Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Others
1D electrospun MOS are basically nanofibers, nanotubes or nanowires, which are made of one or more semiconductor metal oxides or with other dopant material. Polymer/MOS precursor nanofibers that are formed in the electrospinning process are calcined until the polymer is removed and pristine MOS nanostructure is obtained.
- electrospinning
- metal oxides
- nanomaterials
- nanofibers
- nanowires
1. Electrospun 1D MOS in Saving the Natural Environment
Industrialization and increasing consumerism have led to the highest level of warning about environmental pollution and its associated crisis. Industrial waste compared to municipal waste is toxic and non-biodegradable, as it contains heavy metal ions, oils and fats, dyes, phenols and ammonia, which can adversely affect human life and health but also the environment. One possible solution to this problem is to use the process of photocatalysis to break down harmful substances into simpler and environmentally friendly ones. Photocatalysis combines reactions using light and a catalyst, which is usually a semiconductor—it absorbs light and acts as a catalyst for chemical reactions. Therefore, it is necessary to search for semiconductor materials that can help solve this global problem.
Recently, electrospun one-dimensional semiconductor metal oxide nanostructures, predisposed by their unique optical and electrical properties, have attracted the attention of researchers studying photocatalytic pollutant decomposition processes of TiO2, ZnO and SnO2, whose energy gap width, radiation absorption range and mobility rate can be controlled by the parameters of the manufacturing process (Figure 9, Table 3 and Table 4) [86,103,104,105,106,107].
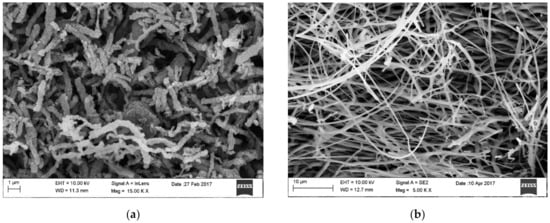
Figure 9. SEM image of surface morphology of (a) TiO2 nanowires after calcination at 400 °C, (b) ZnO nanowires after calcination at 400 °C [own study].
Table 3. Optical properties of selected 1D metal oxides prepared via electrospinning.
Table 4. Optical properties of TiO2 nanowires and ZnO nanowires after calcination at 400 °C [own study].
Z. Wang et al. performed an analysis [111] of the photocatalytic properties of ZnO/SnO2 nanofiber with and without the addition of the P123 precursor, which resulted in a much higher photocatalytic activity in the degradation of methyl orange (MO) in UV light of the composite nanofibers with the addition of P123. C. Zhu et al. in their work [112] showed significantly greater possibilities of photocatalytic decomposition of Rhodamine B in visible light through the use of composite SnO2/Fe2O3 nanofibers compared to the capabilities of the non-admixture SnO2. Electrospun SnO2 nanostructures coated with a 1 nm thick carbon shell fabricated by P. Zhang et al. [113] showed very efficient photocatalytic degradation of 4-Nitrophenol under both UV and visible light (Figure 10). K. Wang et al. in their work [114] reported a study on the photocatalytic activity of mutiheterojunction in the photodegradation of methyl orange (MO) and Cr (VI) ions under visible light. It was observed that the SnO2/Bi2O3/BiOI nanofibers were characterized by better photocatalytic activity than the non-admixture SnO2 and Bi2O3, which the authors attributed to the increased absorption of visible light, electron-hole pair separation and large specific surface area of the nanostructures studied.
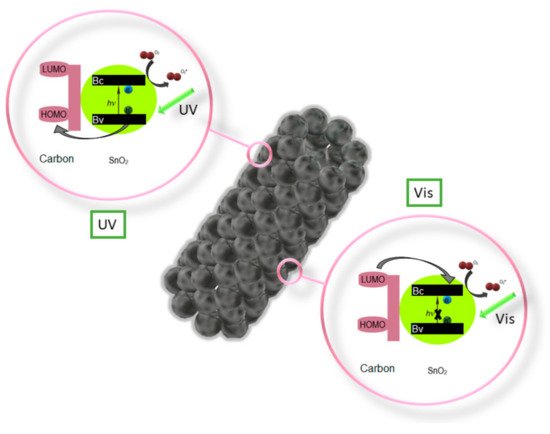
Figure 10. Diagram of the photocatalytic activation of nanotubes SnO2 [113].
