
Video Upload Options
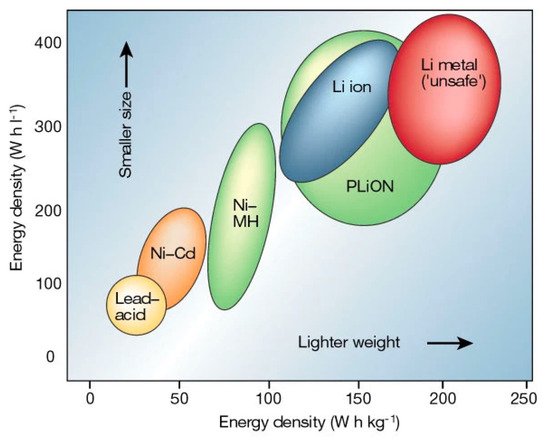
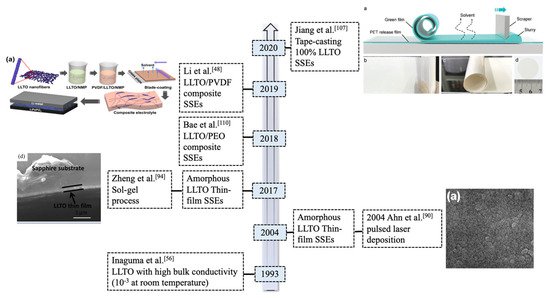
Solid-state lithium metal batteries (LMBs) have become increasingly important in recent years due to their potential to offer higher energy density and enhanced safety compared to conventional liquid electrolyte-based lithium-ion batteries (LIBs). However, they require highly functional solid-state electrolytes (SSEs) and, therefore, many inorganic materials such as oxides of perovskite La2/3−xLi3xTiO3 (LLTO) and garnets La3Li7Zr2O12 (LLZO), sulfides Li10GeP2S12 (LGPS), and phosphates Li1+xAlxTi2−x(PO4)3x (LATP) are under investigation. Among these oxide materials, LLTO exhibits superior safety, wider electrochemical window (8 V vs. Li/Li+), and higher bulk conductivity values reaching in excess of 10−3 S cm−1 at ambient temperature, which is close to organic liquid-state electrolytes presently used in LIBs.
1. Introduction
| SSEs Composition | Anode|Cathode | Ionic Conductivity (S cm−1) | Discharge Capacity/Charging rate/Cycle Number (Capacity Retention Rate) |
|---|---|---|---|
| LLTO/1 PAN/2 SN [45] | 151 mAh g−1 | ||
| Li|LiFePO4 | 2.20 × 10−3 at 30 °C | C/2 | |
| 150 (data unavailable) | |||
| LLTO/3 PEO/LiTFSI/SN [46] | Li|NMC 532 | >10−3 at 55 °C | 143.2 mAh g−1 C/20 30 (data unavailable) |
| LLTO/PEO [47] | 147 mAh g−1 | ||
| Li|LiFePO4 | 3.31 × 10−4 at 7 RT | C/10 | |
| 100 (~98%) | |||
| 15 wt.% LLTO/4 PVDF [48] | Li|LiFePO4 | 5.3 × 10−4 at 25 °C | 121 mAh g−1 1C 100 (~99%) |
| LLTO/PEO/LiTFSI [49] | Li|LiFePO4 | 1.3 × 10−4 at 60 °C | 144.6 mAh g−1 1C 100 (~96%) |
| LLTO/PEO/LiTFSI [50] | Li|LiFePO4 | 1.6 × 10−4 at 60 °C | 135 mAh g−1 2C 300 (79%) |
| 5 wt.% LLTO/PEO/LiTFSI [51] | Li|LiFePO4 | 3.63 × 10−4 at 60 °C | 123 mAh g−1 C/2 100 (94%) |
| 8 wt.% LLTO/PEO/5 PPC/LiTFSI [52] | 135 mAh g−1 | ||
| Li|LiFePO4 | 4.72 × 10−4 at 60 °C | C/2 | |
| 100 (96%) | |||
| 3wt.% LLTO/PEO/LiClO4 [53] | Li|LiFePO4 | 4.01 × 10−4 at 60 °C | 140 mAh g−1 1C 100 (92.4%) |
| LLTO/6 BC [54] | 151.7 mAh g−1 | ||
| Li|LiFePO4 | 1.54 × 10−3 at RT | C/5 | |
