Fungi represent an essential component of ecosystems, functioning as decomposers and biotrophs, and they are one of the most diverse groups of Eukarya. In the tropics, many species are unknown. In this work, high-throughput DNA sequencing was used to discover the biodiversity of soil fungi in the Aguarongo forest reserve, one of the richest biodiversity hotspots in Ecuador. The rDNA metabarcoding analysis revealed the presence of seven phyla: Ascomycota, Basidiomycota, Mortierellomycota, Mucoromycota, Glomeromycota, Chytridiomycota, and Monoblepharomycota. A total of 440 identified species were recorded. They mainly belonged to Ascomycota (263) and Basidiomycota (127). In Mortierellomycota, 12 species were recorded, among which Podila verticillata is extremely frequent and represents the dominant species in the entire mycobiota of Aguarongo.
- Andes
- environmental DNA
- fungal biodiversity
- metabarcoding
- natural reserve
- forest ecosystems tropical mycobiota vulnerable species
1. Introduction
2. Soil Chemical Characteristics
| Sampling Sites | pH | Organic Matter | N | P | K | Mg | Ca | S |
| L1 | 4.9 b | 10.9 a | 0.68 b | 3.6 b | 203.3 b | 425.7 b | 1776.3 b | 846.4 b |
| L2 | 4.8 a | 15.5 a | 0.82 a | 5.9 a | 128.0 a | 162.7 a | 564.7 a | 1012.6 a |
| L3 | 4.6 a | 12.3 a | 0.71 a | 5.3 a | 126.7 a | 177.7 a | 885.3 a | 790.4 a |
| Sampling Sites | Cu | Mn | Zn | Fe | Na | Cl | Al | |
| L1 | 10.5 b | 118.7 b | 16.0 a | 834.3 a | 28.7 a | 11.8 a | 6.4 a | |
| L2 | 8.7 a | 72.7 a | 15.7 a | 926.0 a | 31.3 a | 15.1 a | 6.9 a | |
| L3 | 12.7 a | 107.7 a | 16.0 a | 1197.3 b | 24.3 a | 371.8 b | 7.1 a |
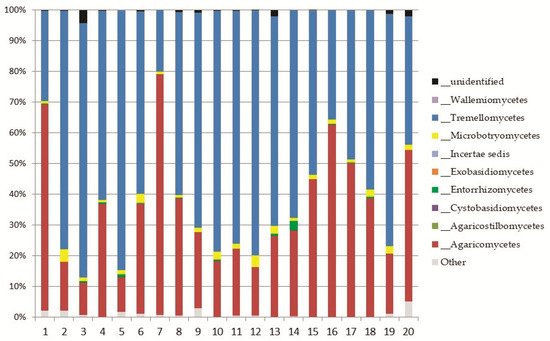
