Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | ISSIFOU AMADOU | + 4944 word(s) | 4944 | 2021-11-15 09:50:45 | | | |
| 2 | Beatrix Zheng | + 177 word(s) | 5121 | 2021-11-22 05:14:16 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Amadou, I. Microbiome and Root Traits in Organic Phosphorus Mobilization. Encyclopedia. Available online: https://encyclopedia.pub/entry/16204 (accessed on 07 February 2026).
Amadou I. Microbiome and Root Traits in Organic Phosphorus Mobilization. Encyclopedia. Available at: https://encyclopedia.pub/entry/16204. Accessed February 07, 2026.
Amadou, Issifou. "Microbiome and Root Traits in Organic Phosphorus Mobilization" Encyclopedia, https://encyclopedia.pub/entry/16204 (accessed February 07, 2026).
Amadou, I. (2021, November 19). Microbiome and Root Traits in Organic Phosphorus Mobilization. In Encyclopedia. https://encyclopedia.pub/entry/16204
Amadou, Issifou. "Microbiome and Root Traits in Organic Phosphorus Mobilization." Encyclopedia. Web. 19 November, 2021.
Copy Citation
Moving toward more sustainable sources for managing phosphorus (P) nutrition in agroecosystems, organic phosphorus (Po) derived from organic inputs and soil is increasingly considered to complement mineral P fertilizer. However, the dynamics of P added by organic input in soil-plant systems is still poorly understood and there is currently no clear information on how the Po composition of these amendments determines P availability through interactions with the soil microbiome and root traits. Here, we review the main mechanisms of rhizosphere microbiome and root traits governing the dynamics of organic input/soil-derived Po pools in the soil-plant system.
agroecology
biogeochemistry
cover crops
organic inputs
organic phosphorus
plant traits
rhizosphere soil
rhizosphere microbiomes
1. Organic Phosphorus Dynamics in Rhizosphere
Rhizosphere microbiomes and root traits involved in P acquisition are known to affect the dynamics of Po in the rhizosphere and, ultimately, its availability to the plant (Figure 1). Although studies have shown that Po can be significantly depleted within the rhizosphere [1][2], the interaction between rhizosphere microbiomes in the rhizosphere, root traits, and their contribution to Po release to plants, have not so far been fully elucidated [3]. Here we summarize and discuss the role of soil microbial processes, root mechanisms, and their interactions, in the fate of Po forms in the organic-input/soil-plant system.
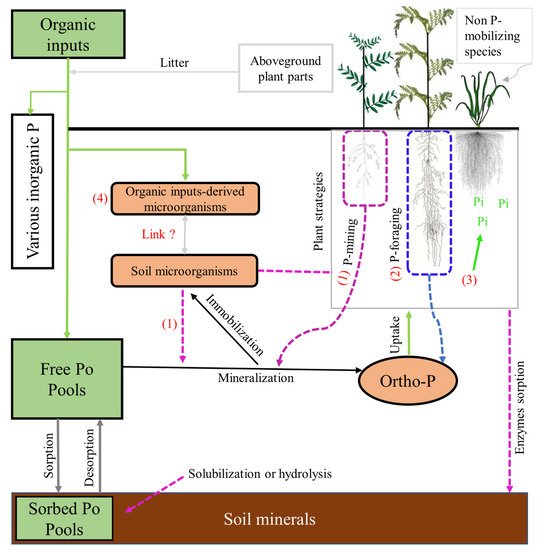
Figure 1. Organic-input/soil-plant-system-related biogeochemical processes that may ultimately modify the dynamics of the Po pool in the rhizosphere: (1) and (2) are the plants P mining and foraging strategies respectively (see Section 3.2 for more details); (3) P-mobilizing crop species improve Po utilization for non-P-mobilizing species, (4) refers to the microorganisms coming from organic input. Indeed, organic inputs involve the addition of carbon sources and often even contain their own microbiota. System modified from [4][5].
1.1. Soil Microbial Processes Involved in Po Mobilization
In organic-input-amended-soils and P-depleted environments, there is generally a proliferation of free rhizosphere microbiome and symbiotic associations with mycorrhizal fungi which have the potential to mobilize and mineralize different forms of available and unavailable Po [4][6] (Table 1). Phyla involved in Po mineralization include the dominant phyla Proteobacteria followed by Acidobacteria and Actinobacteria [7][8]. The order Xanthomonadales of the Xanthomonadaceae family is known to contain several Po mineralizing genes. To obtain P from Po compounds, Streptomyces uses an extracellular alkaline phosphatase encoded by the phoA gene. Other alkaline phosphatase genes, phoD and the phoC, were initially described in the Streptomyces avermitilis and Streptomyces coelicolor genomes, respectively [6]. Stenotrophomonas spp. have been shown to be important contributors to Po solubilization. Controlled experiments using plants inoculated with rhizosphere microbiome have provided further evidence for microbially mediated Po bioavailability to plants. Richardson et al. [9] showed that both grasses and legumes exhibited an improved ability to utilize IHP-P when inoculated with bacteria isolates with high phytase activity. Bacillus spp. and Trichoderma spp. may be able to increase the use of IHP as a P source by plants due to the production of organic anions and phytase [10][11]. Nevertheless, there is little evidence of how these rhizosphere microbiomes may act in the presence of soil minerals that can immobilize both Po and hydrolytic enzymes. Soil minerals such as Fe, Al oxides, and clay minerals are known to considerably reduce the efficiency of applied P. It is assumed that adsorbed Po on Fe and Al oxides is protected from enzymatic hydrolysis leading to its accumulation in soil [12] and its decreased use as a P source by plants. However, García-López et al. [13] showed recently that Bacillus subtilis improved the hydrolytic activity of myo-IHP even in the presence of high Fe oxide concentrations. This led to the conclusion that the rhizosphere microbiome could contribute to an increased hydrolyzing capacity in soil with high Po sorption capacity (Table 1). The currently available research is inadequate to explain the extent to which rhizosphere microbiomes are able to access sorbed forms of P. Future work should be conducted by inoculating microorganisms with sorbed P complexes on major soil minerals such as goethite, gibssite, and major clays, under laboratory conditions and in the field. As most of the legacy P is poorly available to plants, especially the important component corresponding to Po pools [14], understanding the impact of rhizosphere microbiomes in mobilizing this legacy P is crucial to reduce dependence on mined P fertilizers [15]. Moreover, it is important to note that the hydrolysis of adsorbed Po depends to some extent on the release of organic anions or citrate by plant and rhizosphere microbiomes; this promotes the desorption and dissolution of Po making it available for hydrolysis [16].