T. Wang. et al. [86] demonstrated that the use of magnetic field-assisted electrospinning in the fabrication of nanofibers and nanotubes from TiO2 narrowed the band gap to favor photocatalytic performance—TiO2 reduced Rhodamine B (RhB) by 95.8% in 100 min. Q. Zhang et al. [115] proposed the use of 1D composite nanostructures based on In2O3 of admixtured CaIn2O4 in the photocatalytic purification of water from the dye-methylene blue (MB). The degradation rates of MB were 76% and 92%, respectively, under 120 min of simulated sunlight exposure. The efficient separation and transport of photogenerated carriers, as well as the large specific surface area, meant that the CaIn2O4-In2O3 composites were characterized by high photocatalytic efficiency. A. Ahmad et al. [116] by the triaxial electrospinning method produced TiO2 with a structure of nanofiber-in-nanotube (rutile-anatase), with which the photodegradation was carried out for 88.1% of the Sandalfix N. Blue with a 240 min irradiation time.
The diversity of available variations of the electrospinning process makes it possible to obtain MOS with high photocatalytic activity; however, further research is needed to explore the mechanism of this phenomenon.
The growing demand for green energy motivates researchers to look for materials and solutions that can increase the efficiency of existing renewable energy sources (RES), especially photovoltaic cells. So far, the many works that have presented the possibility of using 1D MOS in the construction of modern solar cells mainly focused on the use of TiO2, ZnO and SnO2 [117,118,119,120,121,122,123,124,125].
Favorable optoelectronic properties of crystalline-amorphous hybrid SnO2 nanowires are suggested by W. Matysiak et al. [24] to be used in in modern flexible photovoltaic cells (Table 5, Figure 11). The research group, to which the Authors belong, was awarded a silver medal at the 5th China (Shanghai) International Invention & Innovation Expo in 2021 for the invention “Innovative flexible solid-state solar cell with a hybrid layered architecture”, for which the construction of which SnO2 nanowires were used (Figure 12).
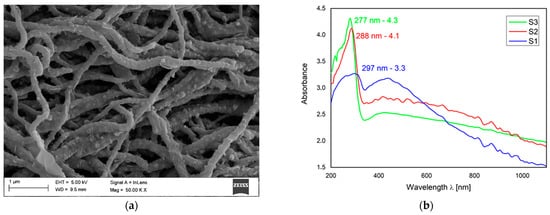
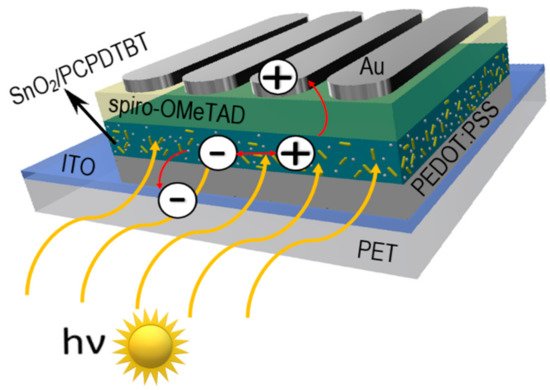
Figure 12. Schematic representation of multilayer flexible photovoltaic architecture manufactured in Department of Engineering Materials and Biomaterials [Poster at the 5th China (Shanghai) International Invention & Innovation Expo: “Innovative flexible solid-state solar cell with a hybrid layered architecture”].
Table 5. Refractive index and dielectric permittivity obtained for the electrospun 1D SnO2 nanomaterials [24].