| 100 (98.5%) | |||
| Sr/Ta co-doped LLTO [55] | Li|LiFePO4 | 1.40 × 10−4 at 25 °C | 83.8 mAh g−1 C/10 80 (89%) |
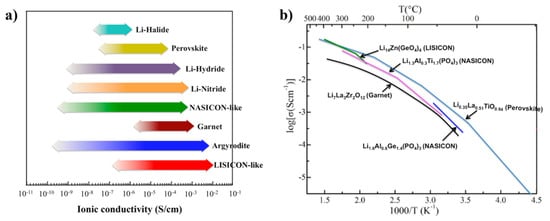
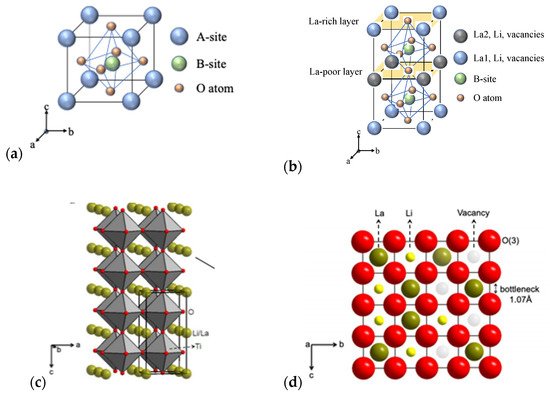
2. Crystal Structure/Composition of LLTO and Relationship to Ionic Conductivity
| Composition | Space Group | Conductivity at RT (S cm−1) | Synthesis Method |
|---|---|---|---|
| Type I: pure LLTO SSEs | |||
| La0.61Li0.17TiO3 | Cmmm | 3.76 × 10−4 | Pulsed Laser Deposition [62] |
| La0.5Li0.5TiO3 | P4/mmm | 3.52 × 10−7 | Spin Coating [63] |
| P4/mmm | 7.2 × 10−7 | Microwave Sintering Method [64] | |
| Type II: composite LLTO SSEs | |||
| La0.5Li0.5TiO3/nano-Ag | Pm3m | 2.8 × 10−5 | Sol-gel Processing [65] |
| La0.5Li0.5TiO3/silica | P4/mmm | 1 × 10−4 | Wet Chemical Method [66] |
| Sr-doped La0.56Li0.33TiO3 | Pm3m | 9.51 × 10−4 | Sol-gel Processing [67] |
| Y-doped La0.46Li0.33TiO3 | P4/mmm | 1.95 × 10−3 | Sol-gel Processing [68] |
| Nb-doped La0.5Li0.5TiO3 | P4/mmm | 1.04 × 10−4 | Solid-state Reaction Method [69] |
| Sr/Co-doped La0.557Li0.33TiO3 | Pmm |
1.4 × 10−4 | Solid-state Reaction Method [55] |
References
- Denholm, P.; Kulcinski, G.L. Life cycle energy requirements and greenhouse gas emissions from large scale energy storage systems. Energy Convers. Manag. 2004, 45, 2153–2172.
- Goodenough, J.B.; Kim, Y. Challenges for Rechargeable Li Batteries. Chem. Mater. 2010, 22, 587–603.
- Goodenough, J.B.; Park, K.-S. The Li-Ion Rechargeable Battery: A Perspective. J. Am. Chem. Soc. 2013, 135, 1167–1176.
- Larcher, D.; Tarascon, J.-M. Towards greener and more sustainable batteries for electrical energy storage. Nat. Chem. 2015, 7, 19–29.
- Liang, Y.; Su, J.; Xi, B.; Yu, Y.; Ji, D.; Sun, Y.; Cui, C.; Zhu, J. Life cycle assessment of lithium-ion batteries for greenhouse gas emissions. Resour. Conserv. Recycl. 2017, 117, 285–293.
- Liu, J.; Bao, Z.; Cui, Y.; Dufek, E.J.; Goodenough, J.B.; Khalifah, P.; Li, Q.; Liaw, B.Y.; Liu, P.; Manthiram, A.; et al. Pathways for practical high-energy long-cycling lithium metal batteries. Nat. Energy 2019, 4, 180–186.
