Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Weronika Smok | + 3051 word(s) | 3051 | 2021-09-14 06:23:20 | | | |
| 2 | Camila Xu | + 231 word(s) | 3282 | 2021-12-10 06:56:28 | | | | |
| 3 | Camila Xu | + 231 word(s) | 3282 | 2021-12-10 07:01:54 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Smok, W. 1D MOS Nanostructures. Encyclopedia. Available online: https://encyclopedia.pub/entry/16899 (accessed on 08 February 2026).
Smok W. 1D MOS Nanostructures. Encyclopedia. Available at: https://encyclopedia.pub/entry/16899. Accessed February 08, 2026.
Smok, Weronika. "1D MOS Nanostructures" Encyclopedia, https://encyclopedia.pub/entry/16899 (accessed February 08, 2026).
Smok, W. (2021, December 08). 1D MOS Nanostructures. In Encyclopedia. https://encyclopedia.pub/entry/16899
Smok, Weronika. "1D MOS Nanostructures." Encyclopedia. Web. 08 December, 2021.
Copy Citation
1D electrospun MOS are basically nanofibers, nanotubes or nanowires, which are made of one or more semiconductor metal oxides or with other dopant material. Polymer/MOS precursor nanofibers that are formed in the electrospinning process are calcined until the polymer is removed and pristine MOS nanostructure is obtained.
electrospinning
metal oxides
nanomaterials
nanofibers
nanowires
1. Electrospun 1D MOS in Saving the Natural Environment
Industrialization and increasing consumerism have led to the highest level of warning about environmental pollution and its associated crisis. Industrial waste compared to municipal waste is toxic and non-biodegradable, as it contains heavy metal ions, oils and fats, dyes, phenols and ammonia, which can adversely affect human life and health but also the environment. One possible solution to this problem is to use the process of photocatalysis to break down harmful substances into simpler and environmentally friendly ones. Photocatalysis combines reactions using light and a catalyst, which is usually a semiconductor—it absorbs light and acts as a catalyst for chemical reactions. Therefore, it is necessary to search for semiconductor materials that can help solve this global problem.
Recently, electrospun one-dimensional semiconductor metal oxide nanostructures, predisposed by their unique optical and electrical properties, have attracted the attention of researchers studying photocatalytic pollutant decomposition processes of TiO2, ZnO and SnO2, whose energy gap width, radiation absorption range and mobility rate can be controlled by the parameters of the manufacturing process (Figure 1, Table 1 and Table 2) [1][2][3][4][5][6].
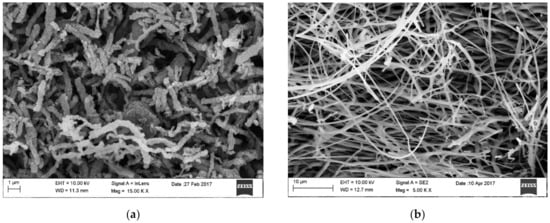
Figure 1. SEM image of surface morphology of (a) TiO2 nanowires after calcination at 400 °C, (b) ZnO nanowires after calcination at 400 °C [own study].
Table 1. Optical properties of selected 1D metal oxides prepared via electrospinning.
Table 2. Optical properties of TiO2 nanowires and ZnO nanowires after calcination at 400 °C [own study].
| Material | Calcination Temperature [°C] | Max. Absorbance | Wavelength [nm] | Eg [eV] |
|---|---|---|---|---|
| TiO2 nanowires | 400 | 2.42 | 248 | 3.73 |
| 500 | 2.34 | 3.83 | ||
| 600 | 2.26 | 3.88 | ||
| ZnO nanowires | 400 | 2.94 | 346 | 3.36 |
| 500 | 3.38 | 3.34 | ||
| 600 | 3.43 | 3.32 |
Z. Wang et al. performed an analysis [13] of the photocatalytic properties of ZnO/SnO2 nanofiber with and without the addition of the P123 precursor, which resulted in a much higher photocatalytic activity in the degradation of methyl orange (MO) in UV light of the composite nanofibers with the addition of P123. C. Zhu et al. in their work [14] showed significantly greater possibilities of photocatalytic decomposition of Rhodamine B in visible light through the use of composite SnO2/Fe2O3 nanofibers compared to the capabilities of the non-admixture SnO2. Electrospun SnO2 nanostructures coated with a 1 nm thick carbon shell fabricated by P. Zhang et al. [15] showed very efficient photocatalytic degradation of 4-Nitrophenol under both UV and visible light (Figure 2). K. Wang et al. in their work [16] reported a study on the photocatalytic activity of mutiheterojunction in the photodegradation of methyl orange (MO) and Cr (VI) ions under visible light. It was observed that the SnO2/Bi2O3/BiOI nanofibers were characterized by better photocatalytic activity than the non-admixture SnO2 and Bi2O3, which the authors attributed to the increased absorption of visible light, electron-hole pair separation and large specific surface area of the nanostructures studied.
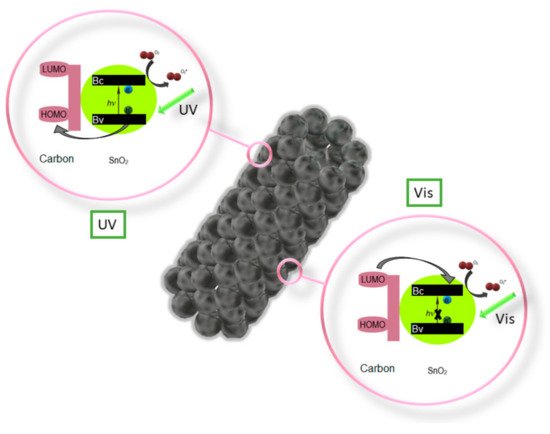
Figure 2. Diagram of the photocatalytic activation of nanotubes SnO2 [15].
T. Wang. et al. [1] demonstrated that the use of magnetic field-assisted electrospinning in the fabrication of nanofibers and nanotubes from TiO2 narrowed the band gap to favor photocatalytic performance—TiO2 reduced Rhodamine B (RhB) by 95.8% in 100 min. Q. Zhang et al. [17] proposed the use of 1D composite nanostructures based on In2O3 of admixtured CaIn2O4 in the photocatalytic purification of water from the dye-methylene blue (MB). The degradation rates of MB were 76% and 92%, respectively, under 120 min of simulated sunlight exposure. The efficient separation and transport of photogenerated carriers, as well as the large specific surface area, meant that the CaIn2O4-In2O3 composites were characterized by high photocatalytic efficiency. A. Ahmad et al. [18] by the triaxial electrospinning method produced TiO2 with a structure of nanofiber-in-nanotube (rutile-anatase), with which the photodegradation was carried out for 88.1% of the Sandalfix N. Blue with a 240 min irradiation time.