Table 1. Possible root traits and microbial activity involved in Po mobilization (solubilization and mineralization).
| Organic Phosphorus Forms | Mode of Action That Root Traits and Microbes Act to Mobilize the Po | Associated Microorganisms | Reference |
|---|---|---|---|
| Glycerophosphate and phytate | Alkaline phosphatase and acid phosphatase; phytase | Bacillus coagulans | [17] |
| Ca-phytate | pH reduced; phytase | Bacillus altitudinis WR10 | [18] |
| Po pools | Alkaline phosphatase | Aphanothece halophytica | [19] |
| Na-phytate | pH reduced; phytase | Tetrathiobacter sp. PB-03 and Bacillus sp. PB-13 | [20] |
| Phytic acid | Phytase | Bacillus amyloliquefaciens US573 Acromobacter sp. PB-01 | [21] |
| Total Po pools | Alkaline phosphatase and acid phosphatase | Bacillus pumilus strain JPVS11 | [22] |
| beta-Glycerophosphate | pH reduced; acid phosphatase | Agrobacterium sp. and Bacillus sp. | [23] |
| 5-bromo-4-chloro-3-indolyl phosphate (BCIP) |
pH reduced; phosphatase | Pantoea agglomerans strain P5 Microbacterium laevaniformans strain P7 and Pseudomonas putida strain P13 |
[24] |
| p-nitrophenyl phosphate (pNPP) and guanosine 5-triphosphate (GTP) |
Alkaline phosphatase/phosphodiesterase activity | Cobetia amphilecti | [25] |
| Lecithin | pH reduced; organic acid | Kushneria sp. YCWA18, Bacillus megaterium | [26][27] |
| Total Po pools | pH reduced, oxalic acid, citric acid, malic acid, succinic acid and acetic acid; alkaline phosphatase |
Alcaligenes faecalis | [28] |
| p-nitrophenyl phosphate |
Malic acid, lactic acid and acetic acid; acid phosphatase, pH reduced, oxalic acid, citric |
Serratia sp., Alcaligenes faecalis | [29] |
| Fe-Po, and lecithin |
pH reduced | Ensifer sesbaniae, Gordonia terrae, Bacillus sp., Acinetobacter sp. |
[30] |
Different enzymes released by the rhizosphere microbiome that are involved in Po hydrolysis, such as phytases and alkaline and acid phosphatases, act specifically on particular Po substrates [8]. Several studies using soil-specific enzyme additions have been published over the past decades [31][32]. Their results show that Po mineralization can be explained by the specificity of enzymatic activities on Po forms. For example, Scyllo-IHP was found to be most resistant to phytase activity [33], while IHP hydrolysis has been reported for bacterial acid phosphomonoesterases [34]. Other phytases from Aspergillus spp. (EC 3.1.3.8) have also been reported to hydrolyze IHP, simple monoesters (G6P, GLY), and phosphoanhydrides, but their ability to hydrolyze diester bonds in nucleic acids [31][35] is contradicted by the results of various studies [36]. Monoesterase enzymes have been found to hydrolyze diester phosphates; however, the release of phosphates from DNA is generally very low. This could be because monoesterases hydrolyze only the 5’ and 3’ phosphate residues of DNA [37], while the other phosphate groups are not accessible [35]. It has previously been shown that acid and alkaline phosphatase and phytase are not active on nucleotide pyrophosphate that contains nucleotide pyrophosphate bonds, nor on RNA and DNA that contain phosphodiester bonds [38]. In organic inputs, nucleotide pyrophosphatase, which hydrolyzes nucleotide pyrophosphate to nicotinamide mononucleotide and AMP, and a P1 nuclease, which cleaves RNA and DNA to produce 5-phospho-monoesters, were used to access Po forms. The result showed that both enzymes (nucleotide pyrophosphatase and P1 nuclease) acted only on their own substrates (nucleotide pyrophosphate, or RNA and DNA, respectively). Therefore, it has been suggested that these enzymes could be used to release specific forms of phosphorus when present in the soil [38].
Soil properties may influence the conversion of Po into Pi by the rhizosphere microbiome. Among the soil properties, soil pH is an especially important factor that affects the efficacy and biochemical availability of enzymes that hydrolyze Po forms in the soil. For instance, at a pH of 7.5, Aspergillus niger phytases remained in solution, but at a pH of 5.5, they were unavailable [35]. However, the optimal pH varies according to the soil microorganism species and the associated plant. Fungal phytases, such as those of Aspergillus fumigatus, require a pH between 4.5 and 6.5, in which 80% of activity takes place [39]. Species such as Rhizoctonia sp. and Fusarium verticillioides can produce phytases at optimal pH of 4.0 and 5.0, respectively [40], while in bacterial phytases the maximum activity was observed at a pH of 6.0–8.0, as in Bacillus sp. [41]. Most of the phytases are acidic and have an optimal pH between 4.5 and 6.0 [42], whereas alkaline phytases in legume seeds [43], lily pollen and cattail pollen, have been reported to have an optimal pH of 8.0 [44]. For example, at pH 7.5, phytases from Aspergillus niger remain available in solution, but at pH 5.5 they are not available [35]. It is evident that phosphatases produced by rhizosphere microbiomes are more sensitive to pH. This pH dependence may be even greater than that of plant phosphatases. However, the optimal pH for Po mobilization varies among rhizosphere microbiome species. It is also important to understand how microorganisms could facilitate P mobilization from organic inputs or soil organic matter within a given pH range. We conclude that the determination of the optimal pH for Po mobilization by microbes and root traits requires careful assessment in future research.
