Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Juan Manuel Monteagudo Ruiz | + 1891 word(s) | 1891 | 2021-12-06 03:21:21 | | | |
| 2 | Bruce Ren | Meta information modification | 1891 | 2021-12-15 02:46:34 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Monteagudo Ruiz, J.M. 2D and 3D Echo in Mitral Stenosis. Encyclopedia. Available online: https://encyclopedia.pub/entry/17086 (accessed on 08 February 2026).
Monteagudo Ruiz JM. 2D and 3D Echo in Mitral Stenosis. Encyclopedia. Available at: https://encyclopedia.pub/entry/17086. Accessed February 08, 2026.
Monteagudo Ruiz, Juan Manuel. "2D and 3D Echo in Mitral Stenosis" Encyclopedia, https://encyclopedia.pub/entry/17086 (accessed February 08, 2026).
Monteagudo Ruiz, J.M. (2021, December 14). 2D and 3D Echo in Mitral Stenosis. In Encyclopedia. https://encyclopedia.pub/entry/17086
Monteagudo Ruiz, Juan Manuel. "2D and 3D Echo in Mitral Stenosis." Encyclopedia. Web. 14 December, 2021.
Copy Citation
Mitral stenosis is an important cause of heart valve disease globally. Echocardiography is the main imaging modality used to diagnose and assess the severity and hemodynamic consequences of mitral stenosis as well as valve morphology. Transthoracic echocardiography (TTE) is sufficient for the management of most patients.
mitral stenosis
echocardiography
3D echocardiography
mitral annulus calcification
1. Introduction
Mitral stenosis is an important cause of heart valve disease globally. Rheumatic heart disease is the most common etiology worldwide [1]. Though its prevalence has been steadily decreasing in developed countries, it is still frequent in low-income countries. The prevalence of degenerative mitral stenosis increases with age, and is a common finding in the elderly population in developed countries [2]. Both types of mitral stenosis are more frequent in females [3]. Less common causes of mitral stenosis include: congenital causes, systemic immune-mediated diseases, carcinoid syndrome, radiotherapy, and some drugs.
Echocardiography is the main imaging modality to diagnose and assess the severity and hemodynamic consequences of mitral stenosis [4][5] as well as valve morphology. Transthoracic echocardiography (TTE) is sufficient for the management of most patients.
2. Two-Dimensional Transthoracic Echocardiography Assessment
Two-dimensional transthoracic echocardiography is the first-line method used to evaluate mitral valve morphology and to diagnose and assess the severity and hemodynamic consequences of mitral stenosis. In order to perform a comprehensive assessment, multiple scanning planes should be explored:
2.1. Parasternal Long-Axis View
The parasternal long-axis view allows for visual assessment of mitral valve morphology and leaflet excursion. It demonstrates A2 and P2 scallops. Leaflet motion and thickness and the presence and extend of calcification should be reported. The characteristic doming of the anterior leaflet is better seen in this view (Figure 1). In mitral stenosis, color Doppler demonstrates diastolic turbulence across the mitral valve in this view.
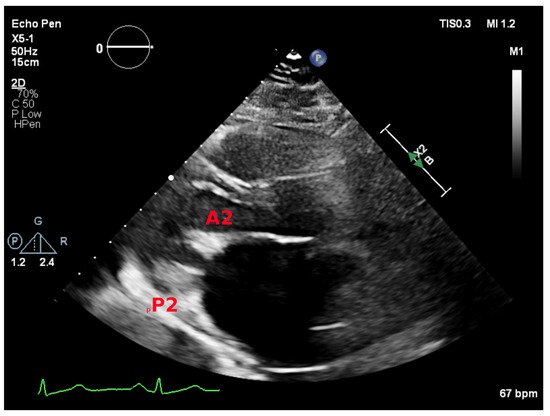
Figure 1. Parasternal long-axis view showing the A2 and P2 mitral scallops.