The diversity of available variations of the electrospinning process makes it possible to obtain MOS with high photocatalytic activity; however, further research is needed to explore the mechanism of this phenomenon.
The growing demand for green energy motivates researchers to look for materials and solutions that can increase the efficiency of existing renewable energy sources (RES), especially photovoltaic cells. So far, the many works that have presented the possibility of using 1D MOS in the construction of modern solar cells mainly focused on the use of TiO2, ZnO and SnO2 [19][20][21][22][23][24][25][26][27].
Favorable optoelectronic properties of crystalline-amorphous hybrid SnO2 nanowires are suggested by W. Matysiak et al. [28] to be used in in modern flexible photovoltaic cells (Table 3, Figure 3). The research group, to which the Authors belong, was awarded a silver medal at the 5th China (Shanghai) International Invention & Innovation Expo in 2021 for the invention “Innovative flexible solid-state solar cell with a hybrid layered architecture”, for which the construction of which SnO2 nanowires were used (Figure 4).
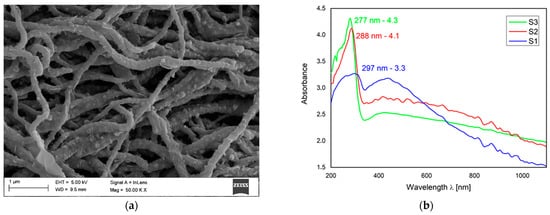
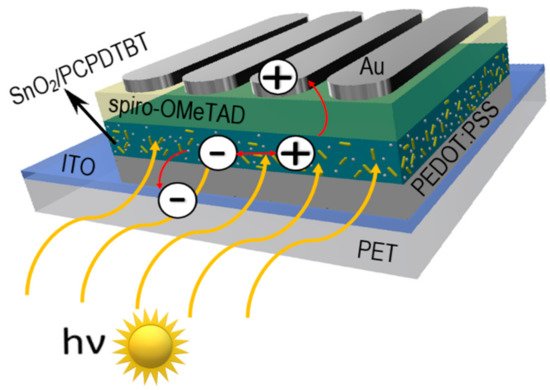
Figure 4. Schematic representation of multilayer flexible photovoltaic architecture manufactured in Department of Engineering Materials and Biomaterials [Poster at the 5th China (Shanghai) International Invention & Innovation Expo: “Innovative flexible solid-state solar cell with a hybrid layered architecture”].
Table 3. Refractive index and dielectric permittivity obtained for the electrospun 1D SnO2 nanomaterials [28].
| Parameter | SnO2 Nanowires | ||
|---|---|---|---|
| Type of Spinning Solutions | |||
| Type 1 | Type 2 | Type 1 | |
| refractive index (n) | 1.51 | 1.52 | 1.51 |
| complex dielectric permeability (ε) | 2.28 | 2.30 | 2.28 |
| Energy band gap (Eg) | 3.3 | 3.8 | 3.9 |
M. Yang et al. in their publication [30] described the effect of graphene oxide (GO) admixture in hybrid SnO2/TiO2 nanofibers on the efficiency of dye-based solar cells (DSCs) constructed with their participation. DSCs along with GO-SnO2/TiO2 as the working electrode were analyzed for efficiency and the following photovoltaic parameters: short-circuit current density, open-circuit voltage and fill factor, which were respectively 11.19 mA/cm2, 0.72 V and 0.67. It was found that the solar-to-electric energy conversion efficiency of GO/SnO2/TiO2 as a photoanode-based device was 5.41%.
Therefore, it is worthwhile to pay attention to the application of MOS in the construction of next-generation photovoltaic cells, as they may provide a solution to the problem of low efficiency of dye-based cells.
2. Electrospun Metal Oxides 1D Nanostructures in Gas Sensors
The most widely studied application of one-dimensional metal oxide-based nanostructures are sensors for gases such as methanol, ethanol, acetone, formaldehyde, xylene and other volatile organic compounds that are highly toxic and dangerous to human health and even life [31][32].
Gas sensors based on semiconductor metal oxides are widely used in many areas, including chemical pollution control in air and rooms, alarms to detect the threat of poisonous substances and even medical diagnostics performed on the basis of a patient’s breath. The popularity of these types of sensors is due to their high sensitivity, low cost and ease of manufacture, as well as their compatibility with modern electronic devices [33][34][35][36][37][38].
The mechanism of gas detection by these MOS can be explained by the fact that the conductivity of the materials is changed by the chemical interaction between the gas and the surface of the nanostructure on which oxygen is adsorbed. Oxygen (O2) molecules are adsorbed on the nanofiber/nanowire surface in air and then they capture electrons from the conductivity band of the oxide so that chemisorbed oxygen ions (O2−) are generated and the formation of a barrier layer at a certain depth of the oxide structure is initiated. When the nanostructures are exposed to gas at an appropriate temperature, the gas reacts with the surface oxygen species and the width of the barrier layer decreases. As a result, the carrier concentration will increase, which ultimately increases the conductivity of the nanofibers/nanowires [39][40][41].
Many scientific reports indicate that the detection of hazardous substances by sensors based on electrospun MOS still needs to be developed—obtaining sensors with a lower substance detection threshold and shorter device response and reaction times. Improvement of these properties can be achieved by admixing with metallic nanoparticles, other MOS and carbon materials, which will affect the conductivity of the MOS. The combination of different materials produces local p-n, n-n or p-p nanojunctions. It is the heterojunctions generated from different materials that directly affect the substance detection mechanism. Several typical morphologies of MOS-based heterostructured materials are most commonly reported in the literature (Figure 5). In addition to non-admixed 1D MOS, hybrid structures consisting of both MOS and admixed crystallites simultaneously stand out. MOS nanowires decorated with nanoparticles or other forms of admixture are another interesting variation. There are also structures with core-shell morphology in which MOS can be either covered or surrounded by other material.
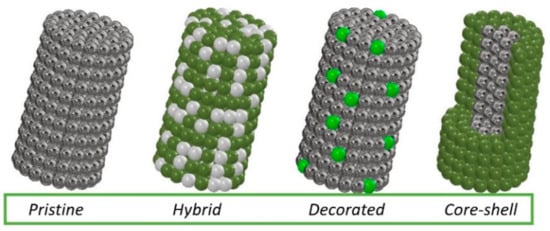
Figure 5. Types of morphology of the most commonly produced 1D nanostructures.