M. Yang et al. in their publication [127] described the effect of graphene oxide (GO) admixture in hybrid SnO2/TiO2 nanofibers on the efficiency of dye-based solar cells (DSCs) constructed with their participation. DSCs along with GO-SnO2/TiO2 as the working electrode were analyzed for efficiency and the following photovoltaic parameters: short-circuit current density, open-circuit voltage and fill factor, which were respectively 11.19 mA/cm2, 0.72 V and 0.67. It was found that the solar-to-electric energy conversion efficiency of GO/SnO2/TiO2 as a photoanode-based device was 5.41%.
Therefore, it is worthwhile to pay attention to the application of MOS in the construction of next-generation photovoltaic cells, as they may provide a solution to the problem of low efficiency of dye-based cells.
2. Electrospun Metal Oxides 1D Nanostructures in Gas Sensors
The most widely studied application of one-dimensional metal oxide-based nanostructures are sensors for gases such as methanol, ethanol, acetone, formaldehyde, xylene and other volatile organic compounds that are highly toxic and dangerous to human health and even life [12,128].
Gas sensors based on semiconductor metal oxides are widely used in many areas, including chemical pollution control in air and rooms, alarms to detect the threat of poisonous substances and even medical diagnostics performed on the basis of a patient’s breath. The popularity of these types of sensors is due to their high sensitivity, low cost and ease of manufacture, as well as their compatibility with modern electronic devices [129,130,131,132,133,134].
The mechanism of gas detection by these MOS can be explained by the fact that the conductivity of the materials is changed by the chemical interaction between the gas and the surface of the nanostructure on which oxygen is adsorbed. Oxygen (O2) molecules are adsorbed on the nanofiber/nanowire surface in air and then they capture electrons from the conductivity band of the oxide so that chemisorbed oxygen ions (O2−) are generated and the formation of a barrier layer at a certain depth of the oxide structure is initiated. When the nanostructures are exposed to gas at an appropriate temperature, the gas reacts with the surface oxygen species and the width of the barrier layer decreases. As a result, the carrier concentration will increase, which ultimately increases the conductivity of the nanofibers/nanowires [135,136,137].
Many scientific reports indicate that the detection of hazardous substances by sensors based on electrospun MOS still needs to be developed—obtaining sensors with a lower substance detection threshold and shorter device response and reaction times. Improvement of these properties can be achieved by admixing with metallic nanoparticles, other MOS and carbon materials, which will affect the conductivity of the MOS. The combination of different materials produces local p-n, n-n or p-p nanojunctions. It is the heterojunctions generated from different materials that directly affect the substance detection mechanism. Several typical morphologies of MOS-based heterostructured materials are most commonly reported in the literature (Figure 13). In addition to non-admixed 1D MOS, hybrid structures consisting of both MOS and admixed crystallites simultaneously stand out. MOS nanowires decorated with nanoparticles or other forms of admixture are another interesting variation. There are also structures with core-shell morphology in which MOS can be either covered or surrounded by other material.
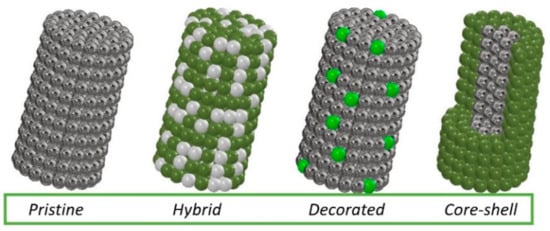
Figure 13. Types of morphology of the most commonly produced 1D nanostructures.
One of the most commonly used MOS as detector anode is tin dioxide, which is characterized by an energy gap width of about 3.6 kV and simultaneous optical transparency and electrical conductivity [136,138,139,140,141,142]. Indium oxide exhibiting similar properties to tin oxide is also increasingly used. These materials are often combined with each other and also admixed with other oxides such as TiO2, ZnO, CuO and NiO (Table 6). The authors of this paper have established a collaboration with the Department of Optoelectronics, which is equipped with laboratories capable of gas detection measurements. Electrospun SnO2 and In2O3 nanowires fabricated in the Department of Engineering Materials and Biomaterials will be plotted on the IDT and tested to detect gases such as NH3, NO2, CO2 and H2.