2.2. Parasternal Short-Axis View
The parasternal short-axis view allows for visual assessment of the leaflets and commissures. Commissural fusion is best seen in this view. The posteromedial (left) and anterolateral (right) commissures are visualized. Scallops one to three are seen from the anterolateral commissure to posteromedial commissure. This view is also used to perform planimetry of the mitral leaflet tips. Tilting from base to apex will demonstrate the narrowest valve orifice (Figure 2).
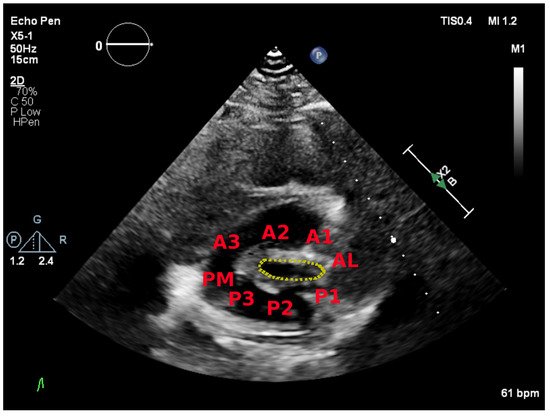
Figure 2. Parasternal short-axis view showing the posteromedial (PM) and anterolateral (AL) commissures and the mitral scallops.
2.3. Apical Four Chamber View
The apical four chamber view typically demonstrates A3, A2, and P1 scallops from left to right (Figure 3A). It allows for visual assessment of the anatomy and mobility of both mitral leaflet. Continuous wave Doppler parallel to the mitral inflow in this view is used to measure transmitral gradients across the valve (Figure 3B). The densest portion of the curve should be used. Calculation of mitral valve area by PHT is also performed in this view (Figure 3C).
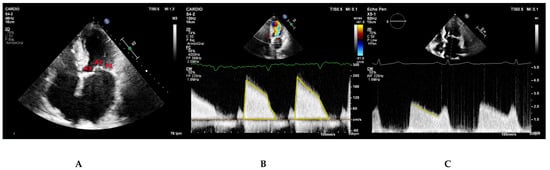
Figure 3. (A) Apical four chamber view depicting the P1, A2, and A3 mitral scallops. (B,C) Continuous wave Doppler parallel to the mitral inflow in apical four-chamber view. The Doppler profile (B) and the deceleration slope of the E wave (C) are traced to obtain the transmitral gradients and the PHT respectively.
2.4. Classification of Mitral Stenosis Severity
Table 1 shows the classification of mitral stenosis severity according to mitral valve area and supportive findings.
Table 1. Classification of mitral stenosis severity at heart rates between 60 and 80 bpm and in sinus rhythm. Adapted from Baumgartnet et al. [6].
| Mild | Moderate | Severe | |
|---|---|---|---|
| Specific Findings Mitral Valve Area (cm2) |
>1.5 | 1.0–1.5 | <1.0 |
| Supportive Findings Mean Transmitral Gradient (mmHg) PSP (mmHg) |
<5 <30 |
5–10 30–50 |
>10 >50 |
2.5. Assessment of Feasibility of Percutaneous Mitral Commissurotomy
Suitability of mitral valve for percutaneous mitral commissurotomy is based on echocardiographic findings. Scoring systems have been developed to help with this task. The Wilkins score [7] (Table 2) is the most widely accepted criteria for patient selection.