One of the most commonly used MOS as detector anode is tin dioxide, which is characterized by an energy gap width of about 3.6 kV and simultaneous optical transparency and electrical conductivity [40][42][43][44][45][46]. Indium oxide exhibiting similar properties to tin oxide is also increasingly used. These materials are often combined with each other and also admixed with other oxides such as TiO2, ZnO, CuO and NiO (Table 4). The authors of this paper have established a collaboration with the Department of Optoelectronics, which is equipped with laboratories capable of gas detection measurements. Electrospun SnO2 and In2O3 nanowires fabricated in the Department of Engineering Materials and Biomaterials will be plotted on the IDT and tested to detect gases such as NH3, NO2, CO2 and H2.
Bai et al. [47] demonstrated that the porous, coreless structure of ZnO-SnO2 nanowires is ideal for detecting very low concentrations (0.023 ppm) of toxic NO2. In addition, good detection properties of NO2 promotes the formation of an n-n heterojunction at the phase boundary of ZnO and SnO2, which results in the formation of an additional barrier layer (Figure 6).
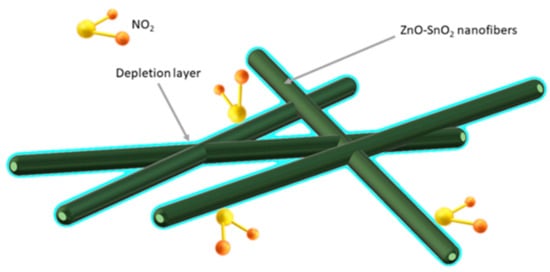
Figure 6. Scheme of hollow structure of gas adsorption and depletion layer for ZnO-SnO2 composite [47].
Zhang et al. [48] observed that the response of sensors in acetone-containing environment can be improved by using heterojunction nanotubes of WO3-SnO2 and admixing it with Pd catalyst. Studies of the sensory properties of the material showed that the addition of Pd increased the response of Pd-WO3-SnO2 sensor more than double the response obtained from WO3-SnO2 sensor in contact with 100 ppm acetone. In addition, the selectivity for detecting acetone in the presence of other gases such as toluene, ammonia, nitrous oxide and pentane was significantly improved.
Du et al. [49] fabricated In2O3 nanofibers with a traditional electrospinning method and then they subjected them to surface modification using low-temperature oxygen and hydrogen radiofrequency plasma. The nanofibers were placed in a plasma reactor chamber and surface modification was performed by increasing the number of pores and channels in the nanofibers (Figure 7). This mechanism enabled more oxygen to be adsorbed on the surface of the indium oxide nanostructures, leading to increased response values and improved selectivity for detecting acetone in the presence of interfering gases such as ethanol, methanol, formaldehyde, benzene, ammonia and nitrogen dioxide.
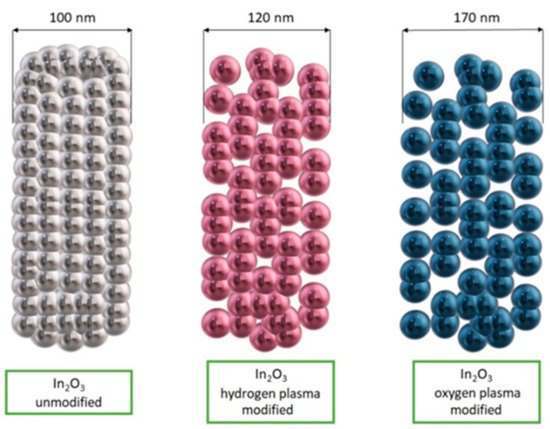
Figure 7. Scheme of In2O3 nanofibers morphology before and after surface modification [49].
Table 4. Selected 1D MOS and their sensing properties.
| Material Type | Polymer | Precursor | Solvent | Calcination | Gas | Conc [ppm] | Response/Recovery Time [s] | Ref. | |
|---|---|---|---|---|---|---|---|---|---|
| Time [h] | Temp [°C] | ||||||||
| ZnO-SnO2 | PVP | SnCl2·2H2O, Zn(AC)2⋅2H2O |
DMF, EtOH | 3 | 600 | Toluene | 1-300 | 6–11/12–23 | [50] |
| NiO-SnO2 | PVP | SnCl2·2H2O, NiCl2·6H2O |
DMF, EtOH | 5 | 600 | Toluene | 50 | 11.2/4 | [51] |
| CuO-SnO2 | PVA | SnCl2·2H2O, CuCl2·2H2O |
DMF, EtOH | 4 | 600 | H2S | 10 | 1/10 | [52] |
| CeO2-SnO2 | PVP | SnCl2·2H2O, Ce(NO3)3·6H2O |
DMF, EtOH | 3 | 600 | EtOH | 200 | 8–10/11–30 | [53] |
| W2O3-SnO2 | PVP | SnCl2·2H2O/ (NH4)6H2W12O40·xH2O |
DMF, EtOH | 1 | 600 | EtOH | 10 | 18.5/282 | [54] |
| Fe2O3-In2O3 | PVP | In(NO3)3·4.5H2O, Fe(NO3)3·9H2O | DMF, EtOH | 2 | 550 | Formaldehyde | 100 | 5/25 | [55] |
| WO3-In2O3 | PVP | In(NO3)3·4.5H2O, WCl6 | DMF, EtOH, AcOH | 2 | 500 | Acetone | 25 | 6/64 | [56] |
| CuO-In2O3 | PVP | In(NO3)3·xH2O, Cu(NO3)2·xH2O | DMF | 2 | 600 | H2S | 5 | 4–30/incomplete recovery | [57] |
| SnO2-In2O3 | PVP | In(NO3)3·4.5H2O, SnCl2·2H2O | DMF, EtOH | 2 | 600 | Formaldehyde | 0.5-50 | ~20/40 | [58] |
| In2O3 (RF plasma modified) | PVP | In(NO3)3·4.5H2O | DMF, EtOH | 3 | 550 | Acetone | 10 | 18–23/55–92 | [49] |
| La2O3-In2O3 | PVP | In(NO3)3·xH2O, La(NO3)3·xH2O | DMF, EtOH, mineral oil | 2 | 550 | Formaldehyde | 50 | 3/19 | [59] |
| In2O3 | PVP | In(NO3)3∙4.5 H2O | DMF | 2 | 800 | NO2 | 5 | 200/1000 | [60] |
The above considerations indicate that electrospun one-dimensional MOS plays a key role in the construction of gas sensors, thus contributing to their development and improving work and life safety in environments exposed to hazardous gases.
