
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Marina Kleopatra Boziki | + 4120 word(s) | 4120 | 2021-11-23 05:17:27 | | | |
| 2 | Peter Tang | Meta information modification | 4120 | 2021-12-01 03:09:31 | | |
Video Upload Options
Sphingosine 1-phosphate (S1P) is a signaling molecule with complex biological functions that are exerted through the activation of sphingosine 1-phosphate receptors 1–5 (S1PR1–5). S1PR expression is necessary for cell proliferation, angiogenesis, neurogenesis and, importantly, for the egress of lymphocytes from secondary lymphoid organs. Since the inflammatory process is a key element of immune-mediated diseases, including multiple sclerosis (MS), S1PR modulators are currently used to ameliorate systemic immune responses.
1. Introduction
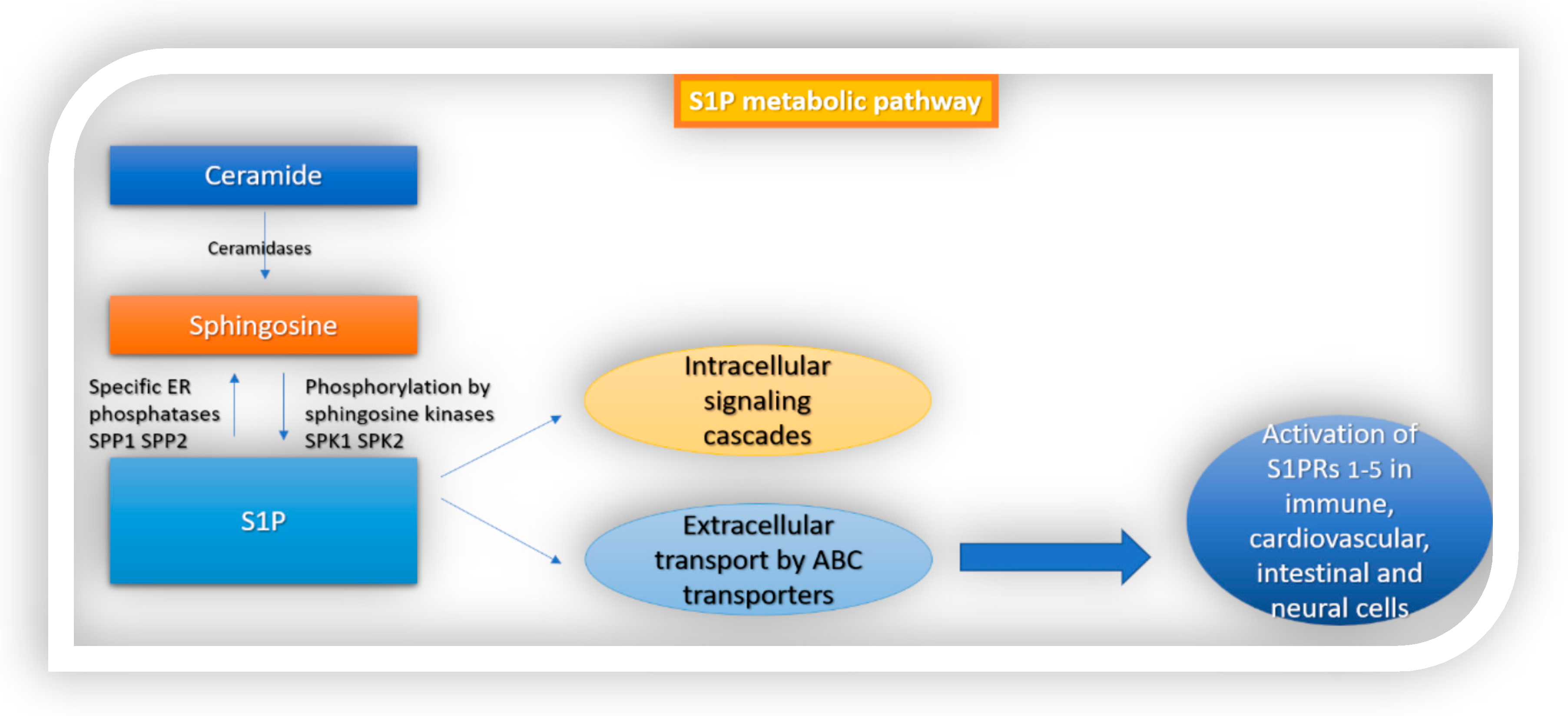
2. S1P Signaling in the Immune System
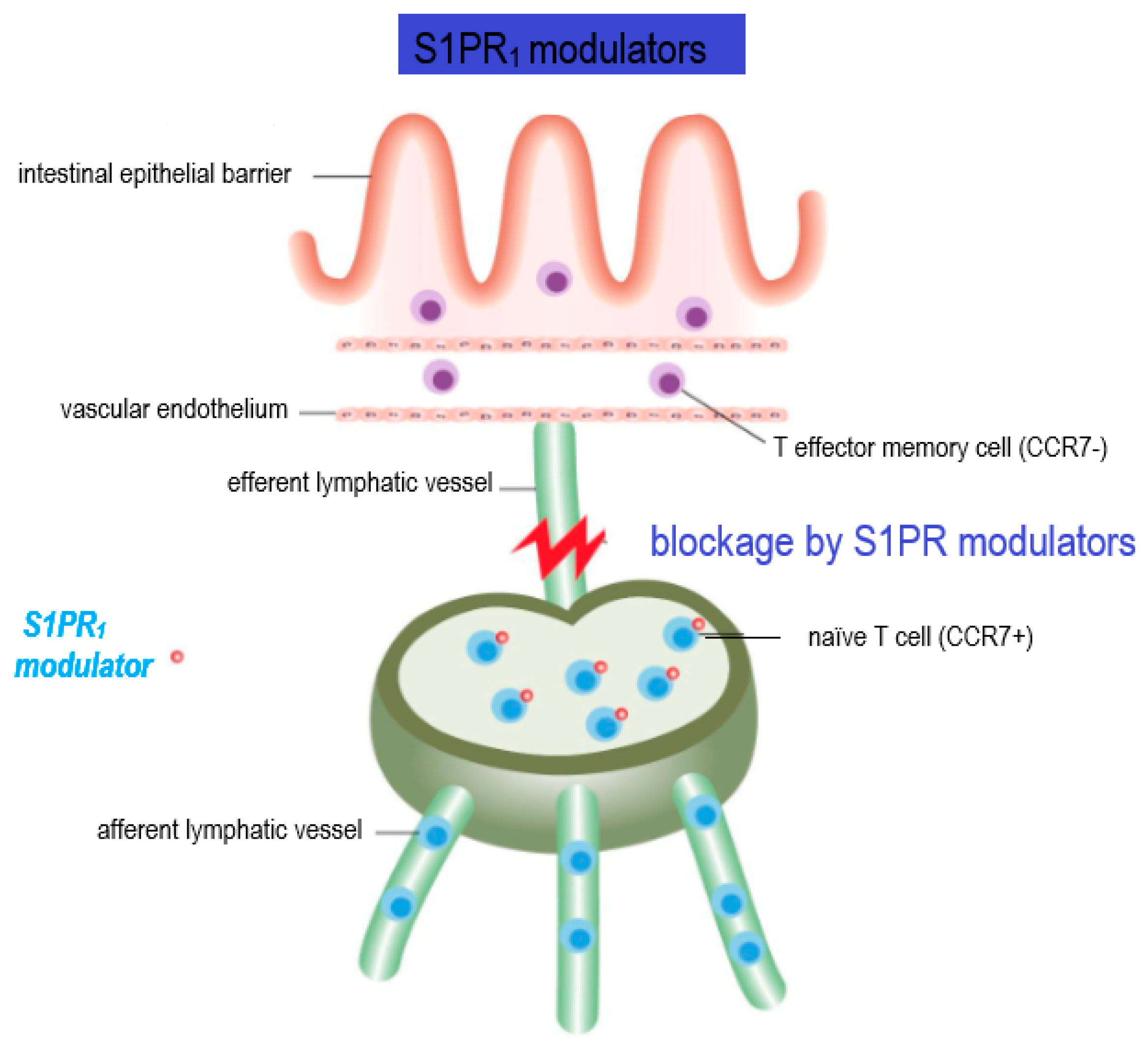
2.1. Lymphocyte Trafficking and Regulation of Adaptive Immune Functions
2.2. Regulation of Innate Immune Cell Trafficking
|
Receptor |
Associated Cell Types |
Functions (Described up to Date) |
|---|---|---|
|
S1PR1 |
Immune system: T cells, B cells Macrophages Neutrophils DCs Monocytes Eosinophils Mast cells NK cells |
Egress from lymph nodes, exit of mature T cells from thymus, migration of natural killer T cells from secondary lymphoid organs to circulation, transfer of immature B cells from bone marrow to circulation Macrophage recruitment Neutrophil migration, recruitment Trafficking of DCs Trafficking of monocytes Eosinophil recruitment Mast cell recruitment |
|
GI: IECs |
Upregulation of intestinal barrier proteins (claudin1, occludin) |
|
|
CNS: Astrocytes Oligodendrocytes Neurons Microglia |