3. Soil Fungal Assemblage Composition
3.1. Subkingdom Dikarya
Ascomycota
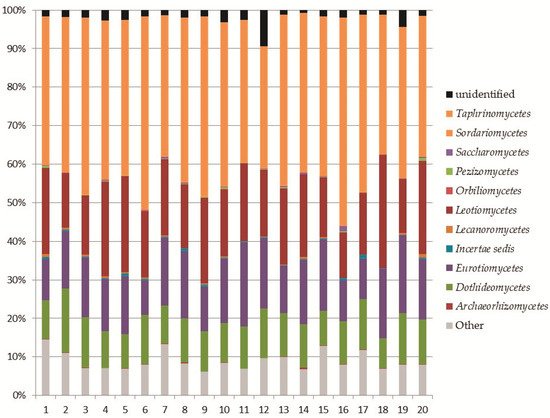
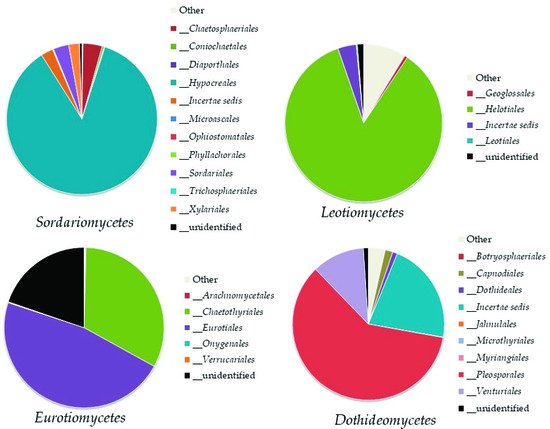
Top Ten Most Abundant Species of Ascomycota
Basidiomycota
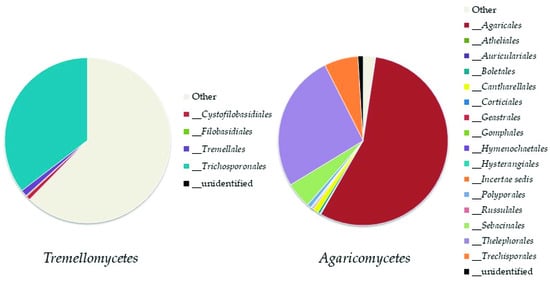
Top Ten Abundant Species of Basidiomycota
3.2. The Subkingdom Mucoromyceta
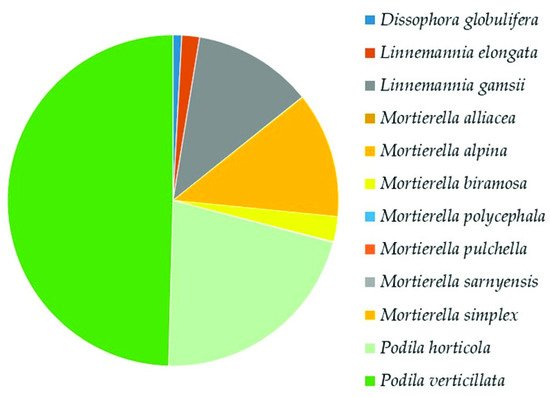
Mortierellomycota
Mucoromycota
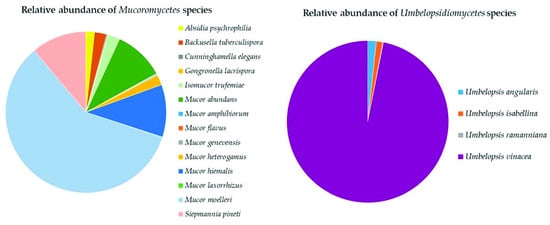
Glomeromycota
The Top Mucoromyceta
3.3. The Subkingdom Chytridiomyceta
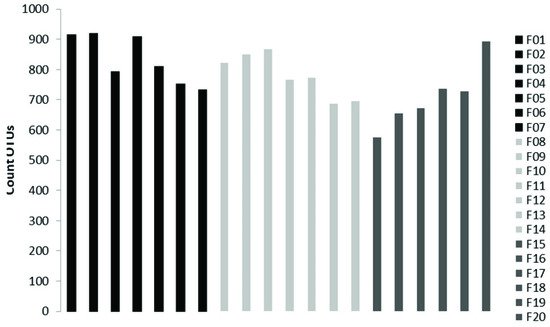
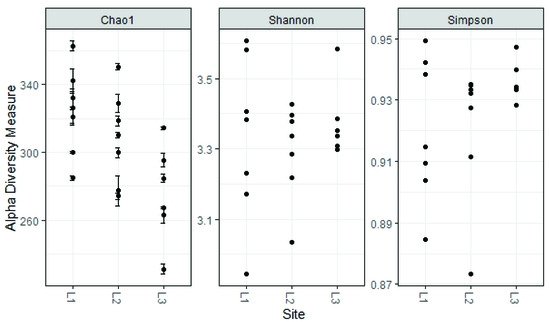
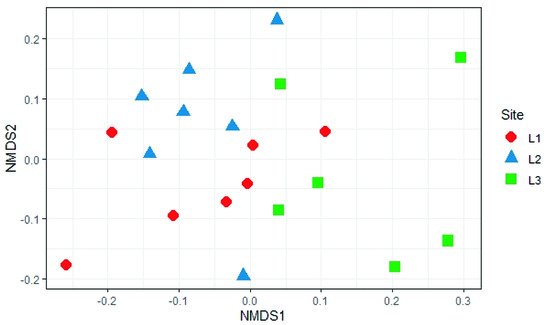
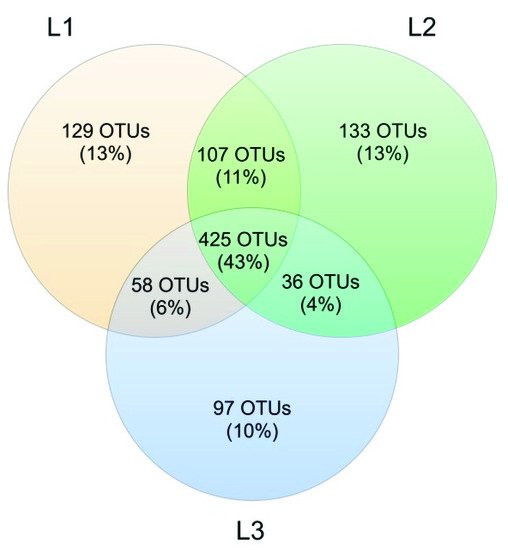
4. Soil Mycobiota Diversity in the Sampling Sites
Discussion
4. Discussion
The Aguarongo forest reserve is one of the most important biodiversity hotspots in Ecuador, and in recent years, it has been the subject of numerous research studies on flora, fauna, use of soils, and water balance [28, 42].
As for all the tropics, Ecuador has huge biodiversity that has yet to be revealed, especially in natural reserves such as the Aguarongo forest. This is true above all for Fungi, a Kingdom often neglected despite its key role in the functionality of terrestrial ecosystems.
Regarding the use of next-generation sequencing in research of soil fungal communities, there are few studies in the tropics; however, a collection of metagenomic data is being implemented [43–46]. Most Ecuadorian studies on soil fungi describe the diversity of arbuscular mycorrhizal fungi (AMF) associated with crops in the Andean part of Loja and Quito [47,48]. There are also studies of the communities of AMF associated with plants growing in contaminated