1. Temperature
Owing to the endothermic nature of the reactions, the reactor temperature needs to be kept as high as 900 °C on the reactor exit. The arrangement of the burners inside the firebox affects the heat flux and the temperature distribution on the reactor wall. It is worth noting that the coke formation inside the reactor tubes is increased by increasing the reactor temperature, resulting in the carbonization of the tube, therefore decreasing the furnace run length and the tube lifetime. Lower temperatures favor the formation of secondary products via oligomerization reactions; thus, it is necessary to apply an optimum temperature profile along the coil to avoid long residence times at low temperatures. Fired tubular reactors are mainly used in commercial pyrolysis units for ethylene production, in which the temperature of the reactants increases continuously from the inlet (500–680 °C) to the outlet (775–875 °C) of the reactor coils. In modern cracking furnaces, a rapid heating system is used at the inlet of the radiant coils, and the temperature rises quickly to the required reaction temperature. However, due to the low temperature, the reaction rate constants are low. In the middle of the coil, the rate of increasing the temperature is lower, but the cracking rates are significantly higher. Owing to the endothermic characteristics of the reaction, most of the heat is transferred to the mixture. At the coil outlet, the rate of the temperature rise increases again, though it is lower than the rate of the temperature rise in the inlet of the coil
[1].
The effects of different operating conditions, including temperature, the steam-to-hydrocarbon ratio (atmospheric gas oil), and residence time, on the yield of the products, were investigated by Depeyre et al.
[2]. As shown in
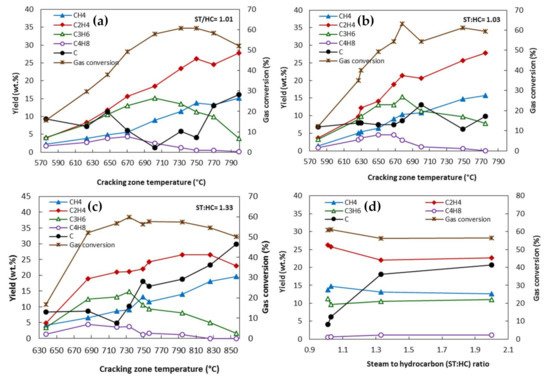
Figure 1a–c, regardless of the steam-to-hydrocarbon ratio, the yields of methane and ethylene increased with the increasing of the temperature, whereas the yields of propylene and butenes reached a maximum and then decreased at higher temperatures. For the different steam-to-hydrocarbon ratios, the highest gas conversion was obtained at about 750 °C. The ratio of the total weight of the produced gas per weight of the injected gas oil used as feedstock is referred to as gas conversion. The amount of deposited carbon also increases with temperature. It has been reported that both radical reactions and molecular secondary reactions are involved in the reaction; however, in a higher-temperature reaction (above 750 °C), the molecular reactions are the dominant reactions. Gál and Lakatos
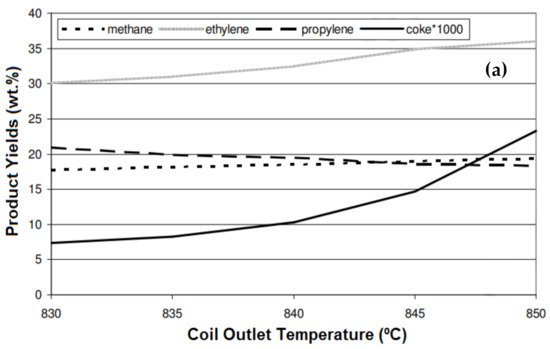
[3] studied the effect of coil outlet temperature (COT) on the product yields and variation in the coke formation rate, using natural gas as a feedstock for the steam cracking process. They reported that by increasing the COT from 830 °C to 845 °C, the ethylene yield increased from 30% to 35%, and increasing the temperature above 845 °C did not cause a considerable increase in the yield (
Figure 2a). The yields of methane and propylene showed little changes when changing the temperature from 830 °C to 845 °C. Interestingly, the coke formation increased significantly (from 7% to 23%) when increasing the temperature.
