Various parameters need to be considered to optimize a pyrolysis reactor for the production of ethylene and propylene. These parameters are included temperature, residence time, steam-to-hydrocarbon ratio, and feedstock composition. A higher reaction temperature results in a higher yield of ethylene, a shorter residence time, and higher reaction velocities. A low operating pressure is preferred for this process because of the gas phase reaction inside the reactor tubes and the production of more gaseous molecules for its reactions to the right. The reactor pressure must be kept as low as possible (between 170 and 250 kPa). The gas pressure in the reactor tube outlet is indirectly adjusted by the suction process gas pressure of the compressor in the compression section, downstream of the process. As mentioned earlier, to reach lower hydrocarbon pressures, process steam is co-fed with the hydrocarbon feed to reduce the hydrocarbon partial pressure and lower coke deposition. The addition of steam decreases the partial pressure of the hydrocarbon feed and favors the formation of primary products.
- olefin production
- steam cracking
- cracking furnace
- Operating parameters
1. Temperature
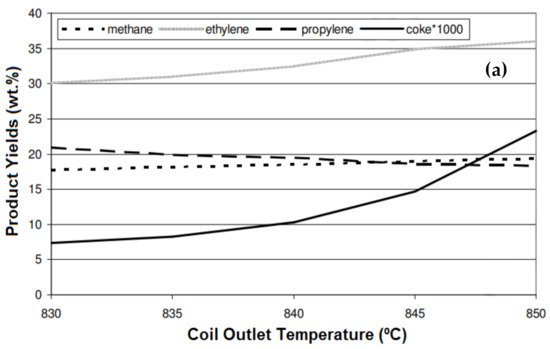
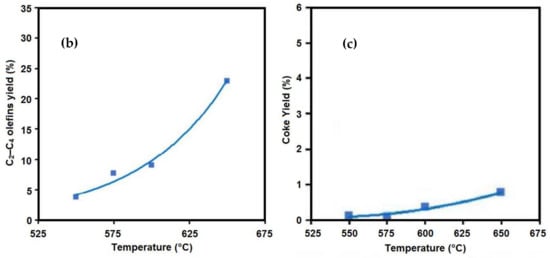
| Product | Temperature (°C) | ||
|---|---|---|---|
| 600 | 650 | 700 | |
| Conversion (%) | 90.67 | 91.55 | 93.97 |
| Gas yield (wt.%) | 18.92 | 27.38 | 35.32 |
| Gas composition (wt.%) | |||
| Methane | 2.91 | 5.01 | 9.44 |
| Ethylene | 5.76 | 6.92 | 6.66 |
| Propylene | 4.76 | 6.38 | 6.95 |
| Butenes | 2.19 | 4.02 | 5.12 |
| C2–C4 olefins | 12.71 | 17.32 | 18.73 |
| Olefinicity (%) | 67.16 | 63.26 | 53.03 |
| Coke yield (wt.%) | 8.20 | 8.49 | 9.13 |
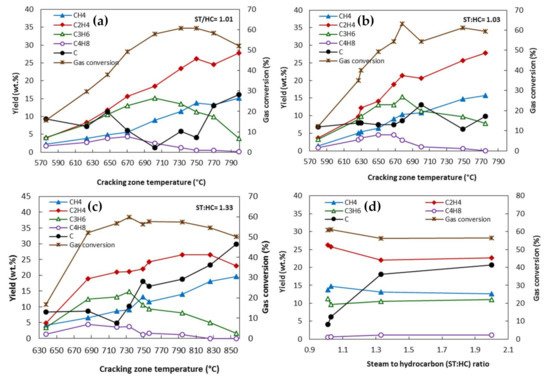
2. Residence Time
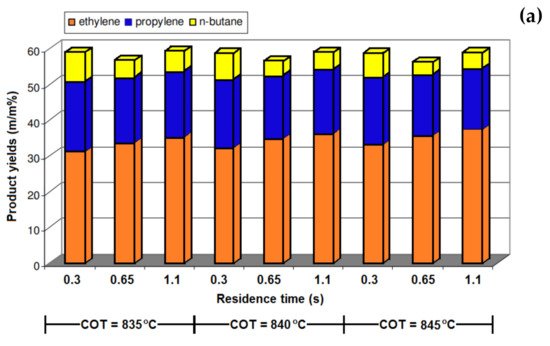
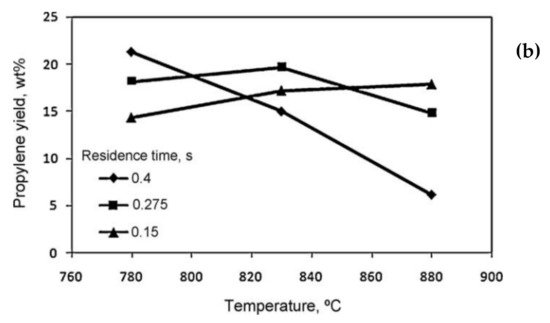
| Products Yields (wt.%) | Residence Time (s) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.22 | 0.30 | 0.51 | ||||||||||