- Dehghani-Sanij, A.R.; Tharumalingam, E.; Dusseault, M.B.; Fraser, R. Study of energy storage systems and environmental challenges of batteries. Renew. Sustain. Energy Rev. 2019, 104, 192–208.
- Yang, Z.; Zhang, J.; Kintner-Meyer, M.C.; Lu, X.; Choi, D.; Lemmon, J.P.; Liu, J. Electrochemical Energy Storage for Green Grid. Chem. Rev. 2011, 111, 3577–3613.
- Ciez, R.E.; Whitacre, J.F. Examining different recycling processes for lithium-ion batteries. Nat. Sustain. 2019, 2, 148–156.
- Ellingsen, L.A.-W.; Hung, C.R.; Strømman, A.H. Identifying key assumptions and differences in life cycle assessment studies of lithium-ion traction batteries with focus on greenhouse gas emissions. Transp. Res. Part D Transp. Environ. 2017, 55, 82–90.
- Nishi, Y. Lithium ion secondary batteries; past 10 years and the future. J. Power Sources 2001, 100, 101–106.
- Tariq, M.; Maswood, A.I.; Gajanayake, C.J.; Gupta, A.K. Aircraft batteries: Current trend towards more electric aircraft. IET Electr. Syst. Transp. 2017, 7, 93–103.
- Lee, J.-W.; Anguchamy, Y.K.; Popov, B.N. Simulation of charge–discharge cycling of lithium-ion batteries under low-earth-orbit conditions. J. Power Sources 2006, 162, 1395–1400.
- Ratnakumar, B.V.; Smart, M.C.; Kindler, A.; Frank, H.; Ewell, R.; Surampudi, S. Lithium batteries for aerospace applications: 2003 Mars Exploration Rover. J. Power Sources 2003, 119, 906–910.
- Miao, Y.; Hynan, P.; Von Jouanne, A.; Yokochi, A. Current Li-Ion Battery Technologies in Electric Vehicles and Opportunities for Advancements. Energies 2019, 12, 1074.
- Scrosati, B.; Garche, J. Lithium batteries: Status, prospects and future. J. Power Sources 2010, 195, 2419–2430.
- Quartarone, E.; Mustarelli, P. Electrolytes for solid-state lithium rechargeable batteries: Recent advances and perspectives. Chem. Soc. Rev. 2011, 40, 2525–2540.
- Chen, S.; Wen, K.; Fan, J.; Bando, Y.; Golberg, D. Progress and future prospects of high-voltage and high-safety electrolytes in advanced lithium batteries: From liquid to solid electrolytes. J. Mater. Chem. A 2018, 6, 11631–11663.
- Zhang, H.; Zhao, H.; Khan, M.A.; Zou, W.; Xu, J.; Zhang, L.; Zhang, J. Recent progress in advanced electrode materials, separators and electrolytes for lithium batteries. J. Mater. Chem. A 2018, 6, 20564–20620.
- Ozdemir, U.; Aktas, Y.O.; Vuruskan, A.; Dereli, Y.; Tarhan, A.F.; Demirbag, K.; Erdem, A.; Kalaycioglu, G.D.; Ozkol, I.; Inalhan, G. Design of a Commercial Hybrid VTOL UAV System. J. Intell. Robot. Syst. 2014, 74, 371–393.
- Sun, Y.; Guan, P.; Liu, Y.; Xu, H.; Li, S.; Chu, D. Recent Progress in Lithium Lanthanum Titanate Electrolyte towards All Solid-State Lithium Ion Secondary Battery. Crit. Rev. Solid State Mater. Sci. 2019, 44, 265–282.
- Sun, C.; Liu, J.; Gong, Y.; Wilkinson, D.P.; Zhang, J. Recent advances in all-solid-state rechargeable lithium batteries. Nano Energy 2017, 33, 363–386.
- Mauger, A.; Julien, C.M.; Paolella, A.; Armand, M.; Zaghib, K. Building Better Batteries in the Solid State: A Review. Materials 2019, 12, 3892.
- US Drive. Electrochemical Energy Storage Technical Team Roadmap (September 2017); US Drive: Washington, WA, USA, 2017.
- Guan, X.; Wu, Q.; Zhang, X.; Guo, X.; Li, C.; Xu, J. In-situ crosslinked single ion gel polymer electrolyte with superior performances for lithium metal batteries. Chem. Eng. J. 2020, 382, 122935.