3. Electrospun Metal Oxides 1D Nanostructures in Other Applications
Supercapacitors and lithium-ion batteries (LIBs) are other devices for which one-dimensional MOS nanostructures can be used. With the rapid progress of civilization and industrialization, there is a growing need for methods, materials and devices to store large amounts of energy [61]. One solution to meet these needs is the development of LIBs with high performance, which is primarily dependent on the performance of the battery’s most important component, the anode. The currently used anode material in the form of graphite is currently no longer able to meet the needs of high energy storage capacity due to its low capacity and low efficiency. Therefore, the search and research of new electrode materials is of great importance for the current demand for high performance LIBs [62][63]. Recently, semiconductor nanomaterials such as ZnO, NiO, SnO2 lub TiO2 nanotubes and nanowires have been of particular interest for 1D, along with heterojunctions formed by combining these materials with carbon materials [64][65][66][67][68][69]. The advantage of using one-dimensional nanomaterials for anodes in LIBs is the much less frequently observed agglomeration of the material than in the case of nanoparticles, which positively affects the electrochemical performance of the battery, and this fact was confirmed in a study by C. K. Chan et al. [70] based on the analysis of a battery based on Si nanowires.
J. Zhu et al. [68] pointed out the high application potential of electrospun ZnO-SnO2 nanofibers as anode material in lithium-ion batteries. It was observed that due to the heterogeneous mesoporous electrode structure based on ZnO-SnO2 nanofibers, they provide excellent performance and reversible capacity at a relatively low cost and with high process repeatability. D. Lei et al. in their work [71] showed that GeO2-SnO2 composite nanofibers with high porosity prepared by the solution electrospinning method have high specific capacitance and good cycling performance, which is mainly due to the porous one-dimensional nanostructure, which can shorten the transport pathway and provide trapping of electrolyte ions to meet the requirements of fast charging and discharging reactions. J. Guo in [72] described the effect of pore distribution on the capacitance of two types of porous C/SnO2 nanofibers produced by electrospinning from solutions based on different precursors, i.e., using tin chloride, the fibers with spherical pores were obtained, while the pores in the form of channels were obtained from acetate (Figure 8). On the basis of a galvanostatic charge-discharge test, it was found that multichannel C/SnO2 nanofibers with a large specific surface area (34.97 m2/g) achieve better charging performance than spherical pore nanofibers and show a more stable capacity retention of about 90% after 50 cycles.
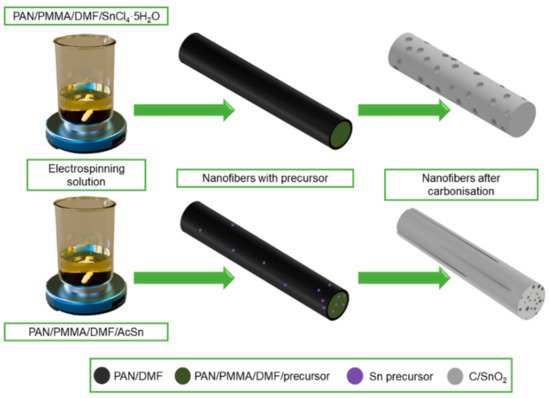
Figure 8. Scheme of the manufacturing process of C/SnO2 nanofibers with a different morphology.
The use of SnO2-ZnO nanofibers in energy storage was presented in the work [69] of J. Zhang et al. The study showed that by using the spinning solution parameters, it is possible to control the morphology and obtain hollow nanotubes, which exhibited good capacity stability in an electrochemical test. In addition, it was observed that the polypyrrole (PPy) polymer coating of SnO2-ZnO nanotubes has made it possible to maintain a high capacity of 626.1 mA hg−1 at 0.2 °C for 100 cycles, and cycle stability has also been improved.
Thus, the electrospinning method with subsequent calcination enables precise control of the electrochemical properties of the fabricated one-dimensional MOS-based nanostructures, thus providing a chance to solve the problem of non-compliant LIBs.
Due to their unique optical, electrical and magnetic properties, they are used in modern devices such as field-effect transistors (FETs) and microwave absorption materials. X. Zhu et al. presented [73] a method to fabricate high-performance field-effect transistors based on electrospun In2O3 nanofibers admixed with Al, Ga and Cr. The devices showed optimal performance at a 10% molar concentration of admixing material (Al, Cr and Ga): low and positive gate-source voltage VGS (<6.0 V), a high ratio of the transistor on current to transistor off current Ion/Ioff (~108), high saturation current (~10–4 A) and carrier mobility on the level of ~2.0 cm2/V−1s−1.
H. Zhang et al. [13] demonstrated that the use of polymorphic anatase-rutile TiO2 nanofibers to build FET showed better transistor characteristics because of a strong synergistic effect compared with pure anatase and rutile TiO2 nanofibers. BioFET created by S. Veeralingam and S. Badhulik [74] based on β–Bi2O3 nanofibers for the detection of serotonin exhibited sensitivity of 51.64 μA/nM over a range of 10 nM−1 μM and a limit of detection of 0.29 nM. Moreover, it maintained excellent sensitivity, stability and reproducibility with a rapid response time of 0.8 s. Using the electrospinning method, K.C.S. Reddy et al. [75] created a self-powered NiO-p/Si-n based ultraviolet photodetector which exhibited a high responsivity of 9.1 mA W−1 at zero bias with a fast photoresponse of less than 0.4 s. X. Huang et al. [76] observed that electrospun bead-like Co-ZnO nanostructures present ferromagnetic properties and an excellent electromagnetic loss performance—the effective microwave absorption of bandwidth with reflection loss less than −10 dB was 11.6 GHz.
For years, medicine has been a priority discipline in which new solutions and biomaterials are constantly being sought. Looking at the disease problems that affect mankind today, the most rapidly developing areas of medicine include cancer therapies, drug delivery, biosensors, medical imaging and tissue engineering. Due to the unsatisfactory properties of conventional biomaterials, it is necessary to search for new material solutions. Production of one-dimensional nanomaterials with controlled dimensions, arrangement of structures with respect to each other or porosity creates many possibilities of using their unique properties for therapeutic purposes. Ceramic nanomaterials, which are based on inert simple oxides, may seem to be a possible solution for some health problems. The most commonly used one-dimensional MOS include TiO2, due to its non-toxicity, environmental friendliness as well as good chemical stability and high corrosion resistance [77][78].