Figure 1. Effect of temperature at different steam-to-hydrocarbon (ST:HC) mass ratios (
a) ST:HC = 1.01, (
b) ST:HC = 1.03, (
c) ST:HC = 1.33; and (
d) the effect of ST:HC on the product yields of the thermal steam cracking of an atmospheric gas oil, reproduced from
[2].
Figure 2. (
a) Effect of coil outlet temperature on the product yields
[3]; (
b) effect of temperature on light olefin yield; and (
c) coke formation for the cracking of Arabian Light crude oil using thermal cracking
[4].
Al-Absi and Al-Khattaf
[4] reported that the conversion in thermal cracking is a function of contact time and temperature during the cracking of Arabian Light (AL) crude oil. Increasing the temperature from 550 °C to 650 °C resulted in a sharp increase in the C
2–C
4 olefin yield from 3.8 wt.% to 22.9 wt.% (
Figure 2b). The main drawback of high-temperature cracking is the rapid coke formation and carburization, resulting in a shorter lifetime of the reactor tube. The important reactions for coke formation are di- and poly-alkenes, the cyclization of alkenes, aromatization, and condensation. Polyaromatic compounds formed via these reactions are the main source of coke during the reaction. Similarly to the results of other reports, coke formation during the cracking of AL crude oil also increased when increasing the temperature (
Figure 2c). However, nowadays, engineers are trying to develop new technologies to reduce coke formation and to use better metallurgy to endure the high temperatures.
Che et al.
[5] performed the thermal cracking of a vacuum residue to investigate the product distribution at different temperatures and a steam-to-hydrocarbon weight ratio of 0.6. By increasing the temperature from 600 °C to 700 °C, the conversion increased from 90.67% to 93.97% (
Table 1). Due to the stronger bond breaking at the higher temperatures, the gas yield also increased gradually with temperature. The yield of C
2–C
4 olefins increased by 47% (from 17.71% to 18.73%) when the temperature increased from 600 °C to 700 °C. The olefinicity (weight ratio of unsaturated open-chain hydrocarbons with at least one double bond, including C
2, C
3, and C
4 olefins, in the cracked gas) of 53–67% confirmed that alkenes are the main components in the gaseous products. Increasing the temperature enhanced the intensity of condensations, and consequently the coke yield increased.
Table 1. Conversion and composition of thermal cracking products of VR
[5].
| Product |
Temperature (°C) |
| 600 |
650 |
700 |
| Conversion (%) |
90.67 |
91.55 |
93.97 |
| Gas yield (wt.%) |
18.92 |
27.38 |
35.32 |
| Gas composition (wt.%) |
|
|
|
| Methane |
2.91 |
5.01 |
9.44 |
| Ethylene |
5.76 |
6.92 |
6.66 |
| Propylene |
4.76 |
6.38 |
6.95 |
| Butenes |
2.19 |
4.02 |
5.12 |
| C2–C4 olefins |
12.71 |
17.32 |
18.73 |
| Olefinicity (%) |
67.16 |
63.26 |
53.03 |
| Coke yield (wt.%) |
8.20 |
8.49 |
9.13 |
2. Residence Time
The other operating parameter is residence time, which can be measured based on the initial contact between the heat source and feedstocks to the time of product quenching
[6]. The produced olefins could be degraded to the lower-value long-chain hydrocarbons due to the occurrence of a multitude of side reactions. A long residence time favors secondary reactions, whereas the yield of the primary products such as ethylene and propylene increases at a short residence time