Table 2. Wilkins score. The total score is the sum of the items (ranging from 4 to 16). Adapted from Wilkins et al. [7].
| Grade | Mobility | Thickening | Subvalvular Thickening |
Calcification |
|---|---|---|---|---|
| 1 | Highly mobile valve with only leaflet tips restricted |
Leaflets near normal in thickness (4–5 mm) |
Minimal thickening just below the mitral leaflets |
A single area of increased echo brightness |
| 2 | Leaflet mid and base portions have normal mobility | Mid leaflets normal, considerable thickening of margins (5–8 mm) | Thickening of chordal structures extending to one third of the chordal length |
Scattered areas of brightness confined to leaflet margins |
| 3 | Valve continues to move forward in diastole, mainly from the base | Thickening extending through the entire leaflet (5–8 mm) | Thickening extended to distal third of the chords | Brightness extending into the mid portions of the leaflets |
| 4 | No or minimal forward movement of the leaflets in diastole |
Considerable thickening of all leaflet tissue (>8–10 mm) |
Extensive thickening and shortening of all chordal structures extending down to the papillary muscles |
Extensive brightness through out much of the leaflet tissue |
Percutaneous mitral commissurotomy is contraindicated in patients with a mitral valve area >1.5cm2, left atrial thrombus, more than mild mitral regurgitation, severe calcification, absence of commissural fusion, severe concomitant aortic valve disease, severe combined tricuspid stenosis and tricuspid regurgitation requiring surgery or concomitant coronary artery disease requiring surgery [4].
3. Two-Dimensional Transesophageal Echocardiography Assessment
Transesophageal echocardiography should be performed to exclude left atrial thrombus before percutaneous mitral commissurotomy or after an embolic event [4]. Due to the absence of interference from the chest wall or the lung, transesophageal echocardiography offers a higher imaging resolution. Thus, it is also useful in patients with limited acoustic window or when clarification regarding valve morphology or suitability of mitral valve for percutaneous mitral commissurotomy (Figure 5).
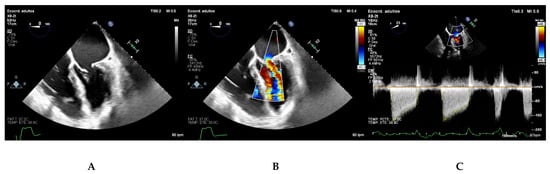
Figure 4. (A) Mid-esophageal four chamber view. (B) Color Doppler at the mitral valve level in a mid-esophageal four chamber view. (C) Continuous wave Doppler parallel to the mitral inflow in mid-esophageal four chamber view.
4. Three-Dimensional Echocardiography Assessment
3D echocardiography represents a key innovation in cardiovascular imaging. The development of matrix-array transducers has allowed for real-time acquisition and online display of 3D images. Nowadays, 3D echocardiography is available on most ultrasound machines, and current transducers are able to acquire both 2D and 3D images. These technological advances have led to a progressive implementation of 3D echocardiography in routine clinical practice. Its main advantages are:
-
It measures all three spatial dimensions. Therefore, it is not reliant on plane positioning and does not require geometric assumptions of cardiac structures.
-
Images can be rotated and viewed from different perspectives. This allows for a better understanding of the relationship between structures and makes 3D images more intuitive.
On the contrary, limitations of 3D echocardiography include reduced spatial and temporal resolution compared to 2D echocardiography and suboptimal images in patients with arrhythmias.
First, 2D images should be used to locate the planes for imaging the mitral valve. Later, 3D imaging of the mitral valve can be performed using multiple 3D imaging modalities.
4.1. Simultaneous Biplane Imaging
Biplane mode allows for the use of a dual screen to display the mitral valve in two planes in real time. The second plane is usually orthogonal, though any rotation angle can be used. Color Doppler imaging can also be used together with biplane imaging. This mode is useful for a preliminary evaluation of the mitral valve. Scallops and segments of the mitral valve can be investigated by manipulating the lateral plane in different angle orientations in order to provide detailed information regarding mitral valve anatomy and the mechanism and etiology of mitral stenosis. In transthoracic echocardiography, biplane imaging can help ensure that planimetry takes place at the leaflet tips. An orthogonal plane can be obtained placing the cursor at the leaflet tips in the parasternal long-axis view. However, in order to avoid an oblique short-axis view, adequate alignment of the mitral orifice in the long-axis view is required.
