5. Discussion
The Aguarongo forest reserve is one of the most important biodiversity hotspots in Ecuador, and in recent years, it has been the subject of numerous research studies on flora, fauna, use of soils, and water balance
[29][30].
As for all the tropics, Ecuador has huge biodiversity that has yet to be revealed, especially in natural reserves such as the Aguarongo forest. This is true above all for Fungi, a Kingdom often neglected despite its key role in the functionality of terrestrial ecosystems.
Regarding the use of next-generation sequencing in research of soil fungal communities, there are few studies in the tropics; however, a collection of metagenomic data is being implemented
[31][32][33][34]. Most Ecuadorian studies on soil fungi describe the diversity of arbuscular mycorrhizal fungi (AMF) associated with crops in the Andean part of Loja and Quito
[35][36]. There are also studies of the communities of AMF associated with plants growing in contaminated soils such as crude-oil-contaminated sites in the Amazon region of Ecuador
[20]. Using modern molecular methods, it was possible to obtain a detailed account of soil fungal biodiversity of the Aguarongo forest and give the first list of soil fungi of this important reserve of biodiversity in Ecuador.
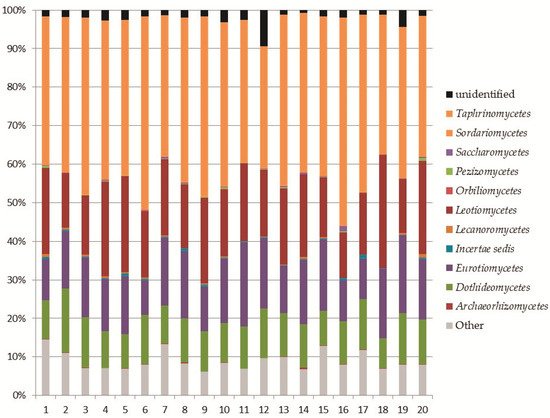
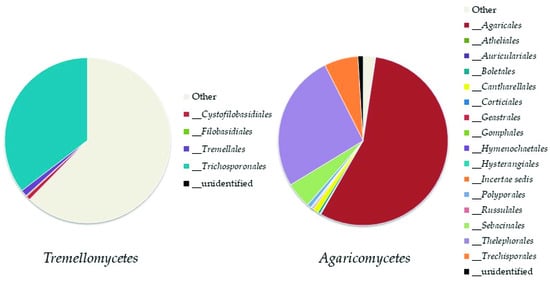
In this work, 408 fungal OTUs were identified at the species level. Together with
Ascomycota, the most abundant phylum in our study was
Mortierellomycota. Its high abundance was expected, as this result has been recorded in other studies on the mycobiota of soil in high altitude environments
[32][34] or from extreme environments such as the Alps
[37] or Antarctica
[38][39][40]. Surprisingly, in the Aguarongo forest, the abundance of
Mortierellomycota is similar to that of
Ascomycota. All the OTUs referring to
Mucoromyceta in this study were updated following the recent paper of Vandepol et al.
[28], in which the phylogeny of
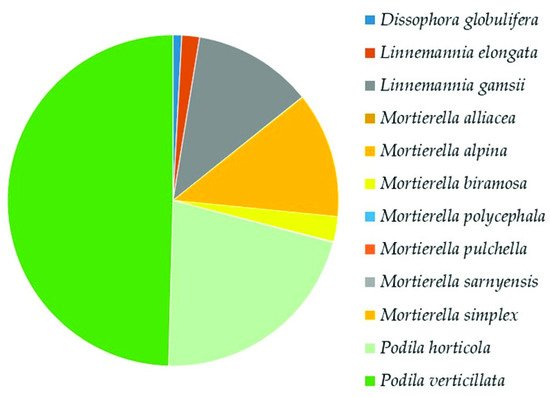
Mortierellaceae was resolved through a synthesis of multigene phylogenetics and phylogenomics. The present work significantly improved the global geography and environmental records of
Mortierellaceae and contributed to the knowledge on the ecology of these species, especially in an under-sampled region such as Ecuador. These data contribute to understanding the ecological function of
Mortierellaceae, which remains mostly unknown. Species of
Mortierellaceae are often isolated from soils, decaying leaves, and insects
[41], due to their saprotrophic nature
[42].