In addition to pH, soil temperature has a strong effect on Po availability though studies have shown contradictory results on the influence of temperature on P solubilization by microbes. White et al. [45] found 20–25 °C to be the optimal temperature for maximum microbial solubilization of P while 28 °C was reported by Chauhan et al. [46] and Alori et al. [47]. Others have reported 30 °C as the optimal temperature for solubilization and mineralization of Po [41][48]. Nautiyal et al. [49] reported solubilization and hydrolysis of P at an extreme temperature of 45 °C in desert soil, while Johri et al. [50] reported a low temperature of 10 °C. The optimum temperature for phytate-degrading enzymes ranges from 35 to 77 °C. In general, plant phytases, such as those from cereals, show maximum activity at lower temperatures than microbial phytases [42]. The phytase from Fusarium verticillioides showed an optimal temperature of 50 °C and stability up to 60 °C [44]. The optimal temperature for phytase activity towards magnesium phytate (Mg-IHP) has been reported to reach 40 °C without and 50 °C with 5 mM Ca2+ [51] Most plant phytases have an optimal temperature of 45–60 °C, as reported by Johnson et al. [52]. The lowest temperature has been reported to be 10 °C [50]. However, it is generally assumed that a higher temperature (>30 °C) has a better effect on Po solubilization and availability as shown by the higher Po solubilization by Bacillus megaterium at 36 °C than at 21 °C. As with pH, phosphatases produced by rhizosphere microbiomes are thermostable. Therefore, changes in the interactions between microbes and root traits as a result of temperature variations and how this could affect Po mobilization processes must be considered in the soil-plant system. Furthermore, understanding the optimal activity of microorganisms as a function of soil temperature is an important challenge for improving biofertilizer management practices and their positive effects on Po hydrolysis and P availability. However, despite the challenge of controlling soil temperature, it is nonetheless possible to identify the optimal dates and seasons to apply biofertilizer to maximize its effect. The effectiveness of microbial enzymatic activity is also influenced by different cations and other constituents in the soil solution. Modelling studies have shown that three classes of phytases, histidine acid phosphatases, β-propellant phytases, and purple acid phosphatases, would be unable to hydrolyze Al3+, Fe2+, Fe3+, Cu2+ salts of IHP, but would be able to hydrolyze Ca2+, Mg2+, and Mn2+ salts [53][54]. This implies that P mobilization will depend on the nature of the cation that precipitates Po. Thus, the accessibility of precipitated Po by enzymes and its mobilization for plants will differ considerably depending on the nature and concentration of electrolytes in the soil.
In summary, the hydrolysis of Po and its release by enzymatic activity is generally affected by the biochemical nature of Po and its ability to interact with soil properties and rhizosphere microbiomes. A wide variety of bacteria, fungi, and endophytes can solubilize Po through the production of organic acids [55]. This solubilization is very important because most forms of Po are high molecular weight compounds that are generally resistant to chemical hydrolysis. However, the mechanisms associated with the transformation of Po to Pi are poorly understood, and further work is needed, especially under field conditions. In most studies, the experimental conditions have suppressed interactions between system components. Therefore, these studies generally only indicate what is possible, but they do not necessarily indicate what is likely [56][57]. Indeed, knowledge of Po transformations is a prerequisite for understanding the potential contribution of lesser-known forms of Po in the organic-input-soil-plant system. In any case, it is evident that Po mineralization occurs in the rhizosphere and could contribute significantly to the requirements for plant growth. Factors affecting the rhizosphere microbiome are likely to influence the lability and stability of their enzymes. Some enzymes become stable through interactions with soil minerals and humic substances and retain some enzyme activity [58]. In general, microbial activity is affected by biological (e.g., the amount and type of substrate, concentration of enzyme, etc.) and physicochemical processes (e.g., interactions with soil constituents pH, temperature, etc.). The former cause changes in enzyme production rates and microbial community composition, while the latter cause changes in adsorption/desorption reactions, substrate diffusion, and enzyme degradation rates [59]. Critical factors affecting microbes and their enzyme activities include the amount and type of Po [60], interactions with soil constituents, pH, temperature, and the concentrations of enzyme and product [61]. In addition, because Po adsorbs rapidly and strongly onto soil particles, the binding processes involved also play a crucial role in the activity of rhizosphere microbiomes [62][63]. To date, there are relatively few studies that explore the enzymatic hydrolysis of adsorbed Po. It has been reported that mineral surfaces protect the majority of adsorbed phosphate esters from enzymatic hydrolysis, but whether this is a general finding remains open [64]. Furthermore, the mechanism of this process is largely unknown in the case where the enzyme can access the adsorbed Po forms. The results of Olsson et al. [65] on the hydrolysis of G1P on α-FeOOH surfaces showed the role of interactions at mineral surfaces with respect to the stabilization of Po molecules in soils [66]. These authors provided a mechanistic explanation of how P can be mobilized via enzymatic activity despite strong interactions with soil minerals. This shed light on previous results showing that microbial stimulation and the resulting enzymatic activity can mobilize adsorbed Po from soil minerals [53]. However, a question is whether the enzyme acts only on the soluble fraction that is reconstituted by desorption of the substrate or whether the hydrolysis reaction occurs at the interface between the aqueous solution and solid particles. We therefore recommend future studies on these issues to better understand the effect of rhizosphere microbiomes on Po dynamics.
Apart from the rhizosphere microbiome, organic-inputs-derived microorganisms also play a major role in the mobilization of Po. Organic inputs imply the addition of carbon sources and often even contain their own microbiota, equivalent to inoculation of microbes, and this is a very important issue that needs more study. Amendment to organic inputs generally increases the diversity of rhizosphere microbiomes and their enzymatic activities in the soil [67]. These positive reactions highlight the role of organic-inputs-derived microorganisms in Po availability and, on the other hand, their high content of organic matter [68] which is the main substrate of most microorganisms [69]. However, complex questions remain about how the addition of organic inputs alters the soil microbial community and especially how this relates to soil Po mineralization. To successfully manage organic inputs, there is a need to develop a consistent procedure to quantitatively compare the potential of these different microorganisms to release orthophosphate from different sources of Po. As the organic-input/soil-plant continuum consists of various forms of Po with different chemical properties, their solubilization and hydrolysis rates would be strongly related to the diversity of soil microbial communities. Therefore, it is crucial to develop and utilize more advanced approaches to support the roles soil microbes, especially via phosphate-solubilizing microorganism-derived enzymes, play in releasing free Pi from Po forms in the soil [47][70]. In sum, the main microbial processes involved in P dynamics that are synthesized and highlighted in this section, and the factors that influence them, greatly affect soil P mobilization processes. They could, therefore, if well understood, contribute to increasing the P use efficiency of organic wastes and those accumulated in agricultural soils. Furthermore, it is known that plant roots inoculated with commercial microorganisms can express synergistic effects to solubilize Pi in the soil. In contrast, little is known about their effect on Po pools. Therefore, further research is needed to evaluate the application and efficacy of commercial microorganisms on various crops with contrasting root traits and fertilized with different P sources under field conditions.