Activation, differentiation, proliferation of astrocytes, astrogliosis Differentiation of oligodendrocytes, process extension, survival of oligodendrocyte progenitors, myelination Growth cone formation, enhancement of neurite extension, synaptic transmission |
|
|
S1PR2 |
Immune system: Macrophages Monocytes Mast cells Eosinophils |
Enhance antibody-mediated phagocytosis, inhibit phagocytosis of bacteria and fungi Degranulation of mast cells |
|
GI: IECs |
Upregulation of c-Myc, cyclin D1, E-cadherin and Zona occludin 1, proliferation of IECs, absorption of NaCl, prevention of IECs apoptosis |
|
|
CNS: Neurons Microglia |
Growth cone formation, inhibition of neurite extension, control of neural excitability |
|
|
S1PR3 |
Immune system: Macrophages Monocytes Neutrophils DCs Eosinophils Mast cells |
Leucocyte rolling on endothelial cells Macrophage’s chemotaxis and killing Neutrophil recruitment DC maturation Eosinophil recruitment |
|
GI: IECs |
||
|
CNS: Astrocytes Microglia |
Activation, differentiation, proliferation of astrocytes, astrogliosis |
|
|
S1PR4 |
Immune system: Macrophages Monocytes Neutrophils DCs Eosinophils Mast cells |
Macrophage migration and cytokine release Neutrophil migration Plasmacytoid DC activation and differentiation |
|
GI: IECs |
||
|
CNS: |
||
|
S1PR5 |
Immune system: Patrolling monocytes NK cells |
Egress of mature NK cells from lymph nodes and bone marrow |
|
GI: IECs |
||
|
CNS: Mature oligodendrocytes Neurons |
Cell survival, process retraction, inhibition of OPC migration, myelination Growth cone formation, inhibition of neurite extension |
3. S1P Signaling in the Intestine
Implication in Intestinal Barrier Homeostasis
4. S1P Signaling in the CNS
Implication in BBB Homeostasis
5. Implication for the Role of S1P in Multiple Sclerosis