- Lv, F.; Wang, Z.; Shi, L.; Zhu, J.; Edström, K.; Mindemark, J.; Yuan, S. Challenges and development of composite solid-state electrolytes for high-performance lithium ion batteries. J. Power Sources 2019, 441, 227175.
- Tan, S.; Walus, S.; Hilborn, J.; Gustafsson, T.; Brandell, D. Poly(ether amine) and cross-linked poly(propylene oxide) diacrylate thin-film polymer electrolyte for 3D-microbatteries. Electrochem. Commun. 2010, 12, 1498–1500.
- Scheers, J.; Fantini, S.; Johansson, P. A review of electrolytes for lithium–sulphur batteries. J. Power Sources 2014, 255, 204–218.
- Mindemark, J.; Lacey, M.J.; Bowden, T.; Brandell, D. Beyond PEO—Alternative host materials for Li+-conducting solid polymer electrolytes. Prog. Polym. Sci. 2018, 81, 114–143.
- Stepniak, I.; Andrzejewska, E.; Dembna, A.; Galinski, M. Characterization and application of N-methyl-N-propylpiperidinium bis(trifluoromethanesulfonyl)imide ionic liquid–based gel polymer electrolyte prepared in situ by photopolymerization method in lithium ion batteries. Electrochim. Acta 2014, 121, 27–33.
- Röchow, E.T.; Coeler, M.; Pospiech, D.; Kobsch, O.; Mechtaeva, E.; Vogel, R.; Voit, B.; Nikolowski, K.; Wolter, M. In Situ Preparation of Crosslinked Polymer Electrolytes for Lithium Ion Batteries: A Comparison of Monomer Systems. Polymers 2020, 12, 1707.
- Ma, C.; Cui, W.; Liu, X.; Ding, Y.; Wang, Y. In situ preparation of gel polymer electrolyte for lithium batteries: Progress and perspectives. InfoMat 2021, 1–16.
- Zaghib, K.; Zhu, W.; Kaboli, S.; Demers, H.; Trudeau, M.; Paolella, A.; Guerfi, A.; Julien, C.M.; Mauger, A.; Armand, M.; et al. (Invited) In Operando and in Situ techniques for Intercalation Compounds in Li-Ion and All-Solid-State Batteries. In ECS Meeting Abstracts; No. 1; IOP Publishing: Bristol, UK, 2020; p. 16.
- Mindemark, J.; Sun, B.; Törmä, E.; Brandell, D. High-performance solid polymer electrolytes for lithium batteries operational at ambient temperature. J. Power Sources 2015, 298, 166–170.
- Wu, H.; Yu, G.; Pan, L.; Liu, N.; McDowell, M.T.; Bao, Z.; Cui, Y. Stable Li-ion battery anodes by in-situ polymerization of conducting hydrogel to conformally coat silicon nanoparticles. Nat. Commun. 2013, 4, 1–6.
- Li, S.; Zhang, S.Q.; Shen, L.; Liu, Q.; Ma, J.B.; Lv, W.; He, Y.; Yang, Q.H. Progress and Perspective of Ceramic/Polymer Composite Solid Electrolytes for Lithium Batteries. Adv. Sci. 2020, 7, 1903088.
- Yao, P.; Yu, H.; Ding, Z.; Liu, Y.; Lu, J.; Lavorgna, M.; Wu, J.; Liu, X. Review on Polymer-Based Composite Electrolytes for Lithium Batteries. Front. Chem. 2019, 7, 522.
- Cao, C.; Li, Z.-B.; Wang, X.-L.; Zhao, X.-B.; Han, W.-Q. Recent Advances in Inorganic Solid Electrolytes for Lithium Batteries. Front. Energy Res. 2014, 2, 25.
- Yu, X.; Manthiram, A. A review of composite polymer-ceramic electrolytes for lithium batteries. Energy Storage Mater. 2021, 34, 282–300.
- Chen, L.; Li, Y.; Li, S.P.; Fan, L.Z.; Nan, C.W.; Goodenough, J.B. PEO/garnet composite electrolytes for solid-state lithium batteries: From “ceramic-in-polymer” to “polymer-in-ceramic”. Nano Energy 2018, 46, 176–184.