soils such as crude-oil-contaminated sites in the Amazon region of Ecuador [20]. Using modern molecular methods, it was possible to obtain a detailed account of soil fungal biodiversity of the Aguarongo forest and give the first list of soil fungi of this important reserve of biodiversity in Ecuador.
In this work, 408 fungal OTUs were identified at the species level. Together with Ascomycota, the most abundant phylum in our study was Mortierellomycota. Its high abundance was expected, as this result has been recorded in other studies on the mycobiota of soil in high altitude environments [44,46] or from extreme environments such as the Alps [49] or Antarctica [50–52]. Surprisingly, in the Aguarongo forest, the abundance of Mortierellomycota is similar to that of Ascomycota. All the OTUs referring to Mucoromyceta in this study were updated following the recent paper of Vandepol et al. [41], in which the phylogeny of Mortierellaceae was resolved through a synthesis of multigene phylogenetics and phylogenomics. The present work significantly improved the global geography and environmental records of Mortierellaceae and contributed to the knowledge on the ecology of these species, especially in an under-sampled region such as Ecuador. These data contribute to understanding the ecological function of Mortierellaceae, which remains mostly unknown. Species of Mortierellaceae are often isolated from soils, decaying leaves, and insects [53], due to their saprotrophic nature [54].
Regarding alpha diversity, it was similar among all sampling sites; the values reported here are similar to those found for other regions of the world with altitudes above 2000 m.a.s.l. [44].