Current cropping models often focus on understanding competition for light and the effects of N or P fertilization, but do not consider interactions with the rhizosphere microbiome and how it affects soil Po forms. Thus, the development of cropping models that consider Po dynamics is needed to determine the efficiency of Po use in multi-species cropping systems and to manage P sustainably in the agroecosystem. Furthermore, it is important to note that current decision support tools do not consider Po sorption, its mineralization kinetics, and the effect of root trait and rhizosphere microbiome interactions on its dynamics. Therefore, in future research aimed at assisting farmers in organic input management, these parameters that govern Po dynamics, should be studied further and integrated into decision support tools. This will not only improve the decision support tools but also make them more focused on Po mobilization in the organic-input/soil-plant system.
Another important factor is the effect of rhizosphere microbial populations on Po mineralization. Some studies have shown that soils taken from the rhizosphere slowed the sorption of phytate or phytase into the rhizosphere, suggesting that rhizosphere soils alter the adsorption of phytase and thus the release of orthophosphate from soils [54]. This is an important observation because Po dynamics are altered in the rhizosphere, so it is possible that Po from organic inputs is more available around plant roots. Therefore, we strongly suggest that, along with the mobilization process by soil microbes, root mechanisms/traits should be exploited to facilitate soil Po mobilization at the field scale.
The hydrolysis of Po and its release by enzymatic activity is influenced by the biochemical nature of Po and its ability to interact with soil properties and rhizosphere microbiomes. However, current work is insufficient to understand the extent to which rhizosphere microbiomes can access sorbed forms of P from both soil constituents and organic matter. Furthermore, investigations into the potential of microbes to mobilize Po have been conducted on cultivable microbes, yet most root-associated rhizosphere microbiomes are not cultivable. We recommend future investigations to screen rhizosphere bacteria in the presence of different forms of Po and different soil properties, to identify those that are effective in Po mobilization.
1.2. Root Mechanisms Involved in the Fate of Po Forms
The contribution of plants to Po availability has been known for many decades. Most plants have developed strategies to increase P acquisition in P-deficient soil or to specifically access different forms of Po and Pi in the soil [71]. These strategies cover a wide range of morphological, architectural, and physiological traits [72]. In general, these traits could be categorized into three types of P acquisition strategies: foraging, mining, and collective microbial-root strategy (Figure 1 and Table 1).
The plant P-foraging strategy relates to the acquisition of P in the soil solution through morphological and architectural traits by maximizing soil exploration. Through this strategy, plants can induce a diffusion gradient which in turn favours the desorption process of the different adsorbed Po forms [73][74]. The phosphorus-foraging strategy would also improve the efficiency of P uptake by slowing the rate at which Po moves from the free or moderately adsorbed form to the strongly adsorbed form on soil compounds [71]. Foraging strategy involves morphological traits such as specific root length, diameter and radius, and architectural traits, including root length density, root biomass, root hairs, and the formation of clustered roots or arbuscular mycorrhizal symbioses that allow plants to increase their foraging capacity [75]. These traits alter the C cost of soil exploration by regulating the extent of competition within and between root systems [76][77]. Among morphological traits, root radius is considered important in the efficiency of P use. Plants with finer/thinner roots can explore and contact a greater volume of soil per unit root area. Gahoonia et Nielsen [1] reported that plant species with finer roots may be more effective in mobilizing Po and absorption of Pi from the soil. Very recently, trade-offs between thicker and thinner roots have been observed by Honvault et al. [78]. Thicker roots are reported to have greater carboxylate release and phosphatase activity in the rhizosphere, affecting the desorption and mineralization of Po [78]. In contrast, thinner roots exhibit the morphological traits (foraging) that favour the exploration and contact of a larger volume of soil to permit P mobilization process [79][80]. These observations are consistent with other results showing that species with finer fibrous roots express higher levels of morphological traits to access more Po in the soil [75][81][82]. However, since fine roots (i.e., root hairs with small root radius) tend to renew faster than large roots [76], the cost of C to produce fine roots could be higher, since they would also need to be replaced more frequently [77]. In addition to root radius, the formation of root groups, such as proteoid and dauciform root groups, commonly found in plant species belonging to the families Proteinaceae and Fabaceae [82] and others, is very strongly related to the dynamics of Po in soil. These roots provide a very dense mat of root hairs and also specialize in the efficient synthesis and secretion of citrate and malate (organic anions) and phosphatases, which help solubilize insoluble Po resources and hydrolyze Po for plant uptake [83]. Various effects of root growth and variation in root hair length on Po dynamics and contribution to P uptake have been reported in several species, including maize, wheat, barley, beans, soybean, and white clover [84][85]. Higher root length density in the upper soil layers was shown to be the most important root trait of wheat for Po mobilisation in response to organic input [86]. Moreover, variation in root growth angle and root hairs may have a significant impact on the total mobilisation of P in the soil [87]. In closely related maize genotypes, the effects of root growth and root hair length variation reportedly led to a 100% increase in total mobilized P and, in other genotypes, to as much as a 600% increase in P mobilization [85]. Such increases occur since root growth maximizes soil exploration and can induce a diffusion gradient and modify soil properties to promote P desorption. Root hairs are smaller in diameter than roots and grow perpendicular to the root axis, forming up to 77% of the root surface [88] of soil/field crops [89][90]. The presence of root hairs can be very important for the effectiveness of Po mobilization through a considerable increase of root area in the soil. It has been reported that root hairs can contribute up to 70% of Po uptake [1][91]. Root hair length and density are highly controlled by P bioavailability. Geometric modeling indicates that root hair responses to P availability interact synergistically to enhance Po and Pi acquisition [92]. Root hairs also aid in the dispersal of root exudates such as organic acids into the rhizosphere, which improves Po bioavailability in many soils [93]. Root morphological traits show a much more significant influence on Po acquisition generally in winter wheat genotypes than biochemical transformation by acid phosphatases [84][94]. These biochemical and morphological changes can vary considerably between and within plant species [4][95]. The potential ability of plants root traits to utilize poorly available Po sources is, however, greatly influenced by genetic makeup.
