
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Christian Baumeier | + 1588 word(s) | 1588 | 2021-11-18 09:11:49 | | | |
| 2 | Dean Liu | -28 word(s) | 1560 | 2021-12-07 01:58:31 | | |
Video Upload Options
The pathophysiology of viral myocarditis and its sequelae leading to severe heart failure with a poor prognosis is not fully understood and represents a significant public health issue globally. Most likely, at a certain point, besides viral persistence, several etiological types merge into a common pathogenic autoimmune process leading to chronic inflammation and tissue remodeling, ultimately resulting in the clinical phenotype of dilated cardiomyopathy.
1. Introduction
2. General Pathophysiological Aspects
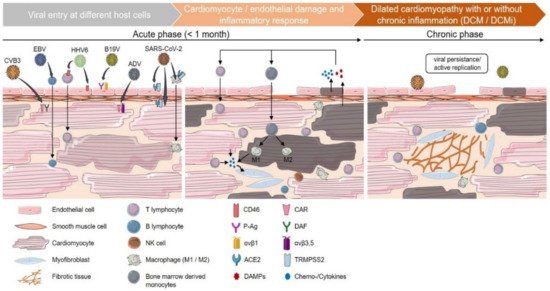
2.1. Phases of Viral-Mediated Myocarditis
2.2. Autoimmunity in Viral Myocarditis
3. Treatment Options
The definitive differentiation between virus positivity/transcriptional activity or virus exclusion, and the evidence of inflammation, its characterization and intensity are a fundamental prerequisite for a specific therapeutic decision based on EMB analyses. The first randomized trial on viral cardiomyopathy was the placebo-controlled phase II multicenter BICC-Trial (Betaferon In Chronic Viral Cardiomyopathy) [7]. Compared to placebo, in enterovirus and adenovirus infections, interferon-β-1b was leading to effective virus clearance in follow-up EMB after treatment, associated with favorable effects on NYHA functional class, improvement in quality of life and global patient assessment.
A recent study demonstrated the benefit of nucleoside analog treatment in controlling B19V replication and reducing viral transcripts, as well as rapidly improving symptoms in patients with active B19V infection [19].
Myocardial inflammation that persists after viral elimination requires immunosuppressive treatment to prevent subsequent autoimmune-mediated myocardial damage. However, viral genomes must be excluded prior to immunosuppressive therapy. Treatment for these patients with post-viral autoimmunological myocarditis consists of corticosteroids, azathioprine or ciclosporin A, in addition to optimal heart failure medication [73][89][168].
References
- Caforio, A.L.P.; Pankuweit, S.; Arbustini, E.; Basso, C.; Blanes, J.G.; Felix, S.B.; Fu, M.; Heliö, T.; Heymans, S.; Jahns, R.; et al. Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: A position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur. Heart J. 2013, 34, 2636–2648.
- Bock, C.-T.; Klingel, K.; Kandolf, R. Human Parvovirus B19–Associated Myocarditis. N. Engl. J. Med. 2010, 362, 1248–1249.
- Sagar, S.; Liu, P.P.; Cooper, L.T., Jr. Myocarditis. Lancet 2012, 379, 738–747.
- Schultheiss, H.-P.; Fairweather, D.; Caforio, A.L.P.; Escher, F.; Hershberger, R.; Lipshultz, S.E.; Liu, P.P.; Matsumori, A.; Mazzanti, A.; McMurray, J.; et al. Dilated cardiomyopathy. Nat. Rev. Dis. Prim. 2019, 5, 32.
- Blauwet, L.A.; Cooper, L.T. Myocarditis. Prog. Cardiovasc. Dis. 2010, 52, 274–288.