In the whole soil mycobiota of Aguarongo, the most frequent species is a Mortierellaceae species, Podila verticillate, representing 12–14% of the total of identified OTUs. This species (reported with its basionym Mortierella verticillata) was first recorded in South America from mountainous environments (1950 m.a.s.l.) in Brazil [55]. Our findings expand the distribution of P. verticillata both in latitude and altitude. This is true for all of the species of Mortierellaceae, which were abundantly found in Aguarongo forest soil up to 3221 m.a.s.l.
Within the identified species of Ascomycetes in Aguarongo, important facultative plant pathogenic fungi were abundantly recorded, for example from the Fusarium oxysporum complex, a well-known group of taxa due to their many pathogenic forms and high frequency of isolation in soil [56], and Curvularia lunata, an important pathogen of maize [57]. In addition to the plant pathogens many saprotrophic fungi were recorded, such as Pleotrichocladium opacum and Penicillium spinulosum, a dominant taxon in the Aguarongo forest.
Beneficial fungi were not lacking, such as Trichoderma asperellum, an important biocontrol agent, applied in agriculture for controlling plant fungal pathogens and plant-growth promoters [58,59], as well as Arthrobotrys musiformis Pochonia bulbillosa, and P. suchlasporia, well-known nematophagous fungi [60,61]. Particularly important in the Aguarongo forest is the presence of the genus Metarhizium, a genus that shows wide biodiversity in this area, with species having high potential as biocontrol agents against insects, specifically M. anisopliae, M. flavoviride, and M. lepidiotae, [62]. Although their presence is occasional, many other species with importance because of their ecological role as entomopathogens or nematopathogens, and potential application for sustainable agriculture [63] are found in the Aguarongo forest. This includes Beauveria brongniartii, B. bassiana, Dactylella mammillata, Hirsutella minnesotensis, H. rhossiliensis, H. vermicola, Paecilomyces farinosus, and P. marquandii.
Basidiomycetes were dominated by Saitozyma podzolica, a common yeast and strong decomposer of dead plant biomass isolated from soils worldwide [64]. Apiotrichum wieringae was also widely present in the soil of Aguarongo, a nonpathogenic member of the Tricosporonaceae family, able to degrade uric acid and aromatic compounds [65]. Tomentella testaceogilva, abundant in Aguarongo, is reported as a terricolous or lignicolous fungus associated with Alnus, but also with moss [66]. Although rarely recorded in the Aguarongo forest, a famous member of Basidiomycetes must be highlighted, namely Fomes fomentarius, an important plant pathogenic fungus rich in pharmacological compounds [67].
Fungi in soils of Aguarongo include members of the phylum Glomeromycota, which remain mainly unidentified. Some identified species are important arbuscular mycorrhizal fungi; the most abundant one was Claroideoglomus claroideum, considered a good plant promoter and heavy metal decontaminant [68]. In the Aguarongo forest, Chytridiomycota were dominated by Rhizophlyctis rosea, a highly effective plant biomass degrader and zoosporic fungus, commonly observed near the soil surface. This species has light-sensitive proteins that allow this fungus to remain in the euphotic zone [69]. Within Monoblepharomycota, only one species was recorded: Monoblepharella mexicana. The southernmost record of this species was reported by Steciow and Arambarri [70], while the present finding can be considered the record of this species at the highest elevation.
The analysis reported in this work is based on a large number of soil samples (100), thus giving a good representation of the fungal biodiversity of Aguarongo forest soil. The collection of samples along an altitudinal gradient did not reveal significant differences among the structure of the soil fungal communities in the three sampling locations (L1, L2, L3). Only Location L3 has a lower abundance of rare taxa with respect to L1, consistent with the Venn diagram.
References
- Tedersoo, L.; Bahram, M.; Põlme, S.; Koljag, U.; Yorou, N.S.; Wijesundera, R.; Virreal Ruiz, L.; Vasco-Palacios, A.M.; Thu, P.Q.; Suija, A.; et al. Global diversity and geography of soil fungi. Science 2014, 346, 1078.