[1]. These side reactions need to be minimized by designing the coils to have a very short residence time and through the rapid cooling of the product gas mixture after it leaves the reactor coil. Old reactors, constructed between 1940 and 1960, had horizontal tubes with a residence time of more than 0.5 s. The reactor tube diameter needs to be decreased to reduce the residence time
[7]. In modern reactors, the residence time is controlled by the tube diameter and reactor flow rate, and they have a residence time of 0.08–0.25 s. A lower residence time could be obtained by decreasing the tube diameter. Gál and Lakatos
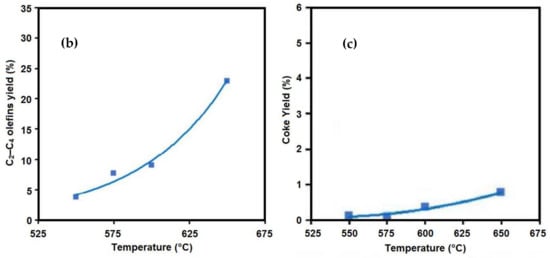
[3] reported that the conversion of n-butane was increased by increasing the residence time from 0.3 s to 0.65 s and 1.1 s (
Figure 3a). The same trend was also observed for the ethylene yield, and compared with the yield at a residence time of 0.3 s, it was 7% and 11.1% higher at the residence times of 0.65 s and 1.1 S, respectively. The coke formation also significantly increased at a higher residence time, and it was 21% and 32% higher at 0.65 s and 1.1 s, respectively. The effect of temperature, residence time, and the steam-to-hydrocarbon ratio on the yield of light olefins in naphtha steam cracking was investigated by Keyvanloo et al.
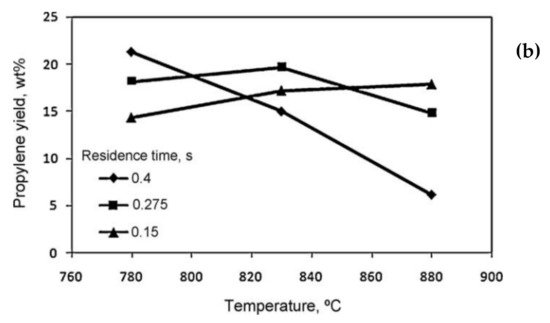
[8]. It was observed that at the short residence time of 0.15 s, the yield of propylene increased when increasing the temperature (
Figure 3b). At a lower reaction temperature, the yield of propylene was higher with a longer residence time, whereas by increasing the reaction temperature, the longer residence time resulted in the formation of secondary reactions and the yield of propylene decreased.
Figure 3. (
a) Effect of residence time on the product yields
[9], (
b) effect of residence time on the propylene yield (steam-to-oil ratio = 0.5 g/g)
[8].
In a study by Han et al.
[10], the influence of the residence time of the yield of light olefins in the steam cracking of a mixture of waste oil and naphtha was studied. By increasing the residence time from 0.3 s to 0.4 s, the yield of ethylene increased, whereas a further increase in the residence time resulted in a decrease in the total yield of C
2–C
4 light olefins. Raw materials have sufficient time for the reaction at longer residence times (up to 0.4 s); however, as mentioned earlier, the secondary reactions also start to occur at longer residence times, resulting in a lower gas yield and higher coke formation. The effect of residence time was more prominent on the yield of ethylene than that of propylene and butadiene. In another study by Karaba et al.
[11], the effect of residence time on the product distribution of the steam cracking process at 815 °C was evaluated. In this study, a mixture of 10 wt.% FT vacuum residue and 90 wt.% hydrocracked vacuum distillate (HCVD) was used as the feedstock. The shorter residence time resulted in higher yields of ethylene and propylene, whereas the yields of ethane, benzene, toluene, and oil decreased (
Table 2). Sedighi et al.
[12] also reported that an increase in the residence time led to a higher rate of ethylene formation, whereas the propylene yield decreased. Accurate control of residence times and temperature are crucial to avoid secondary reactions while still allowing the maximum cracking of the feedstock.