One of many interesting examples of work on the above issue is that presented by I.H.M. Aly et al. [79], who used electrospun TiO2 nanofibers as an admixture to a bioceramic composite based on wollastonite for bone tissue regeneration, which significantly improved the mechanical properties of the composite while not affecting the bioactivity in any way, and proves that this type of material is worth considering and researching for applications in medicine. Mesh with TiO2 nanofibers may also find applications in tissue engineering, as studies have shown that it provides an osteogenic environment—increasing osteoblast production and differentiation [80]. S. Chen et al. confirmed the possibility of using hydrothermal treated nanofibers as delivery systems for the antibiotic tetracycline hydrochloride, whereby nanofibers showed high bactericidal activity against E. coli and S. aureus [81]. N.C. Bezir et al. demonstrated that TiO2 and Ag/TiO2 nanofibers show beneficial antibacterial properties based on measured inhibition zones diameters of S. aureusculture plates [82]. Effective inhibition of B. subtilis and B. cereus through TiO2/GO/CA nanofibers was observed by L. Jia et al. [83]. TiO2 in the form of electrospun one-dimensional nanostructures also shows promising results in promoting apoptosis of cancer cells, e.g., cervical cancer [84]. Other applications of 1D ceramic nanomaterials in medicine include the use of oleic acid-coated ZnO nanowires to fabricate hydrophobic polyvinylidene fluoride (PVDF) membranes, whose self-cleaning properties can be used to construct surgical devices and instruments or artificial blood vessels [85]. ZnO nanofibers, similarly to TiO2 nanofibers, are characterized by tremendous antibacterial activity in S. aureus and E. coli utilization [86][87].
References
- Wang, T.; Gao, Y.; Tang, T.; Bian, H.; Zhang, Z.; Xu, J.; Xiao, H.; Chu, X. Preparation of ordered TiO2 nanofibers/nanotubes by magnetic field assisted electrospinning and the study of their photocatalytic properties. Ceram. Int. 2019, 45, 14404–14410.
- Zhang, J.; Hou, X.; Pang, Z.; Cai, Y.; Zhou, H.; Lv, P.; Wei, Q. Fabrication of hierarchical TiO2 nanofibers by microemulsion electrospinning for photocatalysis applications. Ceram. Int. 2017, 43, 15911–15917.
- Nasr, M.; Balme, S.; Eid, C.; Habchi, R.; Miele, P.; Bechelany, M. Enhanced visible-light photocatalytic performance of electrospun rGO/TiO2 composite nanofibers. J. Phys. Chem. C 2017, 121, 261–269.
- Boyadjiev, S.I.; Kéri, O.; Bárdos, P.; Firkala, T.; Gáber, F.; Nagy, Z.K.; Baji, Z.; Takács, M.; Szilágyi, I.M. TiO2/ZnO and ZnO/TiO2 core/shell nanofibers prepared by electrospinning and atomic layer deposition for photocatalysis and gas sensing. Appl. Surf. Sci. 2017, 424, 190–197.
- Dursun, S.; Koyuncu, S.N.; Kaya, İ.C.; Kaya, G.G.; Kalem, V.; Akyildiz, H. Production of CuO–WO3 hybrids and their dye removal capacity/performance from wastewater by adsorption/photocatalysis. J. Water Process. Eng. 2020, 36, 101390.
- Soares, L.; Alves, A. Photocatalytic properties of TiO2 and TiO2/WO3 films applied as semiconductors in heterogeneous photocatalysis. Mater. Lett. 2018, 211, 339–342.
- Matysiak, W.; Tański, T.; Zaborowska, M. Manufacturing process, characterization and optical investigation of amorphous 1D zinc oxide nanostructures. Appl. Surf. Sci. 2018, 442, 382–389.
- Matysiak, W.; Tański, T. Analysis of the morphology, structure and optical properties of 1D SiO2 nanostructures obtained with sol-gel and electrospinning methods. Appl. Surf. Sci. 2019, 489, 34–43.
- Ch, S.R.; Zhang, L.; Kang, T.; Lin, Y.; Qiu, Y.; Reddy, A.S. Annealing impact on the structural and optical properties of electrospun SnO2 nanofibers for TCOs. Ceram. Int. 2018, 44, 4586–4591.
- Lim, G.-D.; Yoo, J.-H.; Ji, M.; Lee, Y.-I. Visible light driven photocatalytic degradation enhanced by α/β phase heterojunctions on electrospun Bi2O3 nanofibers. J. Alloy. Compd. 2019, 806, 1060–1067.
- Liu, W.; Xie, Y.; Chen, T.; Lu, Q.; Ur Rehman, S.; Zhu, L. Rationally designed mesoporous In2O3 nanofibers functionalized Pt catalysts for high-performance acetone gas sensors. Sens. Actuators B Chem. 2019, 298, 126871.
- Lu, N.; Shao, C.; Li, X.; Miao, F.; Wang, K.; Liu, Y. A facile fabrication of nitrogen-doped electrospun In2O3 nanofibers with improved visible-light photocatalytic activity. Appl. Surf. Sci. 2017, 391, 668–676.
- Wang, Z.; Li, Z.; Zhang, H.; Wang, C. Improved photocatalytic activity of mesoporous ZnO-SnO2 coupled nanofibers. Catal. Commun. 2009, 11, 257–260.
- Zhu, C.; Li, Y.; Su, Q.; Lu, B.; Pan, J.; Zhang, J.; Xie, E.; Lan, W. Electrospinning direct preparation of SnO2/Fe2O3 heterojunction nanotubes as an efficient visible-light photocatalyst. J. Alloy. Compd. 2013, 575, 333–338.
- Zhang, P.; Wang, L.; Zhang, X.; Shao, C.; Hu, J.; Shao, G. SnO2-core carbon-shell composite nanotubes with enhanced photocurrent and photocatalytic performance. Appl. Catal. B Environ. 2015, 166–167, 193–201.
- Wang, K.; Qian, Z.; Guo, W. Multi-heterojunction of SnO2/Bi2O3/BiOI nanofibers: Facile fabrication with enhanced visible-light photocatalytic performance. Mater. Res. Bull. 2019, 111, 202–211.
- Zhang, Q.; Cheah, P.; Han, F.; Dai, Q.; Yan, Y.; Pramanik, A.; Chandra Ray, P. Effects of calcination temperature on crystal structure and photocatalytic activity of CaIn2O4/In2O3 composites. Ceram. Int. 2019, 45, 21851–21857.
- Ahmad, A.; Khan, M.A.; Nazir, A.; Arshad, S.N.; Qadir, M.B.; Khaliq, Z.; Khan, Z.S.; Satti, A.N.; Mushtaq, B.; Shahzad, A. Triaxial electrospun mixed-phased TiO2 nanofiber-in-nanotube structure with enhanced photocatalytic activity. Miroporous Mesoporous Mater. 2021, 320, 111104.