- Mueller, G.M.; Schmit, J.P.; Leacock, P.R.; Buyck, B.; Cifuentes, J.; Dejardin, D.E.; Halling, R.E.; Hjortstam, K.; Iturriaga, T.; Larsson, K.H.; et al. Global diversity and distribution of macrofungi. Biodivers. Conserv. 2007, 16, 37–48.
- Tedersoo, L.; Jairus, T.; Horton, B.M.; Abarenkov, K.; Suvi, T.; Saar, I.; Kõljalg, U. Strong host preference of ectomycorrhizal fungi in a Tasmanian wet sclerophyll forest as revealed by DNA barcoding and taxon-specific primers. New Phytol. 2008, 180, 479–490.
- Thakur, M.P.; Geisen, S. Trophic Regulations of the Soil Microbiome. Trends Microbiol. 2019, 27, 771–780.
- Vandenkoornhuyse, P.; Baldauf, S.L.; Leyval, C.; Straczek, J.; Young, J.P.W. Extensive fungal diversity in plant roots. Science 2002, 295, 2051.
- Banchi, E.; Ametrano, C.G.; Tordoni, E.; Stankovi, D.; Ongaro, S.; Tetiach, M.; Pallavicini, A.; Muggia, L.; Verardo, P.; Tassan, F.; et al. Environmental DNA assessment of airborne plant and fungal seasonal diversity. Sci. Total Environ. 2020, 738, 140249.
- Coleine, C.; Selbmann, L.; Pombubpa, N.; Stajich, J.E. Amplicon sequencing of rock inhabiting microbial communities from Joshua Tree National Park, USA. Microbiol. Resour. Announc. 2021, 10, e00494-21.
- Seifert, K.A. Progress towards DNA barcoding of fungi. Mol. Ecol. Resour. 2009, 9, 83–89.
- Peay, K.; Kennedy, P.; Talbot, J. Dimensions of biodiversity in the Earth mycobiome. Nat. Rev. Microbiol. 2016, 14, 434–447.
- Thomsen, P.F.; Willerslev, E. Environmental DNA—An emerging tool in conservation for monitoring past and present biodiversity. Biol. Conserv. 2015, 83, 4–18.
- Toapanta-Alban, C.E.; Ordoñez, M.E.; Barnes, C.W.; Blanchette, R.A. Taxonomy of the major rhizomorphic species of the “Melanopus group” within Polyporaceae in Yasunı’ National Park, Ecuador. PLoS ONE 2021, 16, e0254567.
- Liu, J.; Haelewaters, D.; Pfliegler, W.P.; Page, R.A.; Dick, C.W.; Aime, M.C. A new species of Gloeandromyces from Ecuador and Panama revealed by morphology and phylogenetic reconstruction, with a discussion of secondary barcodes in Laboulbeniomycetes taxonomy. Mycologia 2020, 112, 1192–1202.
- Crous, P.W.; Wingfield, M.J.; Chooi, Y.H.; Gilchrist, C.L.M.; Lacey, E.; Pitt, J.I.; Roets, F.; Swart, W.J.; Cano-Lira, J.F.; Valenzuela-Lopez, N.; et al. Fungal Planet description sheets: 1042–1111. Persoonia 2020, 44, 301–459.
- Galarza, L.; Akagi, Y.; Takao, K.; Kim, C.S.; Maekawa, N.; Itai, A.; Peralta, E.; Santos, E.; Kodama, M. Characterization of Trichoderma species isolated in Ecuador and their antagonistic activities against phytopathogenic fungi from Ecuador and Japan. J. Gen. Plant. Pathol. 2015, 81, 201–210.
- Baroni, T.J.; Halling, R.E. New species of Rhodocybe from South America with a key to species. Mycologia 1992, 84, 411–421.
- Goh, T.K.; Hyde, K.D. A new species of Palmicola from Ecuador. Mycol. Res. 1996, 100, 714–716.