References
- Thudichum, J.L.W. Treatise on the Chemical Constitution of the Brain: Based Throughout upon Original Researches. Glasgow Med. J. 1884, 22, 363–364.
- An, S.; Bleu, T.; Huang, W.; Hallmark, O.G.; Coughlin, S.R.; Goetzl, E.J. Identification of cDNAs encoding two G protein-coupled receptors for lysosphingolipids. FEBS Lett. 1997, 417, 279–282.
- Lee, M.J.; van Brocklyn, J.R.; Thangada, S.; Liu, C.H.; Hand, A.R.; Menzeleev, R.; Spiegel, S.; Hla, T. Sphingosine-1-phosphate as a ligand for the G protein-coupled receptor EDG-1. Science 1998, 279, 1552–1555.
- Hla, T.; Brinkmann, V. Sphingosine 1-phosphate (S1P): Physiology and the effects of S1P receptor modulation. Neurology 2011, 76, S3–S8.
- Rosen, H.; Gonzalez-Cabrera, P.J.; Sanna, M.G.; Brown, S. Sphingosine 1-phosphate receptor signalling. Annu. Rev. Biochem. 2009, 78, 743–768.
- Prager, B.; Spampinato, S.F.; Ransohoff, R.M. Sphingosine 1-phosphate signaling at the blood-brain barrier. Trends Mol. Med. 2015, 21, 354–363.
- Aoki, M.; Aoki, H.; Ramanathan, R.; Hait, N.C.; Takabe, K. Sphingosine-1-Phosphate Signaling in Immune Cells and Inflammation: Roles and Therapeutic Potential. Mediators Inflamm. 2016, 2016, 8606878.
- Marsolais, D.; Rosen, H. Chemical modulators of sphingosine-1-phosphate receptors as barrier-oriented therapeutic molecules. Nat. Rev. Drug Discov. 2009, 8, 297–307.
- Mendoza, A.; Bréart, B.; Ramos-Perez, W.R.; Pitt, L.A.; Gobert, M.; Sunkara, M.; Lafaille, J.; Morris, A.J.; Schwab, S.R. The transporter Spns2 is required for secretion of lymph but not plasma sphingosine-1- phosphate. Cell Rep. 2012, 2, 1104–1110.
- Cyster, J.G.; Schwab, S.R. Sphingosine-1-Phosphate and Lymphocyte Egress from Lymphoid Organs. Annu. Rev. Immunol. 2012, 30, 69–94.