- Falco, M.; Castro, L.; Nair, J.R.; Bella, F.; Bardé, F.; Meligrana, G.; Gerbaldi, C. UV-Cross-Linked Composite Polymer Electrolyte for High-Rate, Ambient Temperature Lithium Batteries. ACS Appl. Energy Mater. 2019, 2, 1600–1607.
- Falco, M.; Simari, C.; Ferrara, C.; Nair, J.R.; Meligrana, G.; Bella, F.; Nicotera, I.; Mustarelli, P.; Winter, M.; Gerbaldi, C. Understanding the effect of UV-induced cross-linking on the physicochemical properties of highly performing PEO/LiTFSI-based polymer electrolytes. Langmuir 2019, 35, 8210–8219.
- Shin, J.-H.; Henderson, W.A.; Passerini, S. PEO-Based Polymer Electrolytes with Ionic Liquids and Their Use in Lithium Metal-Polymer Electrolyte Batteries. J. Electrochem. Soc. 2005, 152, A978.
- Kim, G.T.; Appetecchi, G.B.; Carewska, M.; Joost, M.; Balducci, A.; Winter, M.; Passerini, S. UV cross-linked, lithium-conducting ternary polymer electrolytes containing ionic liquids. J. Power Sources 2010, 195, 6130–6137.
- Bi, J.; Mu, D.; Wu, B.; Fu, J.; Yang, H.; Mu, G.; Zhang, L.; Wu, F. A hybrid solid electrolyte Li0.33La0.557TiO3/poly(acylonitrile) membrane infiltrated with a succinonitrile-based electrolyte for solid state lithium-ion batteries. J. Mater. Chem. A 2020, 8, 706–713.
- Al-Salih, H.; Huang, A.; Yim, C.-H.; Freytag, A.I.; Goward, G.R.; Baranova, E.; Abu-Lebdeh, Y. A Polymer-Rich Quaternary Composite Solid Electrolyte for Lithium Batteries. J. Electrochem. Soc. 2020, 167, 070557.
- Yan, C.; Zhu, P.; Jia, H.; Zhu, J.; Selvan, R.K.; Li, Y.; Dong, X.; Du, Z.; Angunawela, I.; Wu, N.; et al. High-Performance 3-D Fiber Network Composite Electrolyte Enabled with Li-Ion Conducting Nanofibers and Amorphous PEO-Based Cross-Linked Polymer for Ambient All-Solid-State Lithium-Metal Batteries. Adv. Fiber Mater. 2019, 1, 46–60.
- Li, B.; Su, Q.; Yu, L.; Wang, D.; Ding, S.; Zhang, M.; Du, G.; Xu, B. Li0.35La0.55TiO3 Nanofibers Enhanced Poly(vinylidene fluoride)-Based Composite Polymer Electrolytes for All-Solid-State Batteries. ACS Appl. Mater. Interfaces 2019, 11, 42206–42213.
- Liu, K.; Wu, M.; Wei, L.; Lin, Y.; Zhao, T. A composite solid electrolyte with a framework of vertically aligned perovskite for all-solid-state Li-metal batteries. J. Membr. Sci. 2020, 610, 118265.
- Liu, K.; Zhang, R.; Sun, J.; Wu, M.; Zhao, T. Polyoxyethylene (PEO)|PEO–Perovskite|PEO Composite Electrolyte for All-Solid-State Lithium Metal Batteries. ACS Appl. Mater. Interfaces 2019, 11, 46930–46937.
- Zhu, L.; Zhu, P.; Fang, Q.; Jing, M.; Shen, X.; Yang, L. A novel solid PEO/LLTO-nanowires polymer composite electrolyte for solid-state lithium-ion battery. Electrochim. Acta 2018, 292, 718–726.
- Zhu, L.; Zhu, P.; Yao, S.; Shen, X.; Tu, F. High-performance solid PEO/PPC/LLTO-nanowires polymer composite electrolyte for solid-state lithium battery. Int. J. Energy Res. 2019, 43, 4854–4866.
- He, K.-Q.; Zha, J.-W.; Du, P.; Cheng, S.H.-S.; Liu, C.; Dang, Z.-M.; Li, R.K.Y. Tailored high cycling performance in a solid polymer electrolyte with perovskite-type Li0.33La0.557TiO3 nanofibers for all-solid-state lithium ion batteries. Dalton Trans. 2019, 48, 3263–3269.
