
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Barbara Pawelec | + 1096 word(s) | 1096 | 2021-11-24 04:29:43 | | | |
| 2 | Camila Xu | -321 word(s) | 775 | 2021-11-29 03:17:43 | | |
Video Upload Options
Light olefins (C2-C4) are important C-building blocks which are currently used to produce a variety of chemicals, such as elastomers, medicines, cosmetics, detergents, solvents, etc. They can be produced by steam cracking, fluid catalytic cracking of naphtha, direct/indirect conversion of synthesis gas (CO + H2) or by hydrogenation of CO2 using H2 from renovable energy sources. However, the catalytic production of light olefins from CO2 is difficult due to the chemical inactivity of CO2 molecule, the high C-C coupling barrier and the necesity to limit the formation of C-C bond and methane. Therefore, the catalysts required for this reaction must to be multifunctional and have an optimized amount of active sites.
1. Introduction
The catalytic reaction of hydrogenation of CO2 to olefins is thermodynamically favorable. Nevertheless, there are only a few very efficient catalytic systems exhibiting high selectivity to olefins. The main reason is that the catalyst has to be able to simultaneously catalyze the reverse water-gas shift (RWGS) and modified Fischer-Tropsch to olefins (FTO) reactions working under the same operating conditions, and its final behavior depends on several combined factors, such as the type of metals, the selection of supports, the co-catalyst and the method of catalyst preparation and the selection of operating conditions [1][2][3]. For catalyst design, the selection of metals is limited to Fe, Co, Pd, Ni and Ru, which are the only ones that show high activity in the CO2 hydrogenation upon moderate reaction conditions. Among them, only Fe-based catalysts show relatively high selectivity towards desired products while maintaining low selectivity towards undesired methane [1][2][3]. In addition, Fe-based catalysts are attractive to the industry due to their low cost, which is related to the abundance of iron in nature, and flexible product distribution [4].
2. Reaction Mechanism
There are a large number of theories and hypotheses presented in the literature on the possible mechanism of CO2 hydrogenation to olefins. This is due to the structure sensitivity of CO2 activation [5] and the dependence of CO2 hydrogenation on CO2 coverage and hydrogen adsorption mode [6]. Therefore, the reaction mechanism depends on the catalyst formulation, catalyst morphology, catalyst pre-treatment, reaction conditions employed and proper hydrogen dosing according to the reactor configuration [7][8].
Depending on the catalyst used, the formation of olefins from CO2 can occur via various alternative routes (Figure 1): (1), CO-mediated modified Fischer–Tropsch to olefins (FTO) route; (2), methanol to olefins (MTO) route, and (3), direct CO2 hydrogenation to olefins using promoted and multifunctional catalysts (Equation (1)). The latter reaction pathway is kinetically more difficult and is still under development.
|
nCO2 + 3nH2 → CnH2n + 2nH2O ΔH0573K = 128 kJ mol–1 |
(1) |
In general, it is observed that the FTO route (1) is more efficient in terms of hydrocarbon yield than the MTO route (2).
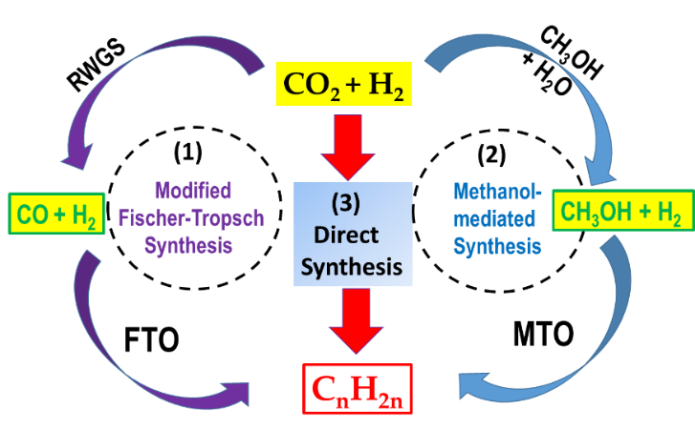
Figure 1. General scheme of the three routes of olefins formation from CO2: (1) modified Fischer–Tropsch to olefins (FTO) route; (2) methanol to olefins (MTO) route; (3) direct olefin formation over multifunctional, promoted catalysts.
