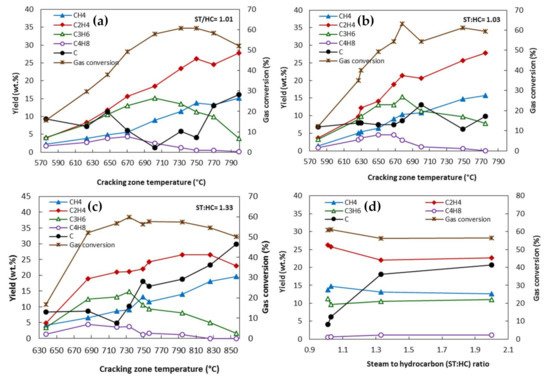
The other operating parameter is residence time, which can be measured based on the initial contact between the heat source and feedstocks to the time of product quenching
[6][47]. The produced olefins could be degraded to the lower-value long-chain hydrocarbons due to the occurrence of a multitude of side reactions. A long residence time favors secondary reactions, whereas the yield of the primary products such as ethylene and propylene increases at a short residence time
[1][23]. These side reactions need to be minimized by designing the coils to have a very short residence time and through the rapid cooling of the product gas mixture after it leaves the reactor coil. Old reactors, constructed between 1940 and 1960, had horizontal tubes with a residence time of more than 0.5 s. The reactor tube diameter needs to be decreased to reduce the residence time
[7][32]. In modern reactors, the residence time is controlled by the tube diameter and reactor flow rate, and they have a residence time of 0.08–0.25 s. A lower residence time could be obtained by decreasing the tube diameter. Gál and Lakatos
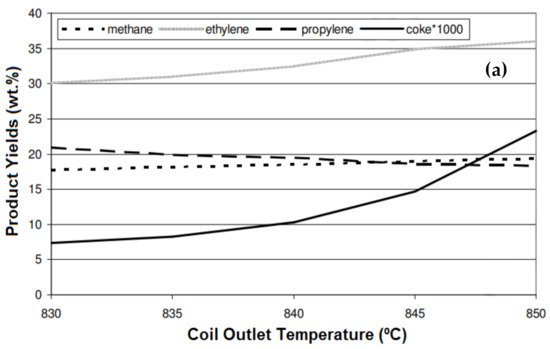
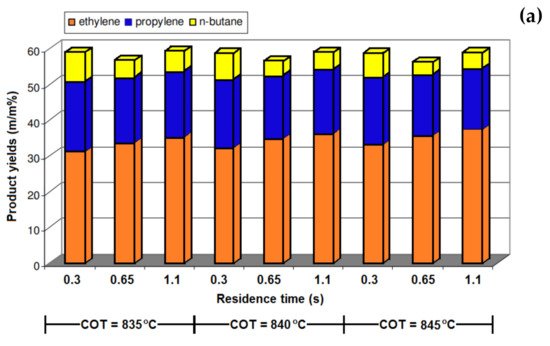
[3][44] reported that the conversion of n-butane was increased by increasing the residence time from 0.3 s to 0.65 s and 1.1 s (
Figure 310a). The same trend was also observed for the ethylene yield, and compared with the yield at a residence time of 0.3 s, it was 7% and 11.1% higher at the residence times of 0.65 s and 1.1 S, respectively. The coke formation also significantly increased at a higher residence time, and it was 21% and 32% higher at 0.65 s and 1.1 s, respectively. The effect of temperature, residence time, and the steam-to-hydrocarbon ratio on the yield of light olefins in naphtha steam cracking was investigated by Keyvanloo et al.
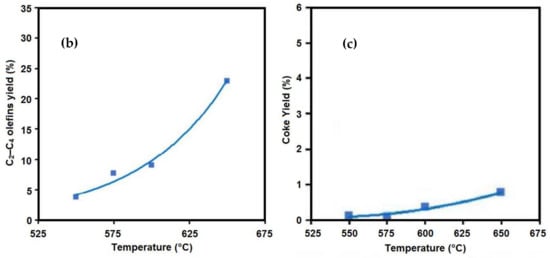
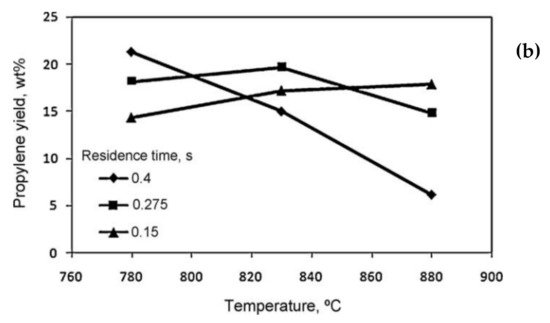
[8][48]. It was observed that at the short residence time of 0.15 s, the yield of propylene increased when increasing the temperature (
Figure 310b). At a lower reaction temperature, the yield of propylene was higher with a longer residence time, whereas by increasing the reaction temperature, the longer residence time resulted in the formation of secondary reactions and the yield of propylene decreased.
In a study by Han et al.
[10][49], the influence of the residence time of the yield of light olefins in the steam cracking of a mixture of waste oil and naphtha was studied. By increasing the residence time from 0.3 s to 0.4 s, the yield of ethylene increased, whereas a further increase in the residence time resulted in a decrease in the total yield of C
2–C
4 light olefins. Raw materials have sufficient time for the reaction at longer residence times (up to 0.4 s); however, as mentioned earlier, the secondary reactions also start to occur at longer residence times, resulting in a lower gas yield and higher coke formation. The effect of residence time was more prominent on the yield of ethylene than that of propylene and butadiene. In another study by Karaba et al.
[11][50], the effect of residence time on the product distribution of the steam cracking process at 815 °C was evaluated. In this study, a mixture of 10 wt.% FT vacuum residue and 90 wt.% hydrocracked vacuum distillate (HCVD) was used as the feedstock. The shorter residence time resulted in higher yields of ethylene and propylene, whereas the yields of ethane, benzene, toluene, and oil decreased (
Table 24). Sedighi et al.
[12][30] also reported that an increase in the residence time led to a higher rate of ethylene formation, whereas the propylene yield decreased. Accurate control of residence times and temperature are crucial to avoid secondary reactions while still allowing the maximum cracking of the feedstock.