The plant P mining strategy, relates to the acquisition of P in the soil solution through physiological traits involving release of substances into the soil from the roots, including carbohydrates, organic and amino acids, phenolic compounds, proteins, fatty acids, sterols, enzymes, polysaccharides and phospholipids [96][97]. Among these compounds, carboxylic acids, PME activity, phenolic and mucilage compounds, and protons are the main physiological traits involved in P mining strategies for Po mobilization [98]. Through this strategy, plants increase the turnover of poorly available Po pools [99][82] by desorption, solubilization, and mineralization processes. Before any hydrolysis process by the enzymes, Po, if not free, must first be desorbed or dissociated from soil minerals or organic matter. The secretion of organic acids by P-mobilizing species improves the availability of Po forms by promoting their desorption from soil constituents to the soil solution, in which they are subsequently mineralized by phosphatases [100]. Organic acids are generally predicted to be strongly linked to Po mobilization in the soil-plant system [101]. Plant roots have the ability to produce organic acids, particularly short-chain organic acids, such as lactate, acetate, oxalate, succinate, fumarate, malate, citrate, isocitrate, and aconitate, which can mobilize both Po and Pi [102]. Among the organic acids, malic and citric acids are the most widespread and abundantly detected in root exudates [103]. Given the highest affinities between Po forms and soil components, chelation between organic acids in root exudates and soil constituents is the main mechanism for Po solubilization by organic acids in soil [104]. Citrate has been shown to chelate Fe and Al oxyhydroxide and Mn and Ca carbonates, which can actively displace adsorbed Po into free forms [105]. Low molecular weight organic acids generally carry one or more negative charges. By complexolysis, negatively charged organic acids can release Po from insoluble forms of Po. These reactions lead to the solubilization of insoluble forms of Po such as Ca-Po, Na-Po etc. [106]. The impact of direct chelation in the solubilization of Po by citrate exudation has, for instance, been demonstrated in rice [107]. In general, citrate and oxalate have a higher potential for mobilization of Po compared to other organic anions [93][101]. After Po is desorbed from soil minerals by organic acids, phosphatases and phytases released by roots can contribute to its efficient use by plants [108][109]. Chickpea, which appears to produce both phosphomonoesterases and diesterases, is thought to improve P nutrition, probably through mining and mineralization of Po forms in soil [110][111]. Various studies have demonstrated that plants have a limited ability to access P in the form of IHP (the main form of Po) due to its low availability in soil solution and low levels of extracellular phosphatase or phytase [112][35]. It has also been shown that wheat and many other species are able to utilize P from G6P, GLY, and phosphodiesters (DNA and RNA), due to their mining capacity, but are limited to acquiring P directly from myo-IHP, although it is abundant in many soils [32]. This suggests that the biological importance of the different forms of Po will be driven by their turnover rates. Therefore, it was considered that plants with optimal mining strategies for phytase release could potentially be used to improve the efficiency of inositol phosphate mobilization. The challenges are to understand the functioning of root-derived phytase activities on Po forms, and the chemical nature, soil properties, and root traits of crop species, to increase Po desorption and hydrolysis.
The collective microbial-root strategy refers to the investment of resources by plants to interact with the microbial community to access Po in soil. Many plants have developed a symbiotic association with vesicular arbuscular mycorrhizal fungi (AMF) that grow out from the root into the surrounding soil, extending the capacity of the root to mineralize Po and take up Pi in soil solution [113]. The association of roots with arbuscular mycorrhizae is thought to be much more related to the mobilization of total P than root hairs and root length activity [114]. The symbiotic association of plant roots with mycorrhizae is reported to extend further from the roots than root hairs, and is also active in areas where P forms are adsorbed onto soil components [115][116]. A significant contribution of AMF to the uptake of P by plants has been reported, particularly in soils with high Po binding capacity. It is also accepted that AMF store Po in their vacuoles, which can be hydrolyzed and transported as Pi in the host plant [117]. In addition, AMF also hydrolyze Po by releasing acid phosphatase in the soil. However, the relative contribution of root-derived extracellular phosphatases in the use of Po is still unclear, as the number and activity of bacteria and fungi are higher in the rhizosphere than in the soil in general [99].
Overall, the roles of P mining, foraging and collective microbial-root strategies are understood and their indirect, and sometimes direct, effects on Po dynamics/mobilization have been demonstrated [92][94]. The processes differ according to the nature of Po forms (e.g., phosphate, mono, or diester), the structure and function of soil microbial communities, and the physicochemical properties of the soil and climate. For instance, the availability of IHP, G6P, GLY, and phosphodiesters (DNA and RNA), as a direct effect of P mining, foraging and collective microbial-root strategies, remains unclear. Although the release of organic acids can make G6P, GLY, and phosphodiesters (DNA and RNA) available to plants, it is less efficient at solubilizing IHP [118], probably due to it binding strongly to soil. Interactions between plant traits in the mobilization of P have been studied but remain poorly understood and unconfirmed. To this end, when developing new crop varieties or cultivars, selection should be based on crops with high Po use efficiency to promote greater P availability. Thus, in future crop breeding programs, traits involved in Po use efficiency should be identified and recorded. Specifically, efficient cultivars with genes and traits that produce strong phosphatase/phytase activities should be identified for better mobilization of the major Po form (Myo-IHP). There may also be trade-offs between physiological and morphological traits [78][82]. These trade-offs have been examined very recently in different crop families and species [78]. Trade-offs between thicker and thinner roots were observed [78], with thicker roots showing greater carboxylate release or phosphatase activity in the rhizosphere. Trade-offs and coordination between traits were strongly influenced by soil type. However, their effect on the availability of Po forms still needs to be elucidated [81]. Thus, we suggest that these strategies can be exploited using combinations of species with contrasting strategies, or by using a single species to better understand their actual effects on Po forms in agroecosystems.
Both plant functional traits and organic input characteristics strongly interact to modulate Po dynamics. However, it is still unclear to what extent these contribute to the mobilization of sparingly available Po forms. Research efforts should focus on understanding the relationships between plant functional traits, Po nature, and organic input properties in order to characterize the dynamics of Po, model its fate in the soil-plant system, and better understand its consequences for P availability. Further, crop models to estimate total soil Po reserves and, at minimum, its specific forms (IHP, G6P, GLY, etc.), in a multi-species cropping system can be developed based on plant traits (i.e., shoot and root morphological and chemical characteristics), including inter- and intraspecific trait variability and soil properties. This trait-based approach to modeling P mobilization can be developed and would be potentially useful in different climates.