The production of olefins from CO2 through the modified Fischer–Tropsch to olefins (FTO) synthesis pathway occurs by redox and associative reaction mechanisms:
(1) Redox mechanism: CO is formed by adsorption and activation of CO2 on metals or metal oxides, and H2 does not participate in the formation of intermediate products (Equation (2));
|
CO2 + H2 → CO+H2O RWGS reaction ΔH0298 = 42.1 kJ mol–1 |
(2) |
(2) Associative mechanism: multi-site adsorption of reagents, chain initiation, chain growth and termination and desorption of the final product (Equation (3)). In this mechanism, the association of hydrogen with CO2 leads to the formation of different intermediate surface species, such as formate, carbonate or carboxylic species, which decompose into final products [9].
|
CO + 2H2 → (-CH2-)+H2O → CnH2n + H2O ΔH0298 = −152.0 kJ mol–1 |
(3) |
The RWGS is an endothermic reaction, so it is favored at higher temperatures, whereas FTO is an exothermic process [10]. Therefore, thermodynamic data indicate that low temperature favors the FTO reaction, while a high temperature is necessary to activate CO2 for the RWGS reaction [1][2].
In general, the modified FT route of CO2 hydrogenation could occur via redox (dissociation of CO2 to CO and O) or via an associative mechanism, in which hydrogen reacts with CO2 to form the intermediate HOCO. This intermediate then decomposes into CO and OH, the latter being hydrogenated to H2O. Unfortunately, in the FTO reaction, product selectivity depends on chain growth, which can be predicted using the chain growth probability (α) of the Anderson–Schulz–Flory (ASF) distribution model [11], which can be calculated by the following equation (4):
|
Wn = n(1-α)2 αn-1 |
(4) |
where Wn is the mass fraction of hydrocarbons with carbon number n in the chain and α is the chain growth probability, which is a function of the rates of chain growth and chain termination [11].
References
- Ma, Z.; Porosoff, M.D. Development of tandem catalysts for CO2 hydrogenation to olefins. ACS Catal. 2019, 9, 2639–2656.
- Ronda-Llioret, M.; Rothenberg, G.; Shiju, N.R. A critical look at direct catalytic hydrogenation of carbon dioxide to olefin. ChemSusChem 2019, 12, 3896–3914
- Al-Dossary, M.; Ismail, A.A.; Fierro, J.L.G.; Bouzid, H.; Al-Sayari, S.A. Effect of Mn loading onto MnFeO nanocomposites for the CO2 hydrogenation reaction. Appl. Catal. B Environ. 2015, 165, 651–660
- Centi, G.; Quadrelli, E.A.; Perathoner, S. Catalysis for CO2 conversion: A key technology for rapid introduction of renewable energy in the value chain of chemical industries. Energy Environ. Sci. 2013, 6, 1711–1731.
- Vogt, C.; Monai, M.; Sterk, E.B.; Palle, J.; Melcherts, A.E.M.; Zijlstra, B.; Groenveld, E.; Berben, P.H.; Boereboom, J.M.; Hensen, E.J.M.; et al. Understanding carbón dioxide activation and carbon-carbon coupling over nickel. Nat. Commun. 2019, 10, 5330, doi:10.1038/s1467-019-12858-3.
- Lin, W.; Stocker, K.M.; Schatz, G.C. Mechanism of hydrogen-assisted CO2 reduction on nickel. J. Am. Chem. Soc. 2017, 139, 4663–4666, doi:10.1021/jacs.7b01538.
- Saedi, S.; Amin, N.A.S.; Rahimpour, M.R. Hydrogenation of CO2 to value-added products—A review and potential future developments. J. CO2 Util. 2014, 5, 66–81, doi:10.1016/j.jcou.2013.12.005.
- González-Carballo, J.M.; Fierro, J.L.G. Fundaments of syngas production and Fischer-Tropsch Synthesis. In Biofuels from Fischer-Tropsch Synthesis; Ojeda, M., Rojas, S., Eds.; Energy Science, Engineering and Technology, Nova. Sci. Pub. Inc.: New York, NY, USA, 2010; ISBN 978-1-61668-366-5.
- Mota, N.; Ordoñez, E.M.; Pawelec, B.; Fierro, J.L.G.; Navarro, R.M. Direct synthesis of dimethyl ether from CO2: Recent advances in bifunctional/hybrid catalytic systems. Catalysts 2021, 11, 411, doi:10.3390/catal11040411.
- Álvarez, A.; Bansode, A.; Urakawa, A.; Bavykina, A.V.; Wezendonk, T.A.; Makkee, M.; Gascon, J.; Kapteijn, F. Challenges in the greener production of formates/formic acid, methanol, and DME by heterogeneously catalyzed CO2 hydrogenation processes. Chem. Rev. 2017, 117, 9804–9838, doi:10.1021/acs.chemrev.6b00816.
- Anderson, R.B. Schulz-Flory equation. J. Catal. 1978, 55, 114–115, doi:10.1016/0021-9517(78)90195-1.