4.2. Full Volume Imaging
Full volume imaging provides the largest sector and allows for an evaluation of the entire mitral valve apparatus. Real-time full volume acquisitions generally result in poor spatial and temporal resolution. Therefore, whenever possible, ECG gating acquisitions should be used. A full volume with gated acquisition is reconstructed by merging several individual volume slices gated to the ECG. This results in high temporal and spatial resolution. However, it can also be susceptible to artefacts due to arrhythmias and/or respiratory motions. ECG should be optimized to show a distinct R wave and the number of acquisition beats should be adjusted attending to the patient’s circumstances. Moreover, images should be obtained with breath-holding. Once acquired, the full volume dataset can be cropped, rotated, viewed in multiple perspectives, or decomposed into 2D cross-sections.
4.3. Real-Time 3D Imaging
Real-time 3D imaging permits a narrow sector and therefore higher spatial and temporal resolution. However, the sector is generally insufficient to visualize the entire mitral valve apparatus. The pyramidal volume should be acquired at a decreased depth and focused on the mitral valve. This allows for an initial 3D evaluation of the mitral valve.
4.4. Focused Wide-Sector (3D Zoom) Imaging
Focused wide-sector imaging allows for a 3D focused view of the mitral valve. In transthoracic echocardiography, 3D zoom of the mitral valve can be acquired from both the parasternal and the apical views (Figure 5). The anterior leaflet is adequately visualized in both views while the posterior leaflet is better seen from the parasternal view [8]. The mitral valve should be oriented with the aortic valve placed superiorly regardless of if the valve is oriented as viewed from the left atrium or the left ventricle (Figure 6) [9].
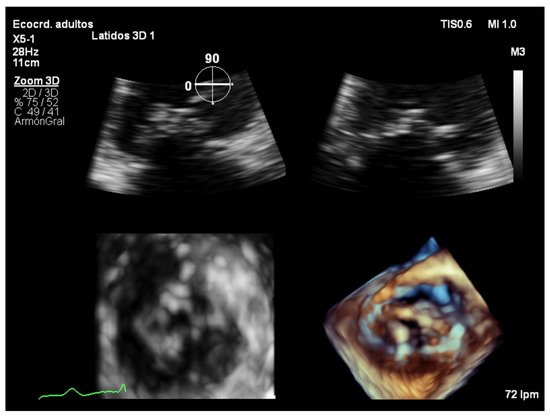
Figure 5. Zoom 3D focused on the mitral valve from the apical four chamber view.
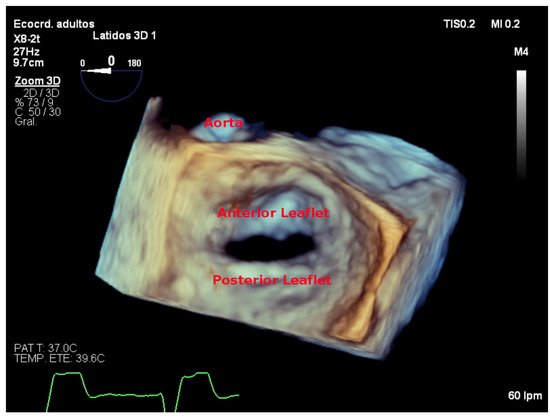
Figure 6. Zoom 3D focused on the mitral valve as view from the left atrium with the aorta placed superiorly (surgical view).
Three-dimensional echocardiography is superior in evaluating mitral stenosis severity, because direct visualization of the valve orifice in multiple planes ensures that the planimetry takes place at the level of the mitral leaflet tips, where the valve orifice is narrowest. Indeed, 3D planimetry has been reported as the most accurate method for mitral valve area evaluation [10][11], and it has become the reference method for mitral valve area quantification.
In order to perform 3D planimetry, the 3D zoom dataset should include the entire mitral annulus and the leaflet height. Later, the frame when leaflet excursion is greater should be identified and multiplane reconstruction should be used to achieve a parallel alignment with the narrowest portion of the mitral valve orifice. Finally, the mitral valve area is obtained tracing the inner edge of the orifice (Figure 7). Modern software permits multiple short-axis slices to be reconstructed to help identify the narrowest mitral valve orifice (Figure 8).