References
- Gahoonia, T.S.; Nielsen, N.E. Root Traits as Tools for Creating Phosphorus Efficient Crop Varieties. Plant Soil 2004, 260, 47–57.
- Chen, C.R.; Condron, L.M.; Davis, M.R.; Sherlock, R.R. Effects of Plant Species on Microbial Biomass Phosphorus and Phosphatase Activity in a Range of Grassland Soils. Biol. Fertil. Soils 2004, 40, 313–322.
- Jakobsen, I.; Leggett, M.E.; Richardson, A.E. Rhizosphere Microorganisms and Plant Phosphorus Uptake. In Agronomy Monographs; Thomas Sims, J., Sharpley, A.N., Eds.; American Society of Agronomy; Crop Science Society of America; Soil Science Society of America: Madison, WI, USA, 2015; pp. 437–494. ISBN 978-0-89118-269-6.
- Sulieman, S.; Mühling, K.H. Utilization of Soil Organic Phosphorus as a Strategic Approach for Sustainable Agriculture. J. Plant Nutr. Soil Sci. 2021, 184, 311–319.
- Hinsinger, P.; Herrmann, L.; Lesueur, D.; Robin, A.; Trap, J.; Waithaisong, K.; Plassard, C. Impact of roots, microorganisms and microfauna on the fate of soil phosphorus in the rhizosphere. In Annual Plant Reviews; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2015; Volume 48, pp. 375–407. ISBN 978-1-118-95884-1.
- Tian, J.; Ge, F.; Zhang, D.; Deng, S.; Liu, X. Roles of Phosphate Solubilizing Microorganisms from Managing Soil Phosphorus Deficiency to Mediating Biogeochemical P Cycle. Biology 2021, 10, 158.
- Gaiero, J.R.; Bent, E.; Fraser, T.D.; Condron, L.M.; Dunfield, K.E. Validating Novel Oligonucleotide Primers Targeting Three Classes of Bacterial Non-Specific Acid Phosphatase Genes in Grassland Soils. Plant Soil 2018, 427, 39–51.
- Annaheim, E.; Frossar, E.; Bünemann, E.K. Characterisation of Organic Phosphorus Compounds in Soil by Phosphatase Hydrolysis. In Proceedings of the 19th World Congress of Soil Science, Soil Solutions for a Changing World, Brisbane, Australia, 1–6 August 2010; pp. 9–11.
- Richardson, A.E.; Lynch, J.P.; Ryan, P.R.; Delhaize, E.; Smith, F.A.; Smith, S.E.; Harvey, P.R.; Ryan, M.H.; Veneklaas, E.J.; Lambers, H.; et al. Plant and Microbial Strategies to Improve the Phosphorus Efficiency of Agriculture. Plant Soil 2011, 349, 121–156.
- Garcia-Lopez, A.M.; Aviles, M.; Delgado, A. Plant Uptake of Phosphorus from Sparingly Available P- Sources as Affected by Trichoderma Asperellum T34. Agric. Food Sci. 2015, 24, 249–260.
- Garcia-Lopez, A.M.; Delgado, A. Effect of Bacillus Subtilis on Phosphorus Uptake by Cucumber as Affected by Iron Oxides and the Solubility of the Phosphorus Source. Agric. Food Sci. 2016, 25, 216–224.
- Stutter, M.I.; Shand, C.A.; George, T.S.; Blackwell, M.S.A.; Dixon, L.; Bol, R.; MacKay, R.L.; Richardson, A.E.; Condron, L.M.; Haygarth, P.M. Land Use and Soil Factors Affecting Accumulation of Phosphorus Species in Temperate Soils. Geoderma 2015, 257–258, 29–39.
- García-López, A.M.; Recena, R.; Delgado, A. The Adsorbent Capacity of Growing Media Does Not Constrain Myo-Inositol Hexakiphosphate Hydrolysis but Its Use as a Phosphorus Source by Plants. Plant Soil 2020.
- Stutter, M.I.; Shand, C.A.; George, T.S.; Blackwell, M.S.A.; Bol, R.; MacKay, R.L.; Richardson, A.E.; Condron, L.M.; Turner, B.L.; Haygarth, P.M. Recovering Phosphorus from Soil: A Root Solution? Environ. Sci. Technol. 2012, 46, 1977–1978.
- Giles, C.; Cade-Menun, B.; Hill, J. The Inositol Phosphates in Soils and Manures: Abundance, Cycling, and Measurement. Can. J. Soil. Sci. 2011, 91, 397–416.
- Celi, L.; Prati, M.; Magnacca, G.; Santoro, V.; Martin, M. Role of Crystalline Iron Oxides on Stabilization of Inositol Phosphates in Soil. Geoderma 2020, 374, 114442.
- Yadav, R.S.; Meena, S.C.; Patel, S.I.; Patel, K.I.; Akhtar, M.S.; Yadav, B.K.; Panwar, J. Bioavailability of Soil P for Plant Nutrition. In Farming for Food and Water Security; Lichtfouse, E., Ed.; Sustainable Agriculture Reviews; Springer: Dordrecht, The Netherlands, 2012; Volume 10, pp. 177–200. ISBN 978-94-007-4499-8.
- Yue, Z.; Shen, Y.; Chen, Y.; Liang, A.; Chu, C.; Chen, C.; Sun, Z. Microbiological Insights into the Stress-Alleviating Property of an Endophytic Bacillus Altitudinis WR10 in Wheat under Low-Phosphorus and High-Salinity Stresses. Microorganisms 2019, 7, 508.
- Kageyama, H.; Tripathi, K.; Rai, A.K.; Cha-um, S.; Waditee-Sirisattha, R.; Takabe, T. An Alkaline Phosphatase/Phosphodiesterase, PhoD, Induced by Salt Stress and Secreted Out of the Cells of Aphanothece Halophytica, a Halotolerant Cyanobacterium. Appl. Environ. Microbiol. 2011, 77, 5178–5183.
- Kumar, V.; Singh, P.; Jorquera, M.A.; Sangwan, P.; Kumar, P.; Verma, A.K.; Agrawal, S. Isolation of Phytase-Producing Bacteria from Himalayan Soils and Their Effect on Growth and Phosphorus Uptake of Indian Mustard (Brassica Juncea). World J. Microbiol. Biotechnol. 2013, 29, 1361–1369.