Bai et al. [143] demonstrated that the porous, coreless structure of ZnO-SnO2 nanowires is ideal for detecting very low concentrations (0.023 ppm) of toxic NO2. In addition, good detection properties of NO2 promotes the formation of an n-n heterojunction at the phase boundary of ZnO and SnO2, which results in the formation of an additional barrier layer (Figure 14).
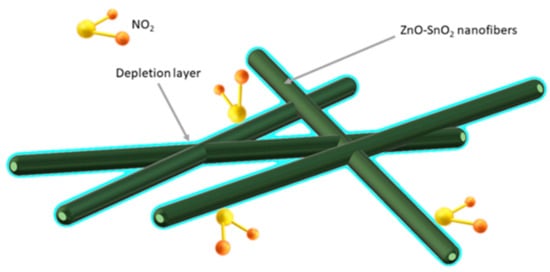
Figure 14. Scheme of hollow structure of gas adsorption and depletion layer for ZnO-SnO2 composite [143].
Zhang et al. [144] observed that the response of sensors in acetone-containing environment can be improved by using heterojunction nanotubes of WO3-SnO2 and admixing it with Pd catalyst. Studies of the sensory properties of the material showed that the addition of Pd increased the response of Pd-WO3-SnO2 sensor more than double the response obtained from WO3-SnO2 sensor in contact with 100 ppm acetone. In addition, the selectivity for detecting acetone in the presence of other gases such as toluene, ammonia, nitrous oxide and pentane was significantly improved.
Du et al. [145] fabricated In2O3 nanofibers with a traditional electrospinning method and then they subjected them to surface modification using low-temperature oxygen and hydrogen radiofrequency plasma. The nanofibers were placed in a plasma reactor chamber and surface modification was performed by increasing the number of pores and channels in the nanofibers (Figure 15). This mechanism enabled more oxygen to be adsorbed on the surface of the indium oxide nanostructures, leading to increased response values and improved selectivity for detecting acetone in the presence of interfering gases such as ethanol, methanol, formaldehyde, benzene, ammonia and nitrogen dioxide.
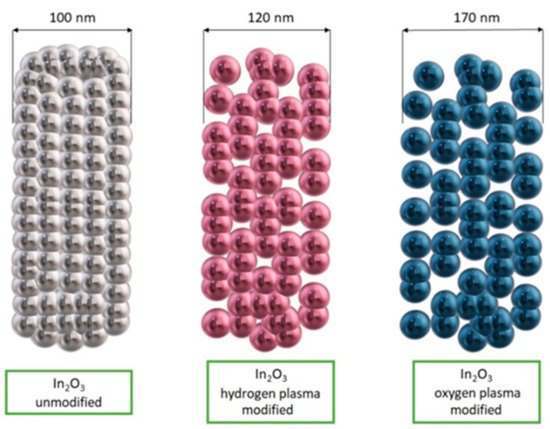
Figure 15. Scheme of In2O3 nanofibers morphology before and after surface modification [145].
Table 6. Selected 1D MOS and their sensing properties.
The above considerations indicate that electrospun one-dimensional MOS plays a key role in the construction of gas sensors, thus contributing to their development and improving work and life safety in environments exposed to hazardous gases.