- Bryan, A.M.; del Poeta, M. Sphingosine-1-phosphate receptors and innate immunity. Cell Microbiol. 2018, 20, e12836.
- Mao-Draayer, Y.; Sarazin, J.; Fox, D.; Schiopu, E. The sphingosine-1-phosphate receptor: A novel therapeutic target for multiple sclerosis and other autoimmune diseases. Clin. Immunol. 2017, 175, 10–15.
- Tsai, H.-C.; Han, M.H. Sphingosine-1-Phosphate (S1P) and S1P Signaling Pathway: Therapeutic Targets in Autoimmunity and Inflammation. Drugs 2016, 76, 1067–1079.
- Obinata, H.; Hla, T. Sphingosine 1-phosphate and inflammation. Int. Immunol. 2019, 23, 617–625.
- Matloubian, M.; Lo, C.G.; Cinamon, G.; Lesneski, M.J.; Xu, Y.; Brinkmann, V.; Allende, M.L.; Proia, R.L.; Cyster, J.G. Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1. Nature 2004, 427, 355.
- Schwab, S.R.; Pereira, J.P.; Matloubian, M.; Xu, Y.; Huang, Y.; Cyster, J.G. Lymphocyte sequestration through S1P lyase inhibition and disruption of S1P gradients. Science 2005, 309, 1735–1739.
- Allende, M.L.; Bektas, M.; Lee, B.G.; Bonifacino, E.; Kang, J.; Tuymetova, G.; Chen, W.; Saba, J.D.; Proia, R.L. Sphingosine-1-phosphate lyase deficiency produces a pro-inflammatory response while impairing neutrophil trafficking. J. Biol. Chem. 2011, 286, 7348–7358.
- Maeda, Y.; Seki, N.; Kataoka, H.; Takemoto, K.; Utsumi, H.; Fukunari, A.; Sugahara, K.; Chiba, K. IL-17-producing Vγ4+ γδ T cells require sphingosine 1-phosphate receptor 1 for their egress from the lymph nodes under homeostatic and inflammatory conditions. J. Immunol. 2015, 195, 1408–1416.
- Czeloth, N.; Bernhardt, G.; Hofmann, F.; Genth, H.; Förster, R. Sphingosine-1-phosphate mediates migration of mature dendritic cells. J. Immunol. 2005, 175, 2960.
- Walzer, T.; Chiossone, L.; Chaix, J.; Calver, A.; Carozzo, C.; Garrigue-Antar, L.; Jacques, Y.; Baratin, M.; Tomasello, E.; Vivier, E. Natural killer cell trafficking in vivo requires a dedicated sphingosine 1-phosphate receptor. Nat. Immunol. 2007, 8, 1337.
- Jenne, C.N.; Enders, A.; Rivera, R.; Watson, S.R.; Bankovich, A.; Pereira, J.; Xu, Y.; Roots, C.M.; Beilke, J.N.; Banerjee, A.; et al. T-bet-dependent S1P5 expression in NK cells promotes egress from lymph nodes and bone marrow. J. Exp. Med. 2009, 206, 2469–2481.
- Massberg, S.; Schaerli, P.; Knezevic-Maramica, I.; Köllnberger, M.; Tubo, N.; Moseman, E.A.; Huff, I.V.; Junt, T.; Wagers, A.J.; Mazo, I.B.; et al. Immunosurveillance by hematopoietic progenitor cells trafficking through blood, lymph, and peripheral tissues. Cell 2007, 131, 994.
- Olesch, C.; Ringel, C.; Brüne, B.; Weigert, A. Beyond Immune Cell Migration: The Emerging Role of the Sphingosine-1-phosphate Receptor S1PR4 as a Modulator of Innate Immune Cell Activation. Mediators Inflamm. 2017, 2017, 1–12.
- Finley, A.; Chen, Z.; Esposito, E.; Cuzzocrea, S.; Sabbadini, R.; Salvemini, D. Sphingosine 1-Phosphate Mediates Hyperalgesia via a Neutrophil-Dependent Mechanism. PLoS ONE 2013, 8, e55255.
- Miyabe, C.; Miyabe, Y.; Komiya, T.; Shioya, H.; Miura, N.N.; Takahashi, K.; Ohno, N.; Tsuboi, R.; Luster, A.D.; Kawai, S.; et al. A sphingosine 1-phosphate receptor agonist ameliorates animal model of vasculitis. Inflamm. Res. 2017, 66, 335–340.