- Boukhris, I.; Farhat-Khemakhem, A.; Blibech, M.; Bouchaala, K.; Chouayekh, H. Characterization of an Extremely Salt-Tolerant and Thermostable Phytase from Bacillus Amyloliquefaciens US573. Int. J. Biol. Macromol. 2015, 80, 581–587.
- Kumar, A.; Singh, S.; Mukherjee, A.; Rastogi, R.P.; Verma, J.P. Salt-Tolerant Plant Growth-Promoting Bacillus Pumilus Strain JPVS11 to Enhance Plant Growth Attributes of Rice and Improve Soil Health under Salinity Stress. Microbiol. Res. 2021, 242, 126616.
- Barua, S.; Tripathi, S.; Chakraborty, A.; Ghosh, S.; Chakrabarti, K. Characterization and Crop Production Efficiency of Diazotrophic Bacterial Isolates from Coastal Saline Soils. Microbiol. Res. 2012, 167, 95–102.
- Malboobi, M.A.; Owlia, P.; Behbahani, M.; Sarokhani, E.; Moradi, S.; Yakhchali, B.; Deljou, A.; Morabbi Heravi, K. Solubilization of Organic and Inorganic Phosphates by Three Highly Efficient Soil Bacterial Isolates. World J. Microbiol. Biotechnol. 2009, 25, 1471–1477.
- Noskova, Y.; Likhatskaya, G.; Terentieva, N.; Son, O.; Tekutyeva, L.; Balabanova, L. A Novel Alkaline Phosphatase/Phosphodiesterase, CamPhoD, from Marine Bacterium Cobetia Amphilecti KMM 296. Mar. Drugs 2019, 17, 657.
- Xiang, W.; Liang, H.; Liu, S.; Luo, F.; Tang, J.; Li, M.; Che, Z. Isolation and Performance Evaluation of Halotolerant Phosphate Solubilizing Bacteria from the Rhizospheric Soils of Historic Dagong Brine Well in China. World J. Microbiol. Biotechnol. 2011, 27, 2629–2637.
- Zhu, F.; Qu, L.; Hong, X.; Sun, X. Isolation and Characterization of a Phosphate-Solubilizing Halophilic Bacterium Kushneria Sp. YCWA18 from Daqiao Saltern on the Coast of Yellow Sea of China. Evid.-Based Complement. Altern. Med. 2011, 2011, e615032.
- Behera, B.C.; Yadav, H.; Singh, S.K.; Sethi, B.K.; Mishra, R.R.; Kumari, S.; Thatoi, H. Alkaline Phosphatase Activity of a Phosphate Solubilizing Alcaligenes Faecalis, Isolated from Mangrove Soil. Biotechnol. Res. Innov. 2017, 1, 101–111.
- Behera, B.C.; Yadav, H.; Singh, S.K.; Mishra, R.R.; Sethi, B.K.; Dutta, S.K.; Thatoi, H.N. Phosphate Solubilization and Acid Phosphatase Activity of Serratia Sp. Isolated from Mangrove Soil of Mahanadi River Delta, Odisha, India. J. Genet. Eng. Biotechnol. 2017, 15, 169–178.
- Jiang, H.; Wang, T.; Chi, X.; Wang, M.; Chen, N.; Chen, M.; Pan, L.; Qi, P. Isolation and Characterization of Halotolerant Phosphate Solubilizing Bacteria Naturally Colonizing the Peanut Rhizosphere in Salt-Affected Soil. Geomicrobiol. J. 2020, 37, 110–118.
- Turner, B.L.; Papházy, M.J.; Haygarth, P.M.; Mckelvie, I.D. Inositol Phosphates in the Environment. Phil. Trans. R. Soc. Lond. B 2002, 357, 449–469.
- George, T.S.; Gregory, P.J.; Hocking, P.; Richardson, A.E. Variation in Root-Associated Phosphatase Activities in Wheat Contributes to the Utilization of Organic P Substrates in Vitro, but Does Not Explain Differences in the P-Nutrition of Plants When Grown in Soils. Environ. Exp. Bot. 2008, 64, 239–249.
- Cosgrove, D.J. The Chemical Nature of Soil Organic Phosphorus. I. Inositol Phosphates. Soil Res. 1963, 1, 203–214.
- Lung, S.-C.; Leung, A.; Kuang, R.; Wang, Y.; Leung, P.; Lim, B.-L. Phytase Activity in Tobacco (Nicotiana tabacum) Root Exudates Is Exhibited by a Purple Acid Phosphatase. Phytochemistry 2008, 69, 365–373.
- George, T.S.; Simpson, R.J.; Gregory, P.J.; Richardson, A.E. Differential Interaction of Aspergillus Niger and Peniophora Lycii Phytases with Soil Particles Affects the Hydrolysis of Inositol Phosphates. Soil Biol. Biochem. 2007, 39, 793–803.
- Annaheim, K.E.; Rufener, C.B.; Frossard, E.; Bünemann, E.K. Hydrolysis of Organic Phosphorus in Soil Water Suspensions after Addition of Phosphatase Enzymes. Biol. Fertil. Soils 2013, 49, 1203–1213.
- Annaheim, K.E.; Doolette, A.L.; Smernik, R.J.; Mayer, J.; Oberson, A.; Frossard, E.; Bünemann, E.K. Long-term addition of organic fertilizers has little effect on soil organic phosphorus as characterized by 31P NMR spectroscopy and enzyme additions. Geoderma 2015, 257, 67–77.
- He, Z.; Honeycutt, C.W. Enzymatic Characterization of Organic Phosphorus in Animal Manure. J. Environ. Qual. 2001, 30, 1685–1692.
- Wyss, M.; Brugger, R.; Kronenberger, A.; Rémy, R.; Fimbel, R.; Oesterhelt, G.; Lehmann, M.; van Loon, A.P.G.M. Biochemical Characterization of Fungal Phytases (Myo-Inositol Hexakisphosphate Phosphohydrolases): Catalytic Properties. Appl. Environ. Microbiol. 1999, 65, 367–373.
- Marlida, Y.; Delfita, R.; Adnadi, P.; Ciptaan, G. Isolation, Characterization and Production of Phytase from Endophytic Fungus Its Application for Feed. Pak. J. Nutr. 2010, 9, 471–474.
- Kim, Y.-O.; Lee, J.-K.; Kim, H.-K.; Yu, J.-H.; Oh, T.-K. Cloning of the Thermostable Phytase Gene (Phy) from Bacillus sp. DS11 and Its Overexpression in Escherichia coli. FEMS Microbiol. Lett. 1998, 162, 185–191.