3. Electrospun Metal Oxides 1D Nanostructures in Other Applications
Supercapacitors and lithium-ion batteries (LIBs) are other devices for which one-dimensional MOS nanostructures can be used. With the rapid progress of civilization and industrialization, there is a growing need for methods, materials and devices to store large amounts of energy [157]. One solution to meet these needs is the development of LIBs with high performance, which is primarily dependent on the performance of the battery’s most important component, the anode. The currently used anode material in the form of graphite is currently no longer able to meet the needs of high energy storage capacity due to its low capacity and low efficiency. Therefore, the search and research of new electrode materials is of great importance for the current demand for high performance LIBs [30,158]. Recently, semiconductor nanomaterials such as ZnO, NiO, SnO2 lub TiO2 nanotubes and nanowires have been of particular interest for 1D, along with heterojunctions formed by combining these materials with carbon materials [159,160,161,162,163,164]. The advantage of using one-dimensional nanomaterials for anodes in LIBs is the much less frequently observed agglomeration of the material than in the case of nanoparticles, which positively affects the electrochemical performance of the battery, and this fact was confirmed in a study by C. K. Chan et al. [165] based on the analysis of a battery based on Si nanowires.
J. Zhu et al. [163] pointed out the high application potential of electrospun ZnO-SnO2 nanofibers as anode material in lithium-ion batteries. It was observed that due to the heterogeneous mesoporous electrode structure based on ZnO-SnO2 nanofibers, they provide excellent performance and reversible capacity at a relatively low cost and with high process repeatability. D. Lei et al. in their work [166] showed that GeO2-SnO2 composite nanofibers with high porosity prepared by the solution electrospinning method have high specific capacitance and good cycling performance, which is mainly due to the porous one-dimensional nanostructure, which can shorten the transport pathway and provide trapping of electrolyte ions to meet the requirements of fast charging and discharging reactions. J. Guo in [167] described the effect of pore distribution on the capacitance of two types of porous C/SnO2 nanofibers produced by electrospinning from solutions based on different precursors, i.e., using tin chloride, the fibers with spherical pores were obtained, while the pores in the form of channels were obtained from acetate (Figure 16). On the basis of a galvanostatic charge-discharge test, it was found that multichannel C/SnO2 nanofibers with a large specific surface area (34.97 m2/g) achieve better charging performance than spherical pore nanofibers and show a more stable capacity retention of about 90% after 50 cycles.
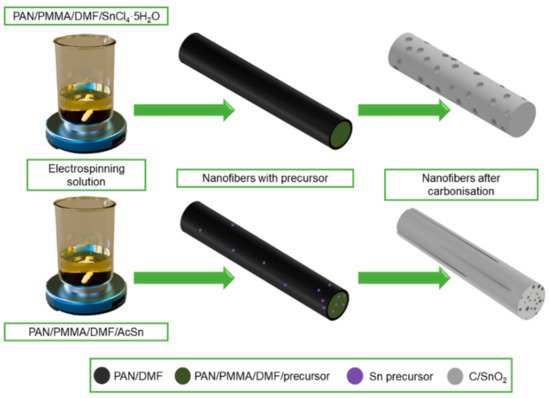
Figure 16. Scheme of the manufacturing process of C/SnO2 nanofibers with a different morphology.
The use of SnO2-ZnO nanofibers in energy storage was presented in the work [164] of J. Zhang et al. The study showed that by using the spinning solution parameters, it is possible to control the morphology and obtain hollow nanotubes, which exhibited good capacity stability in an electrochemical test. In addition, it was observed that the polypyrrole (PPy) polymer coating of SnO2-ZnO nanotubes has made it possible to maintain a high capacity of 626.1 mA hg−1 at 0.2 °C for 100 cycles, and cycle stability has also been improved.
Thus, the electrospinning method with subsequent calcination enables precise control of the electrochemical properties of the fabricated one-dimensional MOS-based nanostructures, thus providing a chance to solve the problem of non-compliant LIBs.
Due to their unique optical, electrical and magnetic properties, they are used in modern devices such as field-effect transistors (FETs) and microwave absorption materials. X. Zhu et al. presented [168] a method to fabricate high-performance field-effect transistors based on electrospun In2O3 nanofibers admixed with Al, Ga and Cr. The devices showed optimal performance at a 10% molar concentration of admixing material (Al, Cr and Ga): low and positive gate-source voltage VGS (<6.0 V), a high ratio of the transistor on current to transistor off current Ion/Ioff (~108), high saturation current (~10–4 A) and carrier mobility on the level of ~2.0 cm2/V−1s−1.