| Methane | 4.6 | 6.8 | 8.9 | |||||||||
| Ethane | 1.6 | 1.8 | 2.6 | |||||||||
| Ethylene | 32.7 | 30.4 | 28.1 | |||||||||
| Propane | 0.3 | 0.5 | 0.4 | |||||||||
| Propylene | 15.8 | 15.3 | 13.7 | |||||||||
| Acetylene | 0.6 | 0.5 | 0.4 | |||||||||
| i-butane | 0.0 | 0.0 | 0.0 | |||||||||
| Propadiene | 0.7 | 0.4 | 0.3 | |||||||||
| n-butane | ||||||||||||
| C=2−C= | 0.0 | 0.1 | 0.1 | |||||||||
| 4 | 9.1 | 14.0 | 18.9 | 9.7 | 15.1 | 19.0 | 9.0 | 15.5 | t-2-butene | 0.3 | 0.3 | 0.3 |
| 1-butene2.9 | 2.9 | 3.0 | ||||||||||
| Oil | 6.6 | 8.2 | 10.3 | |||||||||
3. Steam-to-Hydrocarbon Ratio
| COT (°C) | 835 | 840 | 835 | 840 | 835 | 840 | 835 | 840 |
|---|---|---|---|---|---|---|---|---|
| Reduction of ST:HC Ratio | 5% | 5% | 10% | 10% | 20% | 20% | 30% | 30% |
| Products | ||||||||
| Methane | 18.22 | 18.75 | 18.32 | 18.72 | 18.53 | 19.06 | 19.27 | 19.89 |
| 22.9 | ||||||||
| C=3/C=2 | 1.2 | 1.2 | 1.1 | 1.1 | 1.1 | 1.1 | ||
| 6.6 | 3.6 | 1.3 |
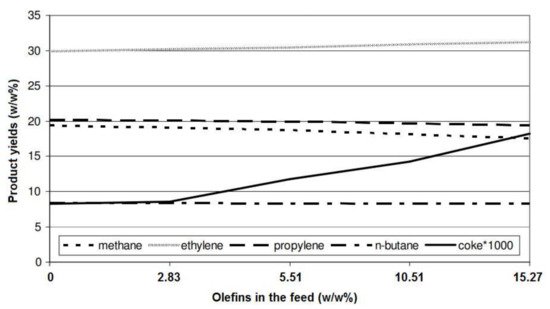
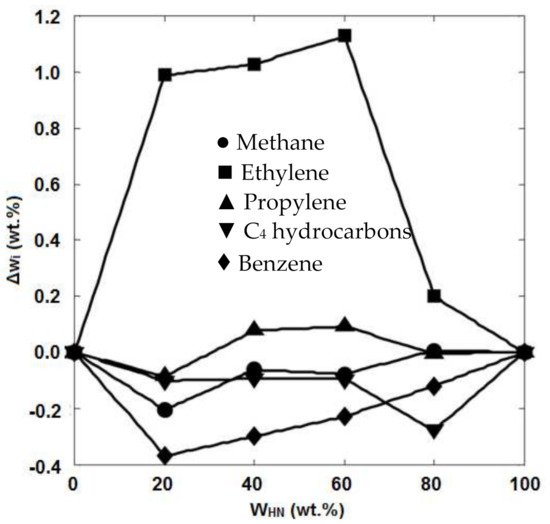
4. Feedstock Composition
| Feed | ASL | AXL | AL | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Temperature (°C) | 600 | 625 | 650 | 600 | 625 | 650 | 600 | 625 | 650 | ||||||||||||
| Conversion (%) | 14.5 | 21.1 | 27.6 | 14.0 | 21.6 | 27.0 | 14.2 | 23.3 | 32.8 | Ethylene | 32.22 | 33.73 | 32.97 | 33.18 | 31.78 | 32.25 | 30.46 | 30.87 | |||
| Product yield (%) | Propylene | 20.63 | 20.15 | 20.68 | 20.34 | ||||||||||||||||
| H2 | 0.1 | 0.1 | 0.120.79 | 20.32 | 0.1 | 0.1 | 0.121.14 | 20.87 | |||||||||||||
| 0.1 | 0.1 | 0.2 | Butadiene | 3.97 | 3.85 | 3.97 | 3.89 | 3.98 | 3.86 | 3.64 | 3.57 | ||||||||||
| C1 | 1.5 | 2.5 | 3.6 | 1.7 | 2.8 | 3.5 | 1.8 | 3.1 | 4.6 | n-butane (Residual) |
8.74 | 7.58 | 8.72 | 7.88 | 8.68 | 7.56 | 8.64 | 7.54 | |||
| C=2 | 2.6 | 4.1 | 6.1 | 3.1 | 5.0 | 6.5 | 2.8 | 5.1 | 7.6 | Benzene + Toluene | 1.61 | 1.63 | 1.71 | 1.74 | 1.81 | 1.89 | 1.98 | 2.05 | |||