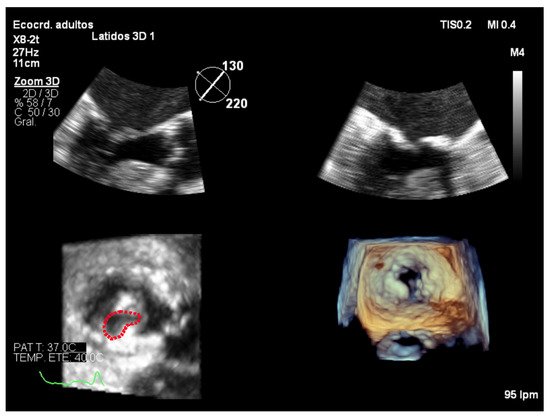
Figure 7. Zoom 3D focused on the mitral valve from the mid-esophageal four chamber view. Multiplanar reconstruction to perform 3D planimetry.
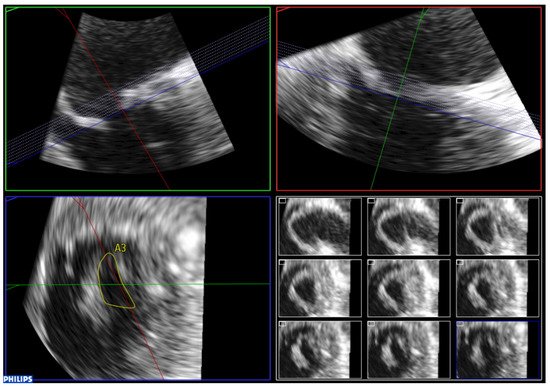
Figure 8. Analysis of zoom 3D dataset focused on the mitral valve obtained from the mid-esophageal four chamber view. Multiple short-axis slices are reconstructed to help identify the narrowest mitral valve orifice.
4.5. Assessment of Feasibility of Percutaneous Mitral Commissurotomy Using 3D Echocardiography
A scoring system based on 3D echocardiography, the Anwar score, has been developed for the evaluation of patients before percutaneous mitral commissurotomy [12] (Table 3).
Table 3. Anwar score. The total score is the sum of the items (ranging from 4 to 16). Mild mitral valve involvement is defined as < 8 points, moderate involvement as 8 to 13 and severe MV involvement as > 14 points. Adapted from Anwar et al. [12].
| Anterior Leaflet | Posterior Leaflet | |||||
| Thickness (0–6) (0 = normal, 1 = thickened) | A1 | A2 | A3 | P1 | P2 | P3 |
| Mobility (0–6) (0 = normal, 1 = limited) | 0–1 | 0–1 | 0–1 | 0–1 | 0–1 | 0–1 |
| Calcification (0–10) (0 = no, 1–2 = calcified) | 0–2 | 0–1 | 0–2 | 0–2 | 0–1 | 0–2 |
| Subvalvular Apparatus | ||||||
| Proximal Third | Middle Third | Distal Third | ||||
| Thickness (0–3) (0 = normal, 1 = thickened) | 0–1 | 0–1 | 0–1 | |||
| Separation (0–6) (0 = normal, 1 = partial, 2 = no) | 0–2 | 0–2 | 0–2 | |||
References
- Seckeler, M.D.; Hoke, T.R. The Worldwide Epidemiology of Acute Rheumatic Fever and Rheumatic Heart Disease. Clin. Epidemiol. 2011, 3, 67–84.
- Abramowitz, Y.; Jilaihawi, H.; Chakravarty, T.; Mack, M.J.; Makkar, R.R. Mitral Annulus Calcification. J. Am. Coll. Cardiol. 2015, 66, 1934–1941.