H. Zhang et al. [111] demonstrated that the use of polymorphic anatase-rutile TiO2 nanofibers to build FET showed better transistor characteristics because of a strong synergistic effect compared with pure anatase and rutile TiO2 nanofibers. BioFET created by S. Veeralingam and S. Badhulik [97] based on β–Bi2O3 nanofibers for the detection of serotonin exhibited sensitivity of 51.64 μA/nM over a range of 10 nM−1 μM and a limit of detection of 0.29 nM. Moreover, it maintained excellent sensitivity, stability and reproducibility with a rapid response time of 0.8 s. Using the electrospinning method, K.C.S. Reddy et al. [169] created a self-powered NiO-p/Si-n based ultraviolet photodetector which exhibited a high responsivity of 9.1 mA W−1 at zero bias with a fast photoresponse of less than 0.4 s. X. Huang et al. [102] observed that electrospun bead-like Co-ZnO nanostructures present ferromagnetic properties and an excellent electromagnetic loss performance—the effective microwave absorption of bandwidth with reflection loss less than −10 dB was 11.6 GHz.
For years, medicine has been a priority discipline in which new solutions and biomaterials are constantly being sought. Looking at the disease problems that affect mankind today, the most rapidly developing areas of medicine include cancer therapies, drug delivery, biosensors, medical imaging and tissue engineering. Due to the unsatisfactory properties of conventional biomaterials, it is necessary to search for new material solutions. Production of one-dimensional nanomaterials with controlled dimensions, arrangement of structures with respect to each other or porosity creates many possibilities of using their unique properties for therapeutic purposes. Ceramic nanomaterials, which are based on inert simple oxides, may seem to be a possible solution for some health problems. The most commonly used one-dimensional MOS include TiO2, due to its non-toxicity, environmental friendliness as well as good chemical stability and high corrosion resistance [170,171].
One of many interesting examples of work on the above issue is that presented by I.H.M. Aly et al. [172], who used electrospun TiO2 nanofibers as an admixture to a bioceramic composite based on wollastonite for bone tissue regeneration, which significantly improved the mechanical properties of the composite while not affecting the bioactivity in any way, and proves that this type of material is worth considering and researching for applications in medicine. Mesh with TiO2 nanofibers may also find applications in tissue engineering, as studies have shown that it provides an osteogenic environment—increasing osteoblast production and differentiation [173]. S. Chen et al. confirmed the possibility of using hydrothermal treated nanofibers as delivery systems for the antibiotic tetracycline hydrochloride, whereby nanofibers showed high bactericidal activity against E. coli and S. aureus [174]. N.C. Bezir et al. demonstrated that TiO2 and Ag/TiO2 nanofibers show beneficial antibacterial properties based on measured inhibition zones diameters of S. aureusculture plates [175]. Effective inhibition of B. subtilis and B. cereus through TiO2/GO/CA nanofibers was observed by L. Jia et al. [176]. TiO2 in the form of electrospun one-dimensional nanostructures also shows promising results in promoting apoptosis of cancer cells, e.g., cervical cancer [177]. Other applications of 1D ceramic nanomaterials in medicine include the use of oleic acid-coated ZnO nanowires to fabricate hydrophobic polyvinylidene fluoride (PVDF) membranes, whose self-cleaning properties can be used to construct surgical devices and instruments or artificial blood vessels [178]. ZnO nanofibers, similarly to TiO2 nanofibers, are characterized by tremendous antibacterial activity in S. aureus and E. coli utilization [179,180].
This entry is adapted from the peer-reviewed paper 10.3390/ma14185139
This entry is offline, you can click here to edit this entry!