Table 2. Effect of residence time on the product yields for the steam cracking of a mixture of 10 wt.% FT residue and 90 wt.% HCVD at 815 °C
[11].
| Products Yields (wt.%) |
Residence Time (s) |
| 0.22 |
0.30 |
0.51 |
| Methane |
4.6 |
6.8 |
8.9 |
| Ethane |
1.6 |
1.8 |
2.6 |
| Ethylene |
32.7 |
30.4 |
28.1 |
| Propane |
0.3 |
0.5 |
0.4 |
| Propylene |
15.8 |
15.3 |
13.7 |
| Acetylene |
0.6 |
0.5 |
0.4 |
| i-butane |
0.0 |
0.0 |
0.0 |
| Propadiene |
0.7 |
0.4 |
0.3 |
| n-butane |
0.0 |
0.1 |
0.1 |
| t-2-butene |
0.3 |
0.3 |
0.3 |
| 1-butene |
6.6 |
3.6 |
1.3 |
| i-butene |
1.5 |
1.5 |
1.2 |
| c-2-butene |
0.3 |
0.4 |
0.3 |
| Propyne |
0.5 |
0.5 |
0.4 |
| 1,3-butadiene |
8.7 |
8.1 |
6.3 |
| Cyclopentadiene + isoprene |
3.6 |
3.2 |
2.8 |
| Benzene |
4.2 |
6.6 |
9.8 |
| Toluene |
2.1 |
3.3 |
4.6 |
| Ethylbenzene |
0.4 |
0.4 |
0.3 |
| Xylenes |
1.3 |
1.8 |
2.6 |
| C5–C6 |
4.6 |
3.5 |
2.2 |
| C7–C12 |
2.9 |
2.9 |
3.0 |
| Oil |
6.6 |
8.2 |
10.3 |
3. Steam-to-Hydrocarbon Ratio
The ratio of steam to hydrocarbon feedstock is another parameter that needs to be considered during the cracking reaction. Steam and feedstock are mixed and preheated in the convection section of the cracking furnace to vaporize the liquid feedstock
[13]. The steam-to-hydrocarbon ratio (ST:HC) depends on the feedstock type and is controlled between 0.3 and 0.7 on a weight basis. Steam is used as an inert that is premixed with the feed to reduce the partial pressure of the hydrocarbons, therefore reducing the coke formation and carbonization rates. The temperature and thermal energy of the feedstock also increase with the addition of steam. The ST:HC ratio is around 0.3 kg
steam/Kg
hydrocarbon for the lighter gaseous feeds, such as ethane, and increases to 0.6 kg
steam/Kg
hydrocarbon for the heavier hydrocarbons, such as naphtha and gas oil
[7]. It is important to have an optimum ratio of steam to hydrocarbon to avoid negative effects on product yields and reduce the specific energy consumption of the production unit. The influence of the steam-to-hydrocarbon ratio in the steam cracking of natural gas was studied by Gál and Lakatos
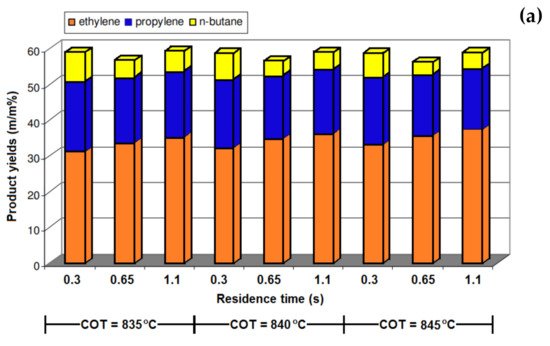
[3], and the basic value for ST:HC was considered to be 0.5 in that study. Their results (
Table 3) showed that reducing the ST:HC ratio up to 10% did not significantly affect the product yields, but a shorter reaction time was expected due to the higher coke formation. A further decrease in the ST:HC ratio (up to 30%) resulted in a decrease in the ethylene formation and increased the coke formation. By reducing the ST:HC ratio at both 835 °C and 840 °C, methane formation showed a rising trend.