- Konietzny, U.; Greiner, R. Molecular and Catalytic Properties of Phytate-Degrading Enzymes (Phytases). Int. J. Food Sci. Technol. 2002, 37, 791–812.
- Scott, J.J. Alkaline Phytase Activity in Nonionic Detergent Extracts of Legume Seeds. Plant Physiol. 1991, 95, 1298–1301.
- Azeem, M.; Riaz, A.; Chaudhary, A.N.; Hayat, R.; Hussain, Q.; Tahir, M.I.; Imran, M. Microbial Phytase Activity and Their Role in Organic P Mineralization. Arch. Agron. Soil Sci. 2015, 61, 751–766.
- White, C.; Sayer, J.A.; Gadd, G.M. Microbial Solubilization and Immobilization of Toxic Metals: Key Biogeochemical Processes for Treatment of Contamination. FEMS Microbiol. Rev. 1997, 20, 503–516.
- Chauhan, B.S.; Mahajan, G.; Randhawa, R.K.; Singh, H.; Kang, M.S. Global Warming and Its Possible Impact on Agriculture in India. In Advances in Agronomy; Elsevier: Amsterdam, The Netherlands, 2014; Volume 123, pp. 65–121. ISBN 978-0-12-420225-2.
- Alori, E.T.; Glick, B.R.; Babalola, O.O. Microbial Phosphorus Solubilization and Its Potential for Use in Sustainable Agriculture. Front. Microbiol. 2017, 8, 971.
- Fasim, F.; Ahmed, N.; Parsons, R.; Gadd, G.M. Solubilization of Zinc Salts by a Bacterium Isolated from the Air Environment of a Tannery. FEMS Microbiol. Lett. 2002, 213, 1–6.
- Nautiyal, C.S.; Bhadauria, S.; Kumar, P.; Lal, H.; Mondal, R.; Verma, D. Stress Induced Phosphate Solubilization in Bacteria Isolated from Alkaline Soils. FEMS Microbiol. Lett. 2000, 182, 291–296.
- Johri, A.K.; Oelmüller, R.; Dua, M.; Yadav, V.; Kumar, M.; Tuteja, N.; Varma, A.; Bonfante, P.; Persson, B.L.; Stroud, R.M. Fungal Association and Utilization of Phosphate by Plants: Success, Limitations, and Future Prospects. Front. Microbiol. 2015, 6.
- Park, I.; Lee, J.; Cho, J. Degradation of Phytate Pentamagnesium Salt by Bacillus sp. T4 Phytase as a Potential Eco-Friendly Feed Additive. Asian-Australas. J. Anim. Sci. 2012, 25, 1466–1472.
- Johnson, S.C.; Yang, M.; Murthy, P.P.N. Heterologous Expression and Functional Characterization of a Plant Alkaline Phytase in Pichia Pastoris. Protein Expr. Purif. 2010, 74, 196–203.
- Tang, J.; Leung, A.; Leung, C.; Lim, B.L. Hydrolysis of Precipitated Phytate by Three Distinct Families of Phytases. Soil Biol. Biochem. 2006, 38, 1316–1324.
- Lim, B.L.; Yeung, P.; Cheng, C.; Hill, J.E. Distribution and Diversity of Phytate-Mineralizing Bacteria. ISME J. 2007, 1, 321–330.
- Adhya, T.K.; Kumar, N.; Reddy, G.; Podile, A.R.; Bee, H.; Samantaray, B. Microbial Mobilization of Soil Phosphorus and Sustainable P Management in Agricultural Soils. Sustain. Phosphorus Manag. 2015, 108, 8.
- Zimmermann, P.; Zardi, G.; Lehmann, M.; Zeder, C.; Amrhein, N.; Frossard, E.; Bucher, M. Engineering the Root-Soil Interface via Targeted Expression of a Synthetic Phytase Gene in Trichoblasts. Plant Biotechnol. J. 2003, 1, 353–360.
- Lung, S.-C.; Lim, B. Assimilation of Phytate-Phosphorus by the Extracellular Phytase Activity of Tobacco (Nicotiana tabacum) Is Affected by the Availability of Soluble Phytate. Plant Soil 2006, 279, 187–199.
- Allison, V.J.; Condron, L.M.; Peltzer, D.A.; Richardson, S.J.; Turner, B.L. Changes in Enzyme Activities and Soil Microbial Community Composition along Carbon and Nutrient Gradients at the Franz Josef Chronosequence, New Zealand. Soil Biol. Biochem. 2007, 39, 1770–1781.
- Wallenstein, M.D.; McMahon, S.K.; Schimel, J.P. Seasonal Variation in Enzyme Activities and Temperature Sensitivities in Arctic Tundra Soils. Glob. Chang. Biol. 2009, 15, 1631–1639.
- Fitriatin, B.N.; Joy, B.; Subroto, T. The Influence of Organic Phosphorous Substrate on Phosphatase Activity of Soil Microbes. In Proceedings of the International Seminar of Chemistry, Jatinangor, Indonesia, 30–31 October 2008; pp. 30–31.
- Sarapatka, B. Phosphatase Activity of Eutric Cambisols (Uppland, Sweden) in Relation to Soil Properties and Farming Systems. Sci. Agric. Bohem. 2002, 33, 18–24.
- Qu, Y.; Tang, J.; Li, Z.; Zhou, Z.; Wang, J.; Wang, S.; Cao, Y. Soil Enzyme Activity and Microbial Metabolic Function Diversity in Soda Saline–Alkali Rice Paddy Fields of Northeast China. Sustainability 2020, 12, 10095.
- Fierer, N. Embracing the Unknown: Disentangling the Complexities of the Soil Microbiome. Nat. Rev. Microbiol. 2017, 15, 579–590.
- Jones, D.L.; Hodge, A.; Kuzyakov, Y. Plant and Mycorrhizal Regulation of Rhizodeposition. New Phytol. 2004, 163, 459–480.
- Olsson, R.; Giesler, R.; Loring, J.S.; Persson, P. Enzymatic Hydrolysis of Organic Phosphates Adsorbed on Mineral Surfaces. Environ. Sci. Technol. 2012, 46, 285–291.
- Bian, J.; Tang, J.; Zhang, L.; Ma, H.; Zhao, J. Arsenic Distribution and Geological Factors in the Western Jilin Province, China. J. Geochem. Explor. 