibiting actin cytoskeleton-dependent cell migration. Am. J. Physiol. Lung Cell. Mol. Physiol. 2021, 321, L189–L203.
- Delorme-Axford, E.; Coyne, C.B. The actin cytoskeleton as a barrier to virus infection of polarized epithelial cells. Viruses 2011, 3, 2462–2477.
- Ridley, A.J. Life at the leading edge. Cell 2011, 145, 1012–1022.
- Heasman, S.J.; Ridley, A.J. Mammalian Rho GTPases: New insights into their functions from in vivo studies. Nat. Rev. Mol. Cell Biol. 2008, 9, 690–701.
- Parsons, J.T.; Horwitz, A.R.; Schwartz, M.A. Cell adhesion: Integrating cytoskeletal dynamics and cellular tension. Nat. Rev. Mol. Cell Biol. 2010, 11, 633–643.
- Spiering, D.; Hodgson, L. Dynamics of the Rho-family small GTPases in actin regulation and motility. Cell Adh. Migr. 2011, 5, 170–180.
- Liu, R.; Woolner, S.; Johndrow, J.E.; Metzger, D.; Flores, A.; Parkhurst, S.M. Sisyphus, the Drosophila myosin XV homolog, traffics within filopodia transporting key sensory and adhesion cargos. Development 2008, 135, 53–63.
- Ye, X.; Lee, Y.C.; Choueiri, M.; Chu, K.; Huang, C.F.; Tsai, W.W.; Kobayashi, R.; Logothetis, C.J.; Yu-Lee, L.Y.; Lin, S.H. Aberrant expression of katanin p60 in prostate cancer bone metastasis. Prostate 2012, 72, 291–300.
- Keren, K.; Pincus, Z.; Allen, G.M.; Barnhart, E.L.; Marriott, G.; Mogilner, A.; Theriot, J.A. Mechanism of shape determination in motile cells. Nature 2008, 453, 475–480.
- Ratner, S.; Sherrod, W.S.; Lichlyter, D. Microtubule retraction into the uropod and its role in T cell polarization and motility. J. Immunol. 1997, 159, 1063–1067.
- Theisen, U.; Straube, E.; Straube, A. Directional persistence of migrating cells requires Kif1C-mediated stabilization of trailing adhesions. Dev. Cell 2012, 23, 1153–1166.
- Andrew, N.; Insall, R.H. Chemotaxis in shallow gradients is mediated independently of PtdIns 3-kinase by biased choices between random protrusions. Nat. Cell Biol. 2007, 9, 193–200.
- Cooper, J.A. Cell biology in neuroscience: Mechanisms of cell migration in the nervous system. J. Cell Biol. 2013, 202, 725–734.
- Eilken, H.M.; Adams, R.H. Dynamics of endothelial cell behavior in sprouting angiogenesis. Curr. Opin. Cell Biol. 2010, 22, 617–625.
- Gupton, S.L.; Gertler, F.B. Filopodia: The fingers that do the walking. Sci. STKE 2007, 2007, re5.
- Clement, C.; Tiwari, V.; Scanlan, P.M.; Valyi-Nagy, T.; Yue, B.Y.; Shukla, D. A novel role for phagocytosis-like uptake in herpes simplex virus entry. J. Cell Biol. 2006, 174, 1009–1021.