- Mansouri, S.; Abbaspour-Fard, M.H.; Meshkini, A. Lily (Iris Persica) pigments as new sensitizer and TiO2 nanofibers as photoanode electrode in dye sensitized solar cells. Optik 2020, 202, 163710.
- Li, X.; Gao, C.; Wang, J.; Lu, B.; Chen, W.; Song, J.; Zhang, S.; Zhang, Z.; Pan, X.; Xie, E. TiO 2 films with rich bulk oxygen vacancies prepared by electrospinning for dye-sensitized solar cells. J. Power Sources 2012, 214, 244–250.
- Anjusree, G.S.; Deepak, T.G.; Nair, S.V.; Nair, A.S. Facile fabrication of TiO2 nanoparticle-TiO2 nanofiber composites by co-electrospinning-electrospraying for dye-sensitized solar cells. J. Energy Chem. 2015, 24, 762–769.
- Mahmood, K.; Khalid, A.; Ahmad, S.W.; Mehran, M.T. Indium-doped ZnO mesoporous nanofibers as efficient electron transporting materials for perovskite solar cells. Surf. Coat. Technol. 2018, 352, 231–237.
- Dinesh, V.P.; Sriram kumar, R.; Sukhananazerin, A.; Mary Sneha, J.; Manoj Kumar, P.; Biji, P. Novel stainless steel based, eco-friendly dye-sensitized solar cells using electrospun porous ZnO nanofibers. Nano-Struct. Nano-Objects 2019, 19, 100311.
- Mohamed, I.M.A.; Dao, V.-D.; Yasin, A.S.; Choi, H.-S.; Barakat, N.A.M. Synthesis of novel SnO2@TiO2 nanofibers as an efficient photoanode of dye-sensitized solar cells. Int. J. Hydrogen Energy 2016, 41, 10578–10589.
- Bakr, Z.H.; Wali, Q.; Ismail, J.; Elumalai, N.K.; Uddin, A.; Jose, R. Synergistic combination of electronic and electrical properties of SnO2 and TiO2 in a single SnO2-TiO2 composite nanofiber for dye-sensitized solar cells. Electrochim. Acta 2018, 263, 524–532.
- Lim, J.M.; Moon, J.; Kim, J.H.; Lee, C.O.; Chi, W.S.; Park, J.T. One-dimensional SnO2 nanotube solid-state electrolyte for fast electron transport and high light harvesting in solar energy conversion. Solid State Ionics 2021, 363, 115584.
- Wei, K.; Gu, X.Y.; Chen, E.Z.; Wang, Y.Q.; Dai, Z.; Zhu, Z.R.; Kang, S.Q.; Wang, A.C.; Gao, X.P.; Sun, G.Z.; et al. Dissymmetric interface design of SnO2/TiO2 side-by-side bi-component nanofibers as photoanodes for dye sensitized solar cells: Facilitated electron transport and enhanced carrier separation. J. Colloid Interface Sci. 2021, 583, 24–32.
- Matysiak, W.; Tański, T.; Smok, W. Study of optical and dielectric constants of hybrid SnO2 electrospun nanostructures. Appl. Phys. A Mater. Sci. Process. 2020, 126, 115.
- Matysiak, W.; Tański, T.; Smok, W.; Polischuk, O. Synthesis of hybrid amorphous/crystalline SnO2 1D nanostructures: Investigation of structure, morphology and optical properties. Sci. Rep. 2020, 10, 14802.
- Yang, M.; Li, X.; Yan, B.; Fan, L.; Yu, Z.; Li, D. Reduced graphene oxide decorated porous SnO2 nanotubes with enhanced sodium storage. J. Alloy. Compd. 2017, 710, 323–330.
- Kim, J.-H.; Lee, J.-H.; Kim, J.-Y.; Mirzaei, A.; Kim, H.W.; Kim, S.S. Enhancement of CO and NO2 sensing in n-SnO2-p-Cu2O core-shell nanofibers by shell optimization. J. Hazard. Mater. 2019, 376, 68–82.
- Wang, Q.; Bai, J.; Huang, B.; Hu, Q.; Cheng, X.; Li, J.; Xie, E.; Wang, Y.; Pan, X. Design of NiCo2O4@SnO2 heterostructure nanofiber and their low temperature ethanol sensing properties. J. Alloy. Compd. 2019, 791, 1025–1032.
- Righettoni, M.; Amann, A.; Pratsinis, S.E. Breath analysis by nanostructured metal oxides as chemo-resistive gas sensors. Mater. Today 2015, 18, 163–171.
- Güntner, A.T.; Pineau, N.J.; Mochalski, P.; Wiesenhofer, H.; Agapiou, A.; Mayhew, C.A.; Pratsinis, S.E. Sniffing entrapped humans with sensor arrays. Anal. Chem. 2018, 90, 4940–4945.
- Tai, H.; Wang, S.; Duan, Z.; Jiang, Y. Evolution of breath analysis based on humidity and gas sensors: Potential and challenges. Sens. Actuators B Chem. 2020, 318, 128104.
- Bhati, V.S.; Hojamberdiev, M.; Kumar, M. Enhanced sensing performance of ZnO nanostructures-based gas sensors: A review. Energy Rep. 2020, 6, 46–62.
- Al-Hashem, M.; Akbar, S.; Morris, P. Role of oxygen vacancies in nanostructured metal-oxide gas sensors: A review. Sens. Actuators B Chem. 2019, 301, 126845.
- Zhang, B.; Gao, P.-X. Metal oxide nanoarrays for chemical sensing: A review of fabrication methods, sensing modes, and their inter-correlations. Front. Mater. 2019, 6, 55.
- Choi, J.-K.; Hwang, I.-S.; Kim, S.J.; Park, J.-S.; Park, S.-S.; Jeong, U.; Kang, Y.C.; Lee, J.-H. Design of selective gas sensors using electrospun Pd-doped SnO2 hollow nanofibers. Sens. Actuators B Chem. 2010, 150, 191–199.
- Xu, S.; Kan, K.; Yang, Y.; Jiang, C.; Gao, J.; Jing, L.; Shen, P.; Li, L.; Shi, K. Enhanced NH3 gas sensing performance based on electrospun alkaline-earth metals composited SnO2 nanofibers. J. Alloy. Compd. 2015, 618, 240–247.
- Wang, J.; Zou, B.; Ruan, S.; Zhao, J.; Chen, Q.; Wu, F. HCHO sensing properties of Ag-doped In2O3 nanofibers synthesized by electrospinning. Mater. Lett. 2009, 63, 1750–1753.