Table 3. Effect of the steam-to-hydrocarbon ratio on the product yields
[3].
| COT (°C) |
835 |
840 |
835 |
840 |
835 |
840 |
835 |
840 |
| Reduction of ST:HC Ratio |
5% |
5% |
10% |
10% |
20% |
20% |
30% |
30% |
| Products |
|
|
|
|
|
|
|
|
| Methane |
18.22 |
18.75 |
18.32 |
18.72 |
18.53 |
19.06 |
19.27 |
19.89 |
| Ethylene |
32.22 |
33.73 |
32.97 |
33.18 |
31.78 |
32.25 |
30.46 |
30.87 |
| Propylene |
20.63 |
20.15 |
20.68 |
20.34 |
20.79 |
20.32 |
21.14 |
20.87 |
| Butadiene |
3.97 |
3.85 |
3.97 |
3.89 |
3.98 |
3.86 |
3.64 |
3.57 |
n-butane
(Residual) |
8.74 |
7.58 |
8.72 |
7.88 |
8.68 |
7.56 |
8.64 |
7.54 |
| Benzene + Toluene |
1.61 |
1.63 |
1.71 |
1.74 |
1.81 |
1.89 |
1.98 |
2.05 |
| Coke |
0.0084 |
0.0089 |
0.0091 |
0.0097 |
0.0107 |
0.012 |
0.0128 |
0.015 |
The product distribution in the thermal cracking of atmospheric gas oil at 800 °C and a residence time of 0.4 s was studied by Abghari et al.
[14]. It was found that by increasing the ST:HC ratio, the yield of methane and ethylene decreased, whereas the propylene yield did not change much. Similarly, a study on the effect of the steam-to-gas-oil ratio in a cracking reaction at 750 °C (
Figure 1d) revealed that the partial pressure of hydrocarbons was reduced via steam dilution; the gas conversion and the yields of methane and ethylene were decreased, whereas the coke formation increased
[2]. In another study by Sedighi et al.
[12][15], the effect of the steam-to-hydrocarbon ratio on the product yields in the steam cracking of heavy liquid hydrocarbon (at 843 °C and a residence time of 0.17 s) was studied, and it was reported that the yields of methane and ethylene increased when increasing the ST:HC ratio, whereas propylene yield decreased slightly. The evaluation of the effect of the ST:HC ratio on the product distribution in the steam cracking of a mixture of waste oil and naphtha by Han et al.
[10] revealed that the yield of light olefins reached a maximum when increasing the ratio from 0.42 to 0.65, then decreased when increasing the ST:HC ratio up to about 0.9. Increasing the ratio above 0.9 did not significantly affect the yields, and they remained unchanged. At constant total pressure, a more diluted feedstock (higher ST:HC ratio) with a lower partial pressure of hydrocarbon results in a higher rate of ethylene formation.
Ethane steam cracking in an industrial tubular reactor was modeled and optimized by Jiang et al.
[16]. By increasing the steam-to-ethane ratio, the selectivity to ethylene increased from 69% to 82%, whereas at the same time, the conversion of ethane decreased from 74% to 55%. The optimum ratio of steam to ethane was found to be in the range of 0.3 to 0.4 in order to obtain the highest ethylene yield. Increasing the steam-to-ethane ratio from 0 to 1.0 resulted in a 46% decrease in coke formation (from 0.0092 to 0.0042 kg/(h.m
2)). It is important to operate at the optimum steam-to-hydrocarbon ratio to achieve the highest possible yields of light olefins and an acceptable coke formation rate.