- Keul, P.; Lucke, S.; Lipinski, K.V.W.; Bode, C.; Gräler, M.; Heusch, G.; Levkau, B. Sphingosine-1-phosphate receptor 3 promotes recruitment of monocyte/macrophages in inflammation and atherosclerosis. Circ. Res. 2011, 108, 314–323.
- Dillmann, C.; Mora, J.; Olesch, C.; Brüne, B.; Weigert, A. S1PR4 is required for plasmacytoid dendritic cell differentiation. Biol. Chem. 2015, 396, 775–782.
- Müller, J.; von Bernstorff, W.; Heidecke, C.-D.; Schulze, T. Differential S1P Receptor Profiles on M1- and M2-Polarized Macrophages Affect Macrophage Cytokine Production and Migration. BioMed Res. Int. 2017, 2017, 1–10.
- Debien, E.; Mayol, K.; Biajoux, V.; Daussy, C.; de Agüero, M.G.; Taillardet, M.; Dagany, N.; Brinza, L.; Henry, T.; Dubois, T.; et al. S1PR5 is pivotal for the homeostasis of patrolling monocytes. Eur. J. Immunol. 2013, 43, 1667–1675.
- Tsai, H.-C.; Nguyen, K.; Hashemi, E.; Engleman, E.; Hla, T.; Han, M.H. Myeloid sphingosine-1-phosphate receptor 1 is important for CNS autoimmunity and neuroinflammation. J. Autoimmun. 2019, 105, 102290.
- Cohen, J.; Bar-Or, A.; Cree, B.A.C.; Mao-Draayer, Y.; Han, M.H.; Singer, B.; Jannu, A.; Kolodny, S.; Meng, X.; Winger, R.C. The FLUENT study design: Investigating immune cell subset and neurofilament changes in patients with relapsing multiple sclerosis treated with fingolimod. Mult. Scler. J. Exp. Transl. Clin. 2019, 5, 2055217318819245.
- Salvo Romero, E.; Alonso Cotoner, C.; Pardo Camacho, C.; Casado Bedmar, M.; Vicario, M. The intestinal barrier function and its involvement in digestive disease. Rev. Esp. Enferm. Dig. 2015, 107, 686–696.
- Klunder, L.J.; Faber, K.N.; Dijkstra, G.; van Ijzendoorn, S.C.D. Mechanisms of Cell Polarity-Controlled Epithelial Homeostasis and Immunity in the Intestine. Cold Spring Harb. Perspect Biol. 2017, 9, a027888.
- Chen, T.; Huang, Z.; Liu, R.; Yang, J.; Hylemon, P.B.; Zhou, H. Sphingosine-1 phosphate promotes intestinal epithelial cell proliferation via S1PR2. Front. Biosci. 2017, 22, 596–608.
- Smith, A.D.; Rao, J.N.; Turner, D.J. Sphingosine-1-Phosphate and the Intestine. Surgery 2012, 2, 1.
- Kunisawa, J.; Kiyono, H. Immunological Function of Sphingosine 1-Phosphate in the Intestine. Nutrients 2012, 4, 154–166.
- Maines, L.W.; Fitzpatrick, L.R.; French, K.J.; Zhuang, Y.; Xia, Z.; Keller, S.N.; Upson, J.J.; Smith, C.D. Suppression of ulcerative colitis in mice by orally available inhibitors of sphingosine kinase. Dig. Dis. Sci. 2008, 53, 997–1012.
- Mazzei, J.C.; Zhou, H.; Brayfield, B.P.; Hontecillas, R.; Bassaganya-Riera, J.; Schmelz, E.M. Suppression of intestinal inflammation and inflammation-driven colon cancer in mice by dietary sphingomyelin: Importance of peroxisome proliferator-activated receptor gamma expression. J. Nutr. Biochem. 2011, 22, 1160–1171.
- Fischbeck, A.; Leucht, K.; Frey-Wagner, I.; Bentz, S.; Pesch, T.; Kellermeier, S.; Krebs, M.; Fried, M.; Rogler, G.; Hausman, M.; et al. Sphingomyelin induces cathepsin D-mediated apoptosis in intestinal epithelial cells and increases inflammation in DSS colitis. Gut 2011, 60, 55–65.
- Nyberg, L.; Duan, R.D.; Nilsson, A. A mutual inhibitory effect on absorption of sphingomyelin and cholesterol. J. Nutr. Biochem. 2000, 11, 244–249.
- Suh, J.H.; Saba, J.D. Sphingosine-1-phosphate in inflammatory bowel disease and colitis-associated colon cancer: The fat’s in the fire. Transl. Cancer Res. 2015, 4, 15.