| C=3 | 3.1 | 5.0 | 6.8 | 3.4 | 5.5 | 6.9 | 3.2 | 5.7 | 8.6 | Coke | 0.0084 | 0.0089 | 0.0091 | 0.0097 | 0.0107 | 0.012 | 0.0128 | 0.015 | 1.1 | 1.1 | 1.1 |
| Coke | 0.2 | 0.3 | 0.3 | 0.3 | 0.4 | i-butene | 1.5 | 1.5 | 1.2 | ||||||||||||
| 0.5 | 0.4 | 0.6 | c-2-butene | 0.3 | 0.4 | 0.3 | |||||||||||||||
| Propyne | 0.5 | 0.5 | 0.4 | ||||||||||||||||||
| 1,3-butadiene | 8.7 | 8.1 | 6.3 | ||||||||||||||||||
| Cyclopentadiene + isoprene | 3.6 | 3.2 | 2.8 | ||||||||||||||||||
| 0.8 | Benzene | 4.2 | 6.6 | 9.8 | |||||||||||||||||
| Toluene | 2.1 | 3.3 | 4.6 | ||||||||||||||||||
| Ethylbenzene | 0.4 | 0.4 | 0.3 | ||||||||||||||||||
| Xylenes | 1.3 | 1.8 | 2.6 | ||||||||||||||||||
| C5–C6 | 4.6 | 3.5 | 2.2 | ||||||||||||||||||
| C7–C12 | |||||||||||||||||||||
| C | |||||||
| = | |||||||
| 4 | |||||||
| 3.4 | |||||||
| 4.9 | 6.0 | 3.2 | 4.6 | 5.6 | 3.0 | 4.7 | 6.7 |
References
- Zimmermann, H.; Walzl, R. Ethylene. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley: Weinheim, Germany, 2009.
- Depeyre, D.; Flicoteaux, C.; Arbabzadeh, F.; Zabaniotou, A. Modeling of Thermal Steam Cracking of an Atomspheric Gas Oil. Ind. Eng. Chem. Res. 1989, 28, 967–976.
- Gál, T.; Lakatos, B.G. Thermal Cracking of Recycled Hydrocarbon Gas-Mixtures for Re-Pyrolysis: Operational Analysis of Some Industrial Furnaces. Appl. Therm. Eng. 2008, 28, 218–225.
- Al-Absi, A.A.; Al-Khattaf, S.S. Conversion of Arabian Light Crude Oil to Light Olefins via Catalytic and Thermal Cracking. Energy Fuels 2018, 32, 8705–8714.
- Che, Y.; Hao, J.; Zhang, J.; Qiao, Y.; Li, D.; Tian, Y. Vacuum Residue Thermal Cracking: Product Yield Determination and Characterization Using Thermogravimetry–Fourier Transform Infrared Spectrometry and a Fluidized Bed Reactor. Energy Fuels 2018, 32, 1348–1357.
- Hulet, C.; Briens, C.; Berruti, F.; Chan, E.W. A Review of Short Residence Time Cracking Processes. Int. J. Chem. React. Eng. 2005, 3, 1–74.
- Sadrameli, S.M. Thermal/Catalytic Cracking of Hydrocarbons for the Production of Olefins: A State-of-the-Art Review I: Thermal cracking review. Fuel 2015, 140, 102–115.