- Andell, P.; Li, X.; Martinsson, A.; Andersson, C.; Stagmo, M.; Zöller, B.; Sundquist, K.; Smith, J.G. Epidemiology of Valvular Heart Disease in a Swedish Nationwide Hospital-Based Register Study. Heart 2017, 103, 1696–1703.
- Vahanian, A.; Beyersdorf, F.; Praz, F.; Milojevic, M.; Baldus, S.; Bauersachs, J.; Capodanno, D.; Conradi, L.; De Bonis, M.; De Paulis, R.; et al. 2021 ESC/EACTS Guidelines for the Management of Valvular Heart Disease. Eur. Heart J. 2021, 60, 727–800.
- Otto, C.M.; Nishimura, R.A.; Bonow, R.O.; Carabello, B.A.; Erwin, J.P.; Gentile, F.; Jneid, H.; Krieger, E.V.; Mack, M.; McLeod, C.; et al. 2020 ACC/AHA Guideline for the Management of Patients With Valvular Heart Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2021, 143, e35–e71.
- Baumgartner, H.; Hung, J.; Bermejo, J.; Chambers, J.B.; Evangelista, A.; Griffin, B.P.; Iung, B.; Otto, C.M.; Pellikka, P.A.; Quiñones, M.; et al. Echocardiographic Assessment of Valve Stenosis: EAE/ASE Recommendations for Clinical Practice. Eur. J. Echocardiogr. 2009, 10, 1–25.
- Wilkins, G.T.; Weyman, A.E.; Abascal, V.M.; Block, P.C.; Palacios, I.F. Percutaneous Balloon Dilatation of the Mitral Valve: An Analysis of Echocardiographic Variables Related to Outcome and the Mechanism of Dilatation. Br. Heart J. 1988, 60, 299–308.
- Sugeng, L.; Coon, P.; Weinert, L.; Jolly, N.; Lammertin, G.; Bednarz, J.E.; Thiele, K.; Lang, R.M. Use of Real-Time 3-Dimensional Transthoracic Echocardiography in the Evaluation of Mitral Valve Disease. J. Am. Soc. Echocardiogr. 2006, 19, 413–421.
- Lang, R.M.; Badano, L.P.; Tsang, W.; Adams, D.H.; Agricola, E.; Buck, T.; Faletra, F.F.; Franke, A.; Hung, J.; de Isla, L.P.; et al. EAE/ASE Recommendations for Image Acquisition and Display Using Three-Dimensional Echocardiography. Eur. Heart J. Cardiovasc. Imaging 2012, 13, 1–46.
- Zamorano, J.; Cordeiro, P.; Sugeng, L.; Perez de Isla, L.; Weinert, L.; Macaya, C.; Rodríguez, E.; Lang, R.M. Real-Time Three-Dimensional Echocardiography for Rheumatic Mitral Valve Stenosis Evaluation: An Accurate and Novel Approach. J. Am. Coll. Cardiol. 2004, 43, 2091–2096.
- Sugeng, L.; Weinert, L.; Lammertin, G.; Thomas, P.; Spencer, K.T.; Decara, J.M.; Mor-Avi, V.; Huo, D.; Feldman, T.; Lang, R.M. Accuracy of Mitral Valve Area Measurements Using Transthoracic Rapid Freehand 3-Dimensional Scanning: Comparison with Noninvasive and Invasive Methods. J. Am. Soc. Echocardiogr. 2003, 16, 1292–1300.
- Anwar, A.M.; Attia, W.M.; Nosir, Y.F.M.; Soliman, O.I.I.; Mosad, M.A.; Othman, M.; Geleijnse, M.L.; El-Amin, A.M.; Ten Cate, F.J. Validation of a New Score for the Assessment of Mitral Stenosis Using Real-Time Three-Dimensional Echocardiography. J. Am. Soc. Echocardiogr. 2010, 23, 13–22.
More
Information
Subjects:
Cardiac & Cardiovascular Systems
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.5K
Revisions:
2 times
(View History)
Update Date:
15 Dec 2021
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