- Ding, C.; Fu, X.; Li, H.; Yang, J.; Lan, J.-L.; Yu, Y.; Zhong, W.-H.; Yang, X. An Ultrarobust Composite Gel Electrolyte Stabilizing Ion Deposition for Long-Life Lithium Metal Batteries. Adv. Funct. Mater. 2019, 29, 1904547.
- Li, R.; Liao, K.; Zhou, W.; Li, X.; Meng, D.; Cai, R.; Shao, Z. Realizing fourfold enhancement in conductivity of perovskite Li0.33La0.557TiO3 electrolyte membrane via a Sr and Ta co-doping strategy. J. Membr. Sci. 2019, 582, 194–202.
- Meesala, Y.; Jena, A.; Chang, H.; Liu, R.-S. Recent Advancements in Li-Ion Conductors for All-Solid-State Li-Ion Batteries. ACS Energy Lett. 2017, 2, 2734–2751.
- Inaguma, Y.; Liquan, C.; Itoh, M.; Nakamura, T.; Uchida, T.; Ikuta, H.; Wakihara, M. High ionic conductivity in lithium lanthanum titanate. Solid State Commun. 1993, 86, 689–693.
- Chen, C.H.; Amine, K. Ionic conductivity, lithium insertion and extraction of lanthanum lithium titanate. Solid State Ion. 2001, 144, 51–57.
- Deng, D. Li-ion batteries: Basics, progress, and challenges. Energy Sci. Eng. 2015, 3, 385–418.
- Kokal, I. Solid State Electrolytes for All Solid State 3D Lithium Ion Batteries. Ph.D. Thesis, Eindhoven University of Technology, Eindhoven, The Netherlands, 6 November 2012.
- Inaguma, Y.; Itoh, M. Influences of carrier concentration and site percolation on lithium ion conductivity in perovskite-type oxides. Solid State Ion. 1996, 86, 257–260.
- Kim, S.; Hirayama, M.; Cho, W.; Kim, K.; Kobayashi, T.; Kaneko, R.; Suzuki, K.; Kanno, R. Low temperature synthesis and ionic conductivity of the epitaxial Li0.17La0.61TiO3film electrolyte. CrystEngComm 2014, 16, 1044–1049.
- Abhilash, K.; Sivaraj, P.; Selvin, P.; Nalini, B.; Somasundaram, K. Investigation on spin coated LLTO thin film nano-electrolytes for rechargeable lithium ion batteries. Ceram. Int. 2015, 41, 13823–13829.
- Geng, H.X.; Mei, A.; Dong, C.; Lin, Y.H.; Nan, C.W. Investigation of structure and electrical properties of Li0.5La0.5TiO3 ceramics via microwave sintering. J. Alloy. Compd. 2009, 481, 555–558.
- Ling, M.; Jiang, Y.; Huang, Y.; Zhou, Y.; Zhu, X. Enhancement of ionic conductivity in Li0.5La0.5TiO3 with Ag nanoparticles. J. Mater. Sci. 2020, 55, 3750–3759.
- Mei, A.; Wang, X.-L.; Lan, J.; Feng, Y.-C.; Geng, H.-X.; Lin, Y.-H.; Nan, C.-W. Role of amorphous boundary layer in enhancing ionic conductivity of lithium–lanthanum–titanate electrolyte. Electrochim. Acta 2010, 55, 2958–2963.
- Zhang, S.; Zhao, H.; Guo, J.; Du, Z.; Wang, J.; Świerczek, K. Characterization of Sr-doped lithium lanthanum titanate with improved transport properties. Solid State Ion. 2019, 336, 39–46.
- Lee, S.-J.; Bae, J.-J.; Son, J.-T. Structural and Electrical Effects of Y-doped Li0.33La0.56−xYxTiO3 Solid Electrolytes on All-Solid-State Lithium Ion Batteries. J. Korean Phys. Soc. 2019, 74, 73–77.
- Jiang, Y.; Huang, Y.; Hu, Z.; Zhou, Y.; Zhu, J.; Zhu, X. Effects of B-site ion (Nb5+) substitution on the microstructure and ionic conductivity of Li0.5La0.5TiO3 solid electrolytes. Ferroelectrics 2020, 554, 89–96.