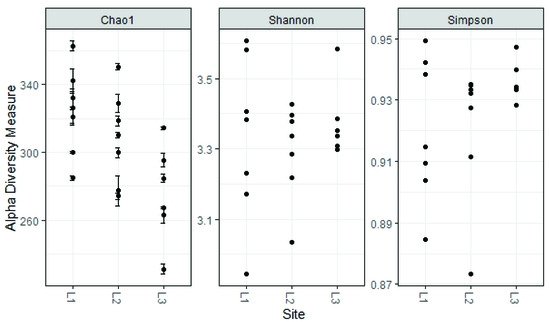
Regarding alpha diversity, it was similar among all sampling sites; the values reported here are similar to those found for other regions of the world with altitudes above 2000 m.a.s.l.
[32].
In the whole soil mycobiota of Aguarongo, the most frequent species is a
Mortierellaceae species,
Podila verticillate, representing 12–14% of the total of identified OTUs. This species (reported with its basionym
Mortierella verticillata) was first recorded in South America from mountainous environments (1950 m.a.s.l.) in Brazil
[43]. Our findings expand the distribution of
P. verticillata both in latitude and altitude. This is true for all of the species of
Mortierellaceae, which were abundantly found in Aguarongo forest soil up to 3221 m.a.s.l.
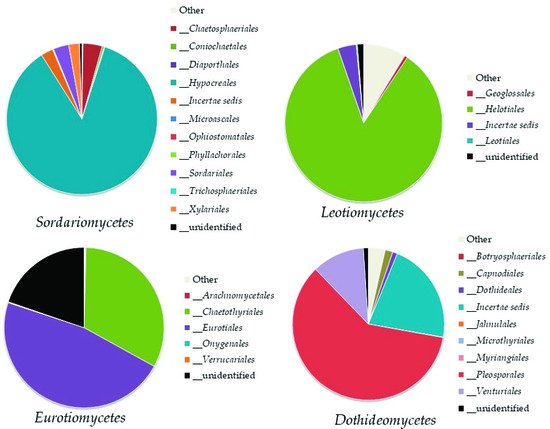
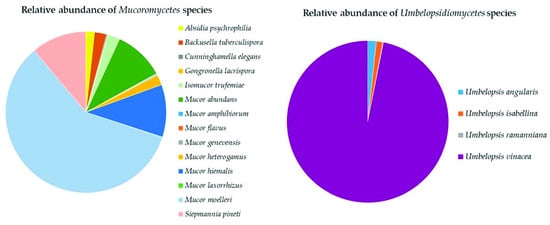
Within the identified species of
Ascomycetes in Aguarongo, important facultative plant pathogenic fungi were abundantly recorded, for example from the
Fusarium oxysporum complex, a well-known group of taxa due to their many pathogenic forms and high frequency of isolation in soil
[44], and
Curvularia lunata, an important pathogen of maize
[45]. In addition to the plant pathogens many saprotrophic fungi were recorded, such as
Pleotrichocladium opacum and
Penicillium spinulosum, a dominant taxon in the Aguarongo forest.
Beneficial fungi were not lacking, such as
Trichoderma asperellum, an important biocontrol agent, applied in agriculture for controlling plant fungal pathogens and plant-growth promoters
[46][47], as well as
Arthrobotrys musiformis Pochonia bulbillosa, and
P. suchlasporia, well-known nematophagous fungi
[48][49]. Particularly important in the Aguarongo forest is the presence of the genus
Metarhizium, a genus that shows wide biodiversity in this area, with species having high potential as biocontrol agents against insects, specifically
M. anisopliae,
M. flavoviride, and
M. lepidiotae,
[50]. Although their presence is occasional, many other species with importance because of their ecological role as entomopathogens or nematopathogens, and potential application for sustainable agriculture
[51] are found in the Aguarongo forest. This includes
Beauveria brongniartii, B. bassiana, Dactylella mammillata, Hirsutella minnesotensis, H. rhossiliensis, H. vermicola, Paecilomyces farinosus, and
P. marquandii.
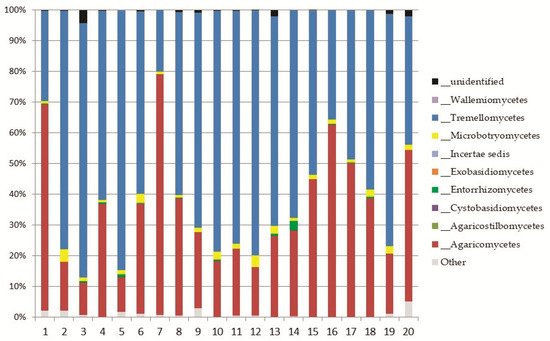
Basidiomycetes were dominated by
Saitozyma podzolica, a common yeast and strong decomposer of dead plant biomass isolated from soils worldwide
[52].