- Wang, B.J.; Ma, S.Y. High response ethanol gas sensor based on orthorhombic and tetragonal SnO2. Vacuum 2020, 177, 109428.
- Zhao, C.; Gong, H.; Niu, G.; Wang, F. Ultrasensitive SO2 sensor for sub-ppm detection using Cu-doped SnO2 nanosheet arrays directly grown on chip. Sens. Actuators B Chem. 2020, 324, 128745.
- Onkar, S.G.; Raghuwanshi, F.C.; Patil, D.R.; Krishnakumar, T. Synthesis, characterization and gas sensing study of SnO2 thick film sensor towards H2S, NH3, LPG and CO2. Mater. Today 2020, 23, 190–201.
- Li, N.; Fan, Y.; Shi, Y.; Xiang, Q.; Wang, X.; Xu, J. A low temperature formaldehyde gas sensor based on hierarchical SnO/SnO2 nano-flowers assembled from ultrathin nanosheets: Synthesis, sensing performance and mechanism. Sens. Actuators B Chem. 2019, 294, 106–115.
- Das, S.; Jayaraman, V. SnO2: A comprehensive review on structures and gas sensors. Prog. Mater. Sci. 2014, 66, 112–255.
- Bai, S.; Fu, H.; Zhao, Y.; Tian, K.; Luo, R.; Li, D.; Chen, A. On the construction of hollow nanofibers of ZnO-SnO2 heterojunctions to enhance the NO2 sensing properties. Sens. Actuators B Chem. 2018, 266, 692–702.
- Zhang, J.; Zhang, L.; Leng, D.; Ma, F.; Zhang, Z.; Zhang, Y.; Wang, W.; Liang, Q.; Gao, J.; Lu, H. Nanoscale Pd catalysts decorated WO3–SnO2 heterojunction nanotubes for highly sensitive and selective acetone sensing. Sens. Actuators B Chem. 2020, 306, 127575.
- Du, H.; Wang, H.; Yao, P.; Wang, J.; Sun, Y. In2O3 nanofibers surface modified by low-temperature RF plasma and their gas sensing properties. Mater. Chem. Phys. 2018, 215, 316–326.
- Wei, S.; Zhang, Y.; Zhou, M. Toluene sensing properties of SnO2ZnO hollow nanofibers fabricated from single capillary electrospinning. Solid State Commun. 2011, 151, 895–899.
- Liu, L.; Zhang, Y.; Wang, G.; Li, S.; Wang, L.; Han, Y.; Jiang, X.; Wei, A. High toluene sensing properties of NiO-SnO2 composite nanofiber sensors operating at 330 °C. Sens. Actuators B Chem. 2011, 160, 448–454.
- Choi, S.-W.; Zhang, J.; Akash, K.; Kim, S.S. H2S sensing performance of electrospun CuO-loaded SnO2 nanofibers. Sens. Actuators B Chem. 2012, 169, 54–60.
- Qin, W.; Xu, L.; Song, J.; Xing, R.; Song, H. Highly enhanced gas sensing properties of porous SnO2-CeO2 composite nanofibers prepared by electrospinning. Sens. Actuators B Chem. 2013, 185, 231–237.
- Li, F.; Gao, X.; Wang, R.; Zhang, T. Design of WO3-SnO2 core-shell nanofibers and their enhanced gas sensing performance based on different work function. Appl. Surf. Sci. 2018, 442, 30–37.
- Chi, X.; Liu, C.; Liu, L.; Li, S.; Li, H.; Zhang, X.; Bo, X.; Shan, H. Enhanced formaldehyde-sensing properties of mixed Fe2O3-In2O3 nanotubes. Mater. Sci. Semicond. Process. 2014, 18, 160–164.
- Feng, C.; Li, X.; Ma, J.; Sun, Y.; Wang, C.; Sun, P.; Zheng, J.; Lu, G. Facile synthesis and gas sensing properties of In2O3-WO3 heterojunction nanofibers. Sens. Actuators B Chem. 2015, 209, 622–629.
- Liang, X.; Kim, T.-H.; Yoon, J.-W.; Kwak, C.-H.; Lee, J.-H. Ultrasensitive and ultraselective detection of H2S using electrospun CuO-loaded In2O3 nanofiber sensors assisted by pulse heating. Sens. Actuators B Chem. 2015, 209, 934–942.
- Du, H.; Wang, J.; Sun, Y.; Yao, P.; Li, X.; Yu, N. Investigation of gas sensing properties of SnO2/In2O3 composite hetero-nanofibers treated by oxygen plasma. Sens. Actuators B Chem. 2015, 206, 753–763.
- Zeng, X.; Liu, L.; Lv, Y.; Zhao, B.; Ju, X.; Xu, S.; Zhang, J.; Tian, C.; Sun, D.; Tang, X. Ultra-sensitive and fast response formaldehyde sensor based on La2O3-In2O3 beaded nanotubes at low temperature. Chem. Phys. Lett. 2020, 746, 137289.
- Zhang, B.; Bao, N.; Wang, T.; Xu, Y.; Dong, Y.; Ni, Y.; Yu, P.; Wei, Q.; Wang, J.; Guo, L.; et al. High-performance room temperature NO2 gas sensor based on visible light irradiated In2O3 nanowires. J. Alloy. Compd. 2021, 867, 159076.
- Li, X.; Wang, J. One-dimensional and two-dimensional synergized nanostructures for high-performing energy storage and conversion. InfoMat 2020, 2, 3–32.
- Song, B.; Loya, P.; Shen, L.; Sui, C.; He, L.; Guo, H.; Guo, W.; Rodrigues, M.-T.F.; Dong, P.; Wang, C.; et al. Quantitative in situ fracture testing of tin oxide nanowires for lithium ion battery applications. Nano Energy 2018, 53, 277–285.
- Schiavi, P.G.; Farina, L.; Zanoni, R.; Altimari, P.; Cojocariu, I.; Rubino, A.; Navarra, M.A.; Panero, S.; Pagnanelli, F. Electrochemical synthesis of nanowire anodes from spent lithium ion batteries. Electrochim. Acta 2019, 319, 481–489.
- Wang, W.; Liang, Y.; Kang, Y.; Liu, L.; Xu, Z.; Tian, X.; Mai, W.; Fu, H.; Lv, H.; Teng, K.; et al. Carbon-coated SnO2@carbon nanofibers produced by electrospinning-electrospraying method for anode materials of lithium-ion batteries. Mater. Chem. Phys. 2019, 223, 762–770.