- Berndt, R. New Puccinia species on Baccharis from Ecuador and Costa Rica. Mycol. Res. 1998, 102, 1108–1112.
- Garcés, F.F.; Fiallos, F.F.; Silva, E.; Martinez, F.; Aime, M.C.; Comstock, J.C.; Glynn, N.C.; Castlebury, L.A. First Report of Orange Rust of Sugarcane Caused by Puccinia kuehnii in Ecuador. Plant. Dis. 2014, 98, 842.
- Dueñas, J.F.; Camenzind, T.; Roy, J.; Hempel, S.; Homeier, J.; Suárez, J.P.; Rillig, M.C. Moderate phosphorus additions consistently affect community composition of arbuscular mycorrhizal fungi in tropical montane forests in southern Ecuador. New Phytol. 2020, 227, 1505–1518.
- Garcés-Ruiz, M.; Senés-Guerrero, C.; Declerck, S.; Cranenbrouck, S. Community composition of arbuscular mycorrhizal fungi associated with native plants growing in a petroleum-polluted soil of the Amazon region of Ecuador. Microbiologyopen 2019, 8, e00703.
- Jaswal, R.; Pathak, A.; Edwards, B., III; Lewis, R., III; Seaman, J.C.; Stothard, P.; Krivushin, K.; Blom, J.; Rupp, O.; Chauhan, A. Metagenomics-Guided Survey, Isolation, and Characterization of Uranium Resistant Microbiota from the Savannah River Site, USA. Genes 2019, 10, 325.
- Rundell, S.M.; Spakowicz, D.J.; Narváez-Trujillo, A.; Strobel, S.A. The Biological Diversity and Production of Volatile Organic Compounds by Stem-Inhabiting Endophytic Fungi of Ecuador. J. Fungi 2015, 1, 384–396.
- Novotná, A.; Ángel Benítez, A.; Herrera, P.; Cruz, D.; Filipczyková, E.; Suárez, J.P. High diversity of root-associated fungi isolated from three epiphytic orchids in southern Ecuador. Mycoscience 2018, 59, 24–32.
- Tedersoo, L.; Sadam, A.; Zambrano, M.; Valencia, R.; Bahram, M. Low diversity and high host preference of ectomycorrhizal fungi in Western Amazonia, a neotropical biodiversity hotspot. ISME J. 2010, 4, 465–471.
- Haug, I.; Setaro, S.; Suárez, J.P. Global AM fungi are dominating mycorrhizal communities in a tropical premontane dry forest in Laipuna, South Ecuador. Mycol. Progress 2021, 20, 837–845.
- Setaro, S.; Suárez, J.P. Species composition of arbuscular mycorrhizal communities changes with elevation in the Andes of South Ecuador. PLoS ONE 2019, 14, e0221091.
- Tedersoo, L.; Sánchez-Ramírez, S.; Kõljalg, U.; Bahram, M.; Doring, M.; Schigel, D.; May, T.; Ryberg, M.; Abarenkov, K. High-level classification of the Fungi and a tool for evolutionary ecological analyses. Fungal Divers. 2018, 90, 135–159.
- Vandepol, N.; Liber, J.; Desirò, A.; Na, H.; Kennedy, M.; Barry, K.; Grigoriev, I.V.; Miller, A.N.; O’Donnell, K.; Stajich, J.E.; et al. Resolving the Mortierellaceae phylogeny through synthesis of multi-gene phylogenetics and phylogenomics. Fungal Divers. 2020, 104, 267–289.
- Dunque-Sarango, P.; Cajamarca-Rivadeneira, R.; Wemple, B.C.; Delgado- Fernández, M.E. Estimation of the water balance of for a small tropical andean catchment. LA GRANJA Rev. Cienc. Vida 2019, 29, 56–69.
- Fredi, F.; Parra, R.P.; Zumba, D.A. Bromeliads of the Aguarongo Protective Forest-Ecuador and Adaptation to Climate Change. J. Eng. Appl. Sci. 2017, 12, 1619–1622.