Apiotrichum wieringae was also widely present in the soil of Aguarongo, a nonpathogenic member of the
Tricosporonaceae family, able to degrade uric acid and aromatic compounds
[53].
Tomentella testaceogilva, abundant in Aguarongo, is reported as a terricolous or lignicolous fungus associated with
Alnus, but also with moss
[54]. Although rarely recorded in the Aguarongo forest, a famous member of
Basidiomycetes must be highlighted, namely
Fomes fomentarius, an important plant pathogenic fungus rich in pharmacological compounds
[55].
Fungi in soils of Aguarongo include members of the phylum
Glomeromycota, which remain mainly unidentified. Some identified species are important arbuscular mycorrhizal fungi; the most abundant one was
Claroideoglomus claroideum, considered a good plant promoter and heavy metal decontaminant
[56]. In the Aguarongo forest,
Chytridiomycota were dominated by
Rhizophlyctis rosea, a highly effective plant biomass degrader and zoosporic fungus, commonly observed near the soil surface. This species has light-sensitive proteins that allow this fungus to remain in the euphotic zone
[57]. Within
Monoblepharomycota, only one species was recorded:
Monoblepharella mexicana. The southernmost record of this species was reported by Steciow and Arambarri
[58], while the present finding can be considered the record of this species at the highest elevation.
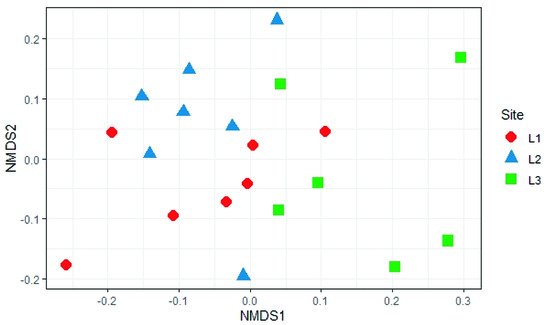
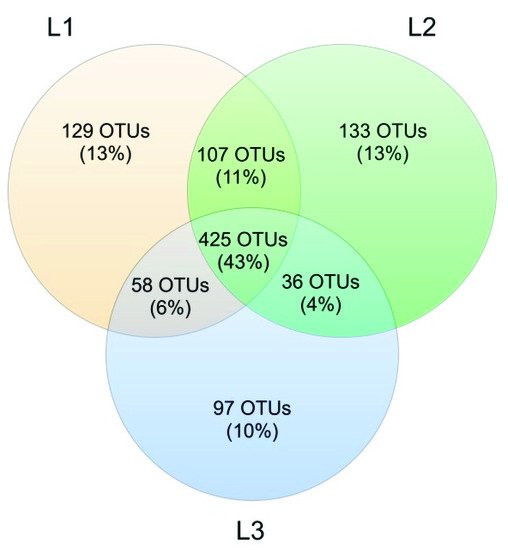
The analysis reported in this work is based on a large number of soil samples (100), thus giving a good representation of the fungal biodiversity of Aguarongo forest soil. The collection of samples along an altitudinal gradient did not reveal significant differences among the structure of the soil fungal communities in the three sampling locations (L1, L2, L3). Only Location L3 has a lower abundance of rare taxa with respect to L1, consistent with the Venn diagram.
The analysis of the records, reported in
Supplementary Table S2, shows the Aguarongo forest soils as a natural area rich in fungal biodiversity, with the main phyla represented by a large number of taxa, known to be beneficial fungi. In addition, the Aguarongo forest is shown to be a rich source of species having strong application potential in agriculture and in the chemical and food industry. This area hides a huge number of unknown species that could be assessed, and its protection is of the utmost importance.