- The Human Protein Atlas, Knut & Alice Wallenberg Foundation. Available online: http://www.proteinatlas.org (accessed on 8 August 2021).
- Greenspon, J.; Li, R.; Xiao, L.; Rao, J.N.; Sun, R.; Strauch, E.D.; Shea-Donohue, T.; Wang, J.; Turner, D.J. Sphingosine-1-phosphate regulates the expression of adherens junction protein E-cadherin and enhances intestinal epithelial cell barrier function. Dig. Dis. Sci. 2011, 56, 1342–1353.
- Chen, T.; Lin, R.; Jin, S.; Chen, R.; Xue, H.; Ye, H.; Huang, Z. The Sphingosine-1-Phosphate/Sphingosine-1-Phosphate Receptor 2 Axis in Intestinal Epithelial Cells Regulates Intestinal Barrier Function During Intestinal Epithelial Cells–CD4+T-Cell Interactions. Cell Physiol. Biochem. 2018, 48, 1188–1200.
- Anbazhagan, A.N.; Priyamvada, S.; Alakkam, A.; Kumar, A.; Borthakur, A.; Saksena, S. Transcriptional Modulation of SLC26A3 (DRA) by Sphingosine-1-Phosphate. Am. J. Physiol. Gastrointest. Liver Physiol. 2016, 310, G1028–G1035.
- Greenspon, J.; Li, R.; Xiao, L.; Rao, J.N.; Marasa, B.S.; Strauch, E.D.; Wang, J.; Turner, D.J. Sphingosine-1-phosphate protects intestinal epithelial cells from apoptosis through the Akt signaling pathway. Dig. Dis. Sci. 2009, 54, 499–510.
- Bavaria, M.N.; Jin, S.; Ray, R.M.; Johnson, L.R. The mechanism by which MEK/ERK regulates JNK and p38 activity in polyamine depleted IEC-6 cells during apoptosis. Apoptosis 2014, 19, 467–479.
- Bordet, R.; Camu, W.; de Seze, J.; Laplaud, D.; Ouallet, J.-C.; Thouvenot, E. Mechanism of action of s1p receptor modulators in multiple sclerosis: The double requirement. Rev. Neurol. 2020, 176, 100–112.
- Martin, R.; Sospedra, M. Sphingosine- 1 phosphate and central nervous system. Curr. Top. Microbiol. Immunol. 2014, 378, 149–170.
- O’Sullivan, S.; Dev, K.K. Sphingosine-1-phosphate receptor therapies: Advances in clinical trials for CNS-related diseases. Neuropharmacology 2017, 113, 597–607.
- Soliven, B.; Miron, V.; Chun, J. The neurobiology of sphingosine 1-phosphate signaling and sphingosine 1-phosphate receptor modulators. Neurology 2011, 76, S9–S14.
- Ng, M.L.; Yarla, N.S.; Menschikowski, M.; Sukocheva, O.A. Regulatory role of sphingosine kinase and sphingosine-1-phosphate receptor signaling in progenitor/stem cells. World J. Stem Cells 2018, 10, 119–133.
- Toman, R.E.; Payne, S.G.; Watterson, K.R.; Maceyka, M.; Lee, N.H.; Milstien, S.; Bigbee, J.W.; Spiegel, S. Differential transactivation of sphingosine-1-phosphate receptors modulates NGF-induced neurite extension. J. Cell Biol. 2004, 166, 381–392.
- Blanc, C.A.; Grist, J.J.; Rosen, H.; Sears-Kraxberger, I.; Steward, O.; Lane, T.E. Sphingosine-1-phosphate receptor antagonism enhances proliferation and migration of engrafted neural progenitor cells in a model of viral-induced demyelination. Am. J. Pathol. 2015, 185, 2819–2832.
- Bieberich, E. There is more to a lipid than just being a fat: Sphingolipid-guided differentiation of oligodendroglial lineage from embryonic stem cells. Neurochem. Res. 2011, 36, 1601–1611.
- Kanno, T.; Nishizaki, T.; Proia, R.L.; Kajimoto, T.; Jahangeer, S.; Okada, T.; Nakamura, S. Regulation of synaptic strength by sphingosine 1-phosphate in the hippocampus. Neuroscience 2010, 171, 973–980.
- Norman, E.; Cutler, R.G.; Flannery, R.; Wang, Y.; Mattson, M.P. Plasma membrane sphingomyelin hydrolysis increases hippocampal neuron excitability by sphingosine-1-phosphate mediated mechanisms. J. Neurochem. 2010, 114, 430–439.