- Vaz, A.B.M.; Fonseca, P.L.C.; Leite, L.R.; Badotti, F.; Salim, A.C.M.; Flavio, M.G.; Araujo, F.M.G.; Cuadros-Orellana, S.C.; Duarte, Â.A.; Rosa, C.A.; et al. Using next-generation sequencing (NGS) to uncover diversity of wood-decaying fungi in neotropical atlantic forests. Phytotaxa 2017, 295, 001–021.
- Landínez-Torres, A.Y.; Panelli, S.; Picco, A.M.; Comandatore, F.; Tosi, S.; Capelli, E. A meta-barcoding analysis of soil myco-biota of the upper Andean Colombian agro-environment. Sci. Rep. 2019, 9, 10085.
- Landínez-Torres, A.Y.; Abril, J.L.B.; Tosi, S.; Nicola, L. Soil Microfungi of the Colombian Natural Regions. Int. J. Environ. Res. Public Heal. 2020, 17, 8311.
- Nicola, L.; Landínez-Torres, A.Y.; Zambuto, F.; Capelli, E.; Tosi, S. The Mycobiota of High Altitude Pear Orchards Soil in Colombia. Biology 2021, 10, 1002.
- Senés-Guerrero, C.; Schüßler, A. A conserved arbuscular mycorrhizal fungal core- species community colonizes potato roots in the Andes. Fungal Divers. 2016, 77, 317–333.
- Loján, P.; Senés-Guerrero, C.; Suárez, J.P.; Kromann, P.; Schüßler, A.; Declerck, S. Potato field-inoculation in Ecuador with Rhizophagus irregularis: No impact on growth performance and associated arbuscular mycorrhizal fungal communities. Symbiosis 2017, 73, 45–56.
- Rodolfi, M.; Longa, C.M.O.; Pertot, I.; Tosi, S.; Savino, E.; Guglielminetti, M.; Altobelli, E.; Del Frate, G.; Picco, A.M. Fungal biodiversity in the periglacial soil of Dosde Glacier (Valtellina, Northern Italy). J. Basic Microbiol. 2016, 56, 263–274.
- Tosi, S.; Casado, B.; Gerdol, R.; Caretta, G. Fungi isolated from antarctic mosses. Polar Biol. 2002, 25, 262–268.
- Santos, J.A.D.; Meyer, E.; Sette, L.D. Fungal Community in Antarctic Soil Along the Retreating Collins Glacier (Fildes Peninsula, King George Island). Microorganisms 2020, 8, 1145.
- Canini, F.; Geml, J.; D’Acqui, L.P.; Selbmann, L.; Onofri, S.; Ventura, S.; Zucconi, L. Exchangeable cations and pH drive diversity and functionality of fungal communities in biological soil crusts from coastal sites of Victoria Land, Antarctica. Fungal Ecol. 2020, 45, 100923.
- Domsch, K.H.; Gams, W.; Anderson, T.H. Compendium of Soil Fungi; Academic Press: London, UK, 1980.
- Tamayo-Vélez, Á.; Osorio, N.W. Soil Fertility Improvement by Litter Decomposition and Inoculation with the Fungus Mortierella sp. in Avocado Plantations of Colombia. Commun. Soil Sci. Plan. 2018, 49, 139–147.
- Gonçalves, C.M.; Oliveira, R.J.V.; Silva, R.M.F.; Souza, C.A.F.; Lima, D.X.; Silva, G.A. Mortierella verticillata Linnem (Mortierellomycota, Mortierellales) isolated from mountainous environments: A first report from South America. Check List 2020, 16, 907–910.
- Smith, S.N. An Overview of Ecological and Habitat Aspects in the Genus Fusarium with Special Emphasis on the Soil Borne Pathogenic Forms. Plant Pathol. Bull. 2007, 16, 97–120.
- Chang, J.; Liu, S.; Shi, J.; Guo, N.; Zhang, H.; Chen, J. A new Curvularia lunata variety discovered in Huanghuaihai Region in China. J. Integr. Agr. 2020, 19, 551–560.