4. Feedstock Composition
The product yield can be affected by the feed composition. This effect is more pronounced in the re-pyrolysis of recycled cracked gases, which can be mixed with the fresh hydrocarbons, and may contain a large amount of olefins and di-olefins. Gál and Lakatos
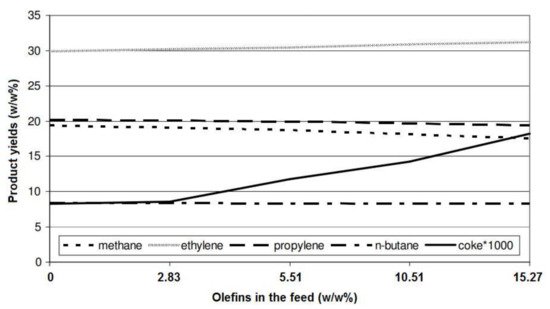
[3] reported that the yield of methane and propylene slightly decreased by increasing the presence of olefins in the feedstock (
Figure 4); lower olefin concentration resulted in lower coke formation. The ethylene yield increased gradually by increasing the presence of unsaturated hydrocarbons in the feedstock. The dependency of product yields and the type of feedstock was studied by Dominov et al.
[17]. The product distribution is changed by varying the feedstock composition. It can be seen that by changing the feedstock from ethane to propane and butane and liquid feedstocks, the ethylene yield decreased by more than 40%, whereas the yield of heavier olefins increased. The feedstocks of ethylene plants usually contain straight and branched alkanes, olefins, aromatics, and naphthenes.
Figure 4. Effect of the olefin concentration in the feedstock on product yields
[3].
Olefins are produced mainly from alkanes and naphthenes in the feedstock. The higher concentration of n-alkanes leads to a higher ethylene yield, and alkanes with even numbers of carbon atoms result in a slightly higher ethylene yields than those with odd numbers of carbon atoms. The yield of propene decreases when increasing the chain length of hydrocarbons in the feedstock
[1]. Compared with n-alkanes, the presence of isoalkanes results in lower ethylene and propene yields and produces higher amounts of hydrogen, methane, C
4, and higher olefins. Simple and aromatic ring compounds, such as benzene, are formed during the cracking process and remain unchanged under normal cracking conditions.
Zimmermann and Walzl
[1] provided a comprehensive investigation of the production of ethylene through steam cracking using different gaseous and liquid feedstocks, including ethane, propane, butane, naphtha, gas oil (atmospheric gas oil (AGO)), and hydrocracker residue (hydrocracker residue (HCR) or heavy vacuum gas oil (HVGO). The effect of residence time on the product distribution was also reported. The ratio of steam to hydrocarbon (ST:HC) was increased by changing the feedstock from light hydrocarbon (ethane) to heavier feedstocks, as follows: ethane, propane (ST:HC = 0.3) < butane (ST:HC = 0.35) < low-severity naphtha, P/E = 0.65 kg/kg (ST:HC = 0.4) < medium-severity naphtha, P/E = 0.55 kg/kg (ST:HC = 0.45) < high-severity naphtha, P/E = 0.45 kg/kg (ST:HC = 0.5) < AGO, P/E = 0.54 kg/kg (ST:HC = 0.8) < AGO, P/E = 0.53 kg/kg (ST:HC = 1.0). When ethane was used as the feedstock, the coil inlet temperature was in the range of 650–680 °C, and the conversion was 60–75% in commercial furnaces. A typical conversion of 90–93% was reported for the propane cracking in the furnace, and conversion increased to 94–96% when using butane as the feedstock. Reducing the residence time resulted in the lower formation of methane and the higher formation of ethylene. Due to the presence of a large amount of condensed polynuclear aromatics in gas oil, heavier products (C
5+) were formed using AGO as the cracking feedstock. During the cracking process, these aromatic components remained unchanged or condensed to other molecules with a higher molecular mass. HCR, the residue of the hydrocrackers, which are working at severe operating conditions, is a highly saturated product with a low content of aromatics and polyaromatics. The yield of ethylene using the HCR or HVGO is almost similar to naphtha cracking (~26%)
[1].
The product distribution in the thermal cracking of different types of crude oil was evaluated by Al-Absi et al.