- Xia, J.; Zhang, X.; Yang, Y.; Wang, X.; Yao, J. Electrospinning fabrication of flexible, foldable, and twistable Sb2S3/TiO2/C nanofiber anode for lithium ion batteries. Chem. Eng. J. 2021, 413, 127400.
- Oh, J.H.; Su Jo, M.; Jeong, S.M.; Cho, C.; Kang, Y.C.; Cho, J.S. New synthesis strategy for hollow NiO nanofibers with interstitial nanovoids prepared via electrospinning using camphene for anodes of lithium-ion batteries. J. Ind. Eng. Chem. 2019, 77, 76–82.
- Wu, X.; Shi, Z.-Q.; Wang, C.-Y.; Jin, J. Nanostructured SiO2/C composites prepared via electrospinning and their electrochemical properties for lithium ion batteries. J. Electroanal. Chem. 2015, 746, 62–67.
- Zhu, J.; Zhang, G.; Gu, S.; Lu, B. SnO2 nanorods on zno nanofibers: A new class of hierarchical nanostructures enabled by electrospinning as anode material for high-performance lithium-ion batteries. Electrochim. Acta 2014, 150, 308–313.
- Zhang, J.; Li, L.; Chen, J.; He, N.; Yu, K.; Liang, C. Controllable SnO2/ hollow nanotubes prepared by electrospinning technology used as anode for lithium ion battery. J. Phys. Chem. Solids 2021, 150, 109861.
- Chan, C.K.; Peng, H.; Liu, G.; McIlwrath, K.; Zhang, X.F.; Huggins, R.A.; Cui, Y. High-performance lithium battery anodes using silicon nanowires. Nat. Nanotechnol. 2008, 3, 31–35.
- Lei, D.; Qu, B.; Lin, H.-T.; Wang, T. Facile approach to prepare porous GeO2/SnO2 nanofibers via a single spinneret electrospinning technique as anodes for Lithium-ion batteries. Ceram. Int. 2015, 41, 10308–10313.
- Guo, J.; Liu, X.; Wang, H.; Sun, W.; Sun, J. Synthesis of hollow tubular reduced graphene oxide/SnO2 composites and their gas sensing properties. Mater. Lett. 2017, 209, 102–105.
- Zhu, X.; Li, Y.; Zhang, H.; Song, L.; Zu, H.; Qin, Y.; Liu, L.; Li, Y.; Wang, F. High-performance field effect transistors based on large ratio metal (Al,Ga,Cr) doped In2O3 nanofibers. J. Alloy. Compd. 2020, 830, 154578.
- Veeralingam, S.; Badhulika, S. Surface functionalized β-Bi2O3 nanofibers based flexible, field-effect transistor-biosensor (BioFET) for rapid, label-free detection of serotonin in biological fluids. Sens. Actuators B Chem. 2020, 321, 128540.
- Reddy, K.C.S.; Sahatiya, P.; Santos-Sauceda, I.; Cortázar, O.; Ramírez-Bon, R. One-step fabrication of 1D p-NiO nanowire/n-Si heterojunction: Development of self-powered ultraviolet photodetector. Appl. Surf. Sci. 2020, 513, 145804.
- Huang, X.; Zhang, M.; Qin, Y.; Chen, Y. Bead-like Co-doped ZnO with improved microwave absorption properties. Ceram. Int. 2019, 45, 7789–7796.
- Doucey, M.A.; Carrara, S. Nanowire sensors in cancer. Trends Biotechnol. 2019, 37, 86–99.
- Chopra, N.; Gavalas, V.G.; Hinds, B.J.; Bachas, L.G. Functional one-dimensional nanomaterials: Applications in nanoscale biosensors. Anal. Lett. 2007, 40, 2067–2096.
- Aly, I.H.M.; Abed Alrahim Mohammed, L.; Al-Meer, S.; Elsaid, K.; Barakat, N.A.M. Preparation and characterization of wollastonite/titanium oxide nanofiber bioceramic composite as a future implant material. Ceram. Int. 2016, 42, 11525–11534.
- Wang, X.; Gittens, R.A.; Song, R.; Tannenbaum, R.; Olivares-Navarrete, R.; Schwartz, Z.; Chen, H.; Boyan, B.D. Effects of structural properties of electrospun TiO2 nanofiber meshes on their osteogenic potential. Acta Biomater. 2012, 8, 878–885.
- Chen, S.; Shen, L.; Huang, D.; Du, J.; Fan, X.; Wei, A.; Jia, L.; Chen, W. Facile synthesis, microstructure, formation mechanism, in vitro biocompatibility, and drug delivery property of novel dendritic TiO2 nanofibers with ultrahigh surface area. Mater. Sci. Eng. C 2020, 115, 111100.
- Bezir, N.Ç.; Evcin, A.; Diker, R.; Özcan, B.; Klr, E.; Akarca, G.; Çetin, E.S.; Kayall, R.; Özen, M.K. Investigation of antibacterial properties of Ag doped TiO2 nanofibers prepared by electrospinning process. Open Chem. 2018, 16, 732–737.
- Jia, L.; Huang, X.; Liang, H.; Tao, Q. Enhanced hydrophilic and antibacterial efficiencies by the synergetic effect TiO2 nanofiber and graphene oxide in cellulose acetate nanofibers. Int. J. Biol. Macromol. 2019, 132, 1039–1043.
- Ramkumar, K.M.; Manjula, C.; Gnanakumar, G.; Kanjwal, M.A.; Sekar, T.V.; Paulmurugan, R.; Rajaguru, P. Oxidative stress-mediated cytotoxicity and apoptosis induction by TiO2 nanofibers in HeLa cells. Eur. J. Pharm. Biopharm. 2012, 81, 324–333.
- Chen, R.; Wan, Y.; Wu, W.; Yang, C.; He, J.-H.; Cheng, J.; Jetter, R.; Ko, F.K.; Chen, Y. A lotus effect-inspired flexible and breathable membrane with hierarchical electrospinning micro/nanofibers and ZnO nanowires. Mater. Des. 2019, 162, 246–248.
- Thangavel, K.; Roshini, T.; Balaprakash, V.; Gowrisankar, P.; Sudha, S.; Mohan, M. Structural, morphological and antibacterial properties of ZnO nanofibers fabricated by electrospinning technique. Mater. Today Proc. 2020, 33, 2160–2166.
- Thakur, S.; Kaur, M.; Lim, W.F.; Lal, M. Fabrication and characterization of electrospun ZnO nanofibers; antimicrobial assessment. Mater. Lett. 2020, 264.
More
Information
Subjects:
Others
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.0K
Revisions:
3 times
(View History)
Update Date:
10 Dec 2021
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