- Watanabe, S.; Kato, H.; Kumakura, K.; Ishibashi, E.; Nagayama, K. Properties and biological control activities of aerial and submerged spores in Trichoderma asperellum SKT-1. J. Pestic. Sci. 2006, 31, 375–379.
- Chagas Junior, A.F.; Chagas, L.F.B.; Miller, L.D.O.; de Oliveira, J.C. Efficiency of Trichoderma asperellum UFT 201 as plant growth promoter in soybean. Afr. J. Agr. Res. 2019, 14, 263–271.
- Nicola, L.; Tosi, S.; Savini, D. In vitro evaluation of nematophagous activity of fungal isolates. J. Basic Microbiol. 2014, 54, 1–5.
- Angeles-Hernández, S.; Torres-Hernández, G.; Alonso-Díaz, M.A.; von Son-de-Fernex, E.; Aguilar-Marcelino, L.; González-Garduño, R.; Becerril-Pérez, C.M.; Alcántara-Carbajal, J.L.; Vargas-López, S.; Olmedo-Juárez, A.; et al. Effect of an Arthrobotrys musiformis (Fungi: Orbiliales) culture filtrate on the population of gastrointestinal parasitic nematode eggs in faeces of grazing lambs. Vet. Parasitol. Reg. Stud. Rep. 2021, 24, 100565.
- Iwanicki, N.S.; Pereira, A.A.; Botelho, A.B.R.Z.; Rezende, J.M.; Moral, R.D.; Zucchi, M.I.; Delalibera, I. Monitoring of the field application of Metarhizium anisopliae in Brazil revealed high molecular diversity of Metarhizium spp in insects, soil and sugarcane roots. Sci. Rep. 2019, 9, 4443.
- Amaresan, N.; Senthil Kumar, M.; Sankaranarayanan, M. Beneficial Microbes in Agro-Ecology: Bacteria and Fungi; Academic Press: Amsterdam, The Netherlands, 2020.
- Mašínová, T.; Bahnmann, B.D.; Větrovský, T.; Tomšovský, M.; Merunková, K.; Baldrian, P. Drivers of yeast community composition in the litter and soil of a temperate forest. FEMS Microbiol. Ecol. 2017, 93, fiw223.
- Middelhoven, W.J. Trichosporon wieringae sp. nov., an anamorphic basidiomycetous yeast from soil, and assimilation of some phenolic compounds, polysaccharides and other non-conventional carbon sources by saprophytic Trichosporon species. Antonie van Leeuwenhoek 2004, 86, 329–337.
- Larsen, M.J.A. Contribution to the Taxonomy of the Genus Tomentella; New York Botanical Garden: New York, NY, USA, 1974; p. 145.
- Gáper, J.; Gáperová, S.; Pristas, P.; Naplavova, K. Medicinal Value and Taxonomy of the Tinder Polypore, Fomes fomentarius (Agaricomycetes): A Review. Int. J. Med. Mushrooms 2016, 18, 851–859.
- Pérez, R.; Yasna Tapia, Y.; Antilén, M.; Manuel Casanova, M.; Vidal, C.; Santander, C.; Aponte, H.; Cornejo, P. Interactive effect of compost application and inoculation with the fungus Claroideoglomus claroideum in Oenothera picensis plants growing in mine tailings. Ecotoxicol. Environ. Safe 2021, 208, 111495.
- Gleason, F.H.; Pilgaard, B.; Henderson, L.; Lange, L. The key ecological role and biology of Rhizophlyctis rosea, a zoosporic, early lineage fungus in soil ecosystems. Curr. Trends Microbiol. 2019, 60, 67–80.
- Steciow, M.M.; Arambarri, A.M. Southernmost occurrence of a tropical fungus: Monoblepharella mexicana (Gonapodyaceae, Chytridiomycota). Nova Hedwig. 2000, 70, 107–112.