[18]. The crude oils with different API gravities of 34° (Arab Light: AL), 39° (Arab Extra Light: AXL), and 51° (Arab Super Light: ASL) were used as the cracking feedstock. A lower API gravity indicates a heavier crude with a higher density. The density of the selected feedstocks at 15 °C were in the following order: AL (892 kg/m
3) > AXL (828 kg/m
3) > ASL (774 kg/m
3). The paraffin, isoparaffin, olefin, naphthene, and aromatic (PIONA) contents of the naphtha fraction of these crude oils were: 34/32/0/19/15 wt.%, 34/31/0/8/27 wt.%, and 46/18/0/9/27 wt.% for ASL, AXL, and AL, respectively. There was a linear relationship between the conversion of the feedstocks and temperature and this was increased by increasing the temperature (
Table 4).
Table 4. Conversion and yields of product for the thermal cracking of different crude oils
[18].
| Feed |
ASL |
AXL |
AL |
| Temperature (°C) |
600 |
625 |
650 |
600 |
625 |
650 |
600 |
625 |
650 |
| Conversion (%) |
14.5 |
21.1 |
27.6 |
14.0 |
21.6 |
27.0 |
14.2 |
23.3 |
32.8 |
| Product yield (%) |
|
|
|
|
|
|
|
|
|
| H2 |
0.1 |
0.1 |
0.1 |
0.1 |
0.1 |
0.1 |
0.1 |
0.1 |
0.2 |
| C1 |
1.5 |
2.5 |
3.6 |
1.7 |
2.8 |
3.5 |
1.8 |
3.1 |
4.6 |
| C=2 |
2.6 |
4.1 |
6.1 |
3.1 |
5.0 |
6.5 |
2.8 |
5.1 |
7.6 |
| C=3 |
3.1 |
5.0 |
6.8 |
3.4 |
5.5 |
6.9 |
3.2 |
5.7 |
8.6 |
| C=4 |
3.4 |
4.9 |
6.0 |
3.2 |
4.6 |
5.6 |
3.0 |
4.7 |
6.7 |
| C=2−C=4 |
9.1 |
14.0 |
18.9 |
9.7 |
15.1 |
19.0 |
9.0 |
15.5 |
22.9 |
| C=3/C=2 |
1.2 |
1.2 |
1.1 |
1.1 |
1.1 |
1.1 |
1.1 |
1.1 |
1.1 |
| Coke |
0.2 |
0.3 |
0.3 |
0.3 |
0.4 |
0.5 |
0.4 |
0.6 |
0.8 |
The conversions of all feedstocks were about 14% at 600 °C and the highest conversion (32.8%) belonged to the heaviest feedstock (AL) at 650 °C. Similarly to the conversion, the yield of C
2–C
4 olefins increased by increasing the temperature. The ethylene formation obeyed the free radical and beta-scission mechanisms. At higher temperatures, the cleavage of C–C bond uncharged molecules resulted in the formation of very reactive free radicals. These free radicals undergo beta-scission and produce ethylene and primary free radicals. The yields of propylene and butenes also followed the same trend as ethylene and increased when increasing the temperature
[18].
The effect of feedstock composition on the product distribution in the steam cracking process, as studied by Geerts et al.
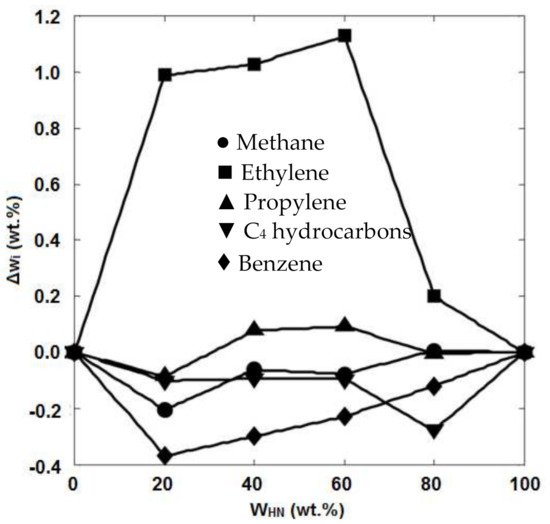
[19], also revealed that the steam cracking of the naphtha fraction resulted in higher yields of ethylene and propylene (36%) than the yields of these products (28.3%) obtained from the steam cracking of wide-range gas oil (WRGO). However, the yields of pyrolysis fuel oil (PFO) and pyrolysis gasoline (Pygas) were higher when WRGO was used as the feedstock. The pyrolysis of light and heavy naphtha feedstocks and their mixtures was evaluated by Karaba et al.