- Keyvanloo, K.; Towfighi, J.; Sadrameli, S.M.; Mohamadalizadeh, A. Investigating the Effect of Key Factors, Their Interactions and Optimization of Naphtha Steam Cracking by Statistical Design of Experiments. J. Anal. Appl. Pyrolysis 2010, 87, 224–230.
- Wang, Q.; Xu, H.; Pan, L.; Sun, L. Active Disturbance Rejection Control of Boiler Forced Draft System: A Data-Driven Practice. Sustainability 2020, 12, 4171.
- Han, L.; Ding, C.Q.; Lui, H. Studies on Olefin Production by Steam Cracking of Waste Oil Blended with Naphtha. Appl. Mech. Mater. 2013, 291, 738–743.
- Karaba, A.; Rozhon, J.; Patera, J.; Hájek, J.; Zámostný, P. Fischer-Tropsch Wax from Renewable Resources as an Excellent Feedstock for the Steam-Cracking Process. Chem. Eng. Technol. 2021, 44, 329–338.
- Sedighi, M.; Keyvanloo, K.; Towfighi, D.J. Olefin Production from Heavy Liquid Hydrocarbon Thermal Cracking: Kinetics and Product Distribution. Iran. J. Chem. Chem. Eng. 2010, 29, 135–147.
- Abbasali, S.M.Z.; Farsi, M.; Rahimpour, M.R. Simulation and Dynamic Optimization of an Industrial Naphtha Thermal Cracking Furnace Based on Time Variant Feeding Policy. Chem. Prod. Process Model. 2018, 13, 1–15.
- Abghari, S.Z.; Darian, J.T.; Karimzadeh, R.; Omidkhah, M.R. Determination of Yield Distribution in Olefin Production by Thermal Cracking of Atmospheric Gasoil. Korean J. Chem. Eng. 2008, 25, 681–692.
- Sedighi, M.; Keyvanloo, K.; Towfighi, J. Experimental Study and Optimization of Heavy Liquid Hydrocarbon Thermal Cracking to Light Olefins by Response Surface Methodology. Korean J. Chem. Eng. 2010, 27, 1170–1176.
- Jiang, B.-B.; Liao, Z.-W.; Mohsin, A.; Sun, J.-Y.; Wang, J.-D.; Yang, Y.; Yang, Y.-R. Modeling and Optimization of Ethane Steam Cracking Process in an Industrial Tubular Reactor with Improved Reaction Scheme. China Petrol. Process. Petrochem. Technol. 2020, 22, 117–125.
- Dominov, P.; Gilyazetdinova, R.; Zhirnov, B.; Tarasov, I.; Khlestkin, R. Overview world technologies of pyrolysis and perspective of development. Electron. Sci. J. Oil Gas Bus. 2009, 1, 23–32. Available online: http://ogbus.ru/files/ogbus/eng/authors/Dominov/Dominov_1.pdf (accessed on 29 January 2021).
- Al-Absi, A.A.; Aitani, A.M.; Al-Khattaf, S.S. Thermal and Catalytic Cracking of Whole Crude Oils at High Severity. J. Anal. Appl. Pyrolysis 2020, 145, 104705.
- Geerts, M.; Ristic, N.; Djokic, M.; Ukkandath Aravindakshan, S.; Marin, G.B.; Van Geem, K.M. Crude to Olefins: Effect of Feedstock Composition on Coke Formation in a Bench-Scale Steam Cracking Furnace. Ind. Eng. Chem. Res. 2020, 59, 2849–2859.
- Karaba, A.; Dvořáková, V.; Patera, J.; Zámostný, P. Improving the Steam-Cracking Efficiency of Naphtha Feedstocks by Mixed/Separate Processing. J. Anal. Appl. Pyrolysis 2020, 146, 104768.
- Zámostný, P.; Bělohlav, Z.; Starkbaumová, L.; Patera, J. Experimental Study of Hydrocarbon Structure Effects on the Composition of its Pyrolysis Products. J. Anal. Appl. Pyrolysis 2010, 87, 207–216.
- Hou, X.; Ma, Z.; Chen, B.; Zhang, J.; Ning, Y.; Zhao, L.; Yuan, E. Role of normal/cyclo-alkane in hydrocarbons pyrolysis process and product distribution. J. Anal. Appl. Pyrolysis 2021, 156, 105130.