- MacLennan, A.J.; Carney, P.R.; Zhu, W.J.; Chaves, H.; Garcia, J.; Grimes, J.R.; Anderson, K.J.; Roper, S.N.; Lee, N. An essential role for the H218/AGR16/Edg-5/LP(B2) sphingosine 1-phosphate receptor in neuronal excitability. Eur. J. Neurosci. 2001, 14, 203–209.
- Ishii, I.; Ye, X.; Friedman, B.; Kawamura, S.; Contos, J.J.A.; Kingsbury, M.A.; Yang, A.H.; Zhang, G.; Brown, J.H.; Chun, J. Marked perinatal lethality and cellular signaling deficits in mice null for the two sphingosine 1-phosphate (S1P) receptors, S1P/LP(B2)/EDG-5 and S1P/LP(B3)/EDG-3. J. Biol. Chem. 2002, 277, 25152–25159.
- Yu, N.; Lariosa-Willingham, K.D.; Lin, F.F.; Webb, M.; Rao, T.S. Characterization of lysophosphatidic acid and sphingosine-1-phosphate-mediated signal transduction in rat cortical oligodendrocytes. Glia 2004, 45, 17–27.
- Miron, V.E.; Durafourt, B.A.; Antel, J.P.; Kennedy, T.E. Assessment of sphingosine-1-phosphate receptor expression and associated intracellular signaling cascades in primary cells of the human central nervous system. Methods Mol. Biol. 2012, 874, 141–154.
- Novgorodov, A.S.; El-Alwani, M.; Bielawski, J.; Obeid, L.M.; Gudz, T.I. Activation of sphingosine-1-phosphate receptor S1P5 inhibits oligodendrocyte progenitor migration. FASEB J. 2007, 21, 1503–1514.
- Miron, V.E.; Jung, C.G.; Kim, H.J.; Kennedy, T.E.; Soliven, B.; Antel, J.P. FTY720 modulates human oligodendrocyte progenitor process extension and survival. Ann. Neurol. 2008, 63, 61–71.
- Jaillard, C.; Harrison, S.; Stankoff, B.; Aigrot, M.S.; Calver, A.R.; Duddy, G.; Walsh, F.S.; Pangalos, M.N.; Arimura, N.; Kaibuchi, K.; et al. Edg8/S1P5: An oligodendroglial receptor with dual function on process retraction and cell survival. J. Neurosci. 2005, 25, 1459–1469.
- Kim, H.J.; Miron, V.E.; Dukala, D.; Proia, R.L.; Ludwin, S.K.; Traka, M.; Antel, J.P.; Soliven, B. Neurobiological effects of sphingosine 1-phosphate receptor modulation in the cuprizone model. FASEB J. 2011, 25, 1509–1518.
- Jung, C.G.; Kim, H.J.; Miron, V.E.; Cook, S.; Kennedy, T.E.; Foster, C.A.; Antel, J.P.; Soliven, B. Functional consequences of S1P receptor modulation in rat oligodendroglial lineage cells. Glia 2007, 55, 1656–1667.
- Fischer, I.; Alliod, C.; Martinier, N.; Newcombe, J.; Brana, C.; Pouly, S. Sphingosine kinase 1 and sphingosine 1-phosphate receptor 3 are functionally upregulated on astrocytes under pro-inflammatory conditions. PLoS ONE 2011, 6, e23905.
- Rao, T.S.; Lariosa-Willingham, K.D.; Lin, F.; Yu, N.; Tham, C.; Chun, J.; Webb, M. Growth factor pretreatment differentially regulates phosphoinositide turnover downstream of lysophospholipid receptor and metabotropic glutamate receptors in cultured rat cerebrocortical astrocytes. Int. J. Dev. Neurosci. 2004, 22, 131–135.
- Osinde, M.; Mullershausen, F.; Dev, K.K. Phosphorylated FTY720 stimulates ERK phosphorylation in astrocytes via S1P receptors. Neuropharmacology 2007, 52, 1210–1218.
- Choi, J.W.; Chun, J. Lysophospholipids and their receptors in the central nervous system. Biochim. Biophys. Acta 2013, 1831, 20–32.
- Wu, Y.P.; Mizugishi, K.; Bektas, M.; Sandhoff, R.; Proia, R.L. Sphingosine kinase 1/S1P receptor signaling axis controls glial proliferation in mice with Sandhoff disease. Hum. Mol. Genet. 2008, 17, 2257–2264.
- O’Sullivan, S.A.; O’Sullivan, C.; Healy, L.M.; Dev, K.K.; Sheridan, G.K. Sphingosine 1-phosphate receptors regulate TLR4-induced CXCL5 release from astrocytes and microglia. J. Neurochem. 2018, 144, 736–747.
- Tham, C.; Lin, F.; Rao, T.; Yu, N.; Webb, M. Microglial activation state and lysophospholipid acid receptor expression. Int. J. Dev. Neurosci. 2003, 21, 431–443.
- Camerer, E.; Regard, J.B.; Cornelissen, I.; Srinivasan, Y.; Duong, D.N.; Palmer, D.; Pham, T.H.; Wong, J.S.; Pappu, R.; Coughlin, S.R. Sphingosine-1-phosphate in the plasma compartment regulates basal and inflammation-induced vascular leak in mice. J. Clin. Investig. 2009, 119, 1871–1879.
- Christensen, P.M.; Liu, C.H.; Swendeman, S.L.; Obinata, H.; Qvortrup, K.; Nielsen, L.B.; Hla, T.; Di Lorenzo, A.; Christoffersen, C. Impaired endothelial barrier function in apolipoprotein M-deficient mice is dependent on sphingosine-1-phosphate receptor 1. FASEB J. 2016, 30, 2351.
- Yanagida, K.; Liu, C.H.; Faraco, G.; Galvani, S.; Smith, H.K.; Burg, N.; Anrather, J.; Sanchez, T.; Iadecola, C.; Hla, T. Size-selective opening of the blood-brain barrier by targeting endothelial sphingosine 1-phosphate receptor 1. Proc. Natl. Acad. Sci. USA 2017, 114, 4531–4536.
- Lee, M.-J.; Thangada, S.; Claffey, K.P.; Ancellin, N.; Liu, C.H.; Kluk, M.; Volpi, M.; Sha’afi, R.I.; Hla, T. Vascular endothelial cell adherens junction assembly and morphogenesis induced by sphingosine-1-phosphate. Cell 1999, 99, 301–312.
- Garcia, J.G.; Liu, F.; Verin, A.D.; Birukova, A.; Dechert, M.A.; Gerthoffer, W.T.; Bamberg, J.R.; English, D. Sphingosine 1-phosphate promotes endothelial cell barrier integrity by Edg-dependent cytoskeletal rearrangement. J. Clin. Investig. 2001, 108, 689–701.
- Galvani, S.; Sanson, M.; Blaho, V.A.; Swendeman, S.L.; Obinata, H.; Conger, H.; Dahlbäck, B.; Kono, M.; Proia, R.L.; Smith, J.D.; et al. HDL-bound sphingosine 1-phosphate acts as a biased agonist for the endothelial cell receptor S1P1 to limit vascular inflammation. Sci. Signal. 2015, 8, ra79.
- Groves, A.; Kihara, Y.; Chun, J. Fingolimod: Direct CNS effects of sphingosine 1-phosphate (S1P) receptor modulation and implications in multiple sclerosis therapy. J. Neurol. Sci. 2013, 328, 9–18.
- Heliopoulos, I.; Patousi, A. Therapeutic Monoclonal Antibodies and Multiple Sclerosis: The Essentials. Med. Chem. 2018, 14, 144–154.
- Dendrou, C.A.; Fugger, L.; Friese, M.A. Immunopathology of multiple sclerosis. Nat. Rev. Immunol. 2015, 15, 545–558.
- Dargahi, N.; Katsara, M.; Tselios, T.; Androutsou, M.E.; de Courten, M.; Matsoukas, J.; Apostolopoulos, V. Multiple Sclerosis: Immunopathology and treatment update. Brain Sci. 2017, 7, 78.
- Bsibsi, M.; Peferoen, L.; Holtman, I.; Nacken, P.; Gerritsen, W.; Witte, M.; van Horssen, J.; Eggen, B.J.; van der Valk, P.; Amor, S.; et al. Demyelination during multiple sclerosis is associated with combined activation of microglia/macrophages by IFN-gamma and alpha B-crystallin. Acta Neuropathol. 2014, 128, 215–229.
- Faissner, S.; Plemel, J.R.; Gold, R.; Yong, V.W. Progressive multiple sclerosis: From pathophysiology to therapeutic strategies. Nat. Rev. Drug Discov. 2019, 18, 905–922.
- Sospedra, M. B cells in multiple sclerosis. Curr. Opin. Neurol. 2018, 31, 256–262.
- Stadelmann, C.; Wegner, C.; Bruck, W. Inflammation, demyelination, and degeneration—Recent insights from MS pathology. Biochim. Biophys. Acta 2011, 1812, 275–282.