[20]. It was reported that the product distribution depended on the feedstock’s properties, and light naphtha could lead to higher yields of the light olefins. Pyrolysis of the feedstock containing 20–60 wt.% of heavy naphtha resulted in a higher ethylene yield (around 1.1 wt.% higher), whereas the addition of light naphtha to the heavy naphtha feedstock caused a decrease in the yields of methane, C
4 hydrocarbons, and benzene (
Figure 5). Even this slight increase in the ethylene yield could make a significant improvement at the industrial scale. For example, for an ethylene plant with 30 tons per hour of naphtha feedstock, this 1.1 wt.% increase in the ethylene yield results in an additional 330 kg of ethylene per hour per one steam cracker, which means about 300 EUR per hour per one steam cracker
[20].
Figure 5. Potential product yield increases (T: 810 °C, P: 4 bar, F: 65 Nml/min) depending on the content of heavy naphtha in the blend with light naphtha
[20].
Zámostný et al.
[21] studied the pyrolysis of feedstocks with different hydrocarbon chain lengths at 810 °C, 400 kPa, and residence times of 0.2–0.4 s in the reaction zone. They found that longer-chain hydrocarbons with a higher number of carbon atoms showed a higher probability of the hydrogen abstraction reaction; thus, the hydrogen transfer rate could increase and result in a higher conversion rate. The feedstock conversion increased from 78% for n-pentane to 99% for n-hexane. The yield of ethylene also increased for longer hydrocarbon chains because despite the original position of the unpaired electron in the formed radicals, they can produce more ethylene molecules through the successive-beta scission of C–C bonds. However, in very-long-chain hydrocarbons, the beta-scission of C–H bonds could interrupt the beta-scission of the C–C bonds and result in the increased formation of 1-alkene. Due to the more evident effect of alkyl radicals with odd carbon numbers produced during the cracking process, the yield of propylene and methane decreased when increasing the chain length
[21].
Recently, the effect of normal/cyclo-alkane in the product distribution of hydrocarbon pyrolysis was studied by Hou et al.
[22]. Pyrolysis of n-hexane, n-heptane, cyclohexane, and methylcyclohexane was performed at 650–830 °C, under atmospheric pressure. The molecular structures could determine the initial cleavage of C–C bonds and the formation of radicals, which affect the hydrogen abstraction, beta-scission, secondary cracking, radical combination aromatization, and Diels–Alder reactions in the pyrolysis process. The direct cleavage of C–C bonds and the production of alkyl radicals were observed for the n-hexane feedstock, while cyclohexane with the cyclic structure with the consecutive reactions of ring-opening, isomerization, and decomposition led to the formation of alkyl and alkenyl radicals. A lower conversion and a higher selectivity to 1,3-butadiene and aromatics were observed in the pyrolysis of cyclohexane. Similarly to the results reported by Zámostný et al.
[21], a longer hydrocarbon chain length weakened the C–C bonds and thus increased the number of free radicals, resulting in higher conversion and selectivity to heavier products. A demethylation reaction was observed in the presence of the methyl substituent, which can improve the formation of radicals (methyl, cycloalkyl, and methylcycloalkyl). A higher conversion rate was obtained with the pyrolysis of methylcyclohexane than with cyclohexane. Pyrolysis of the mixture of n-hexane and cyclohexane revealed that the formation of radicals and hydrogen abstraction were decreased by increasing the cyclohexane content. Due to the competition of cyclohexane and n-hexane for the radicals in hydrogen abstraction, the n-hexane decomposition could be suppressed with a higher content of cyclohexane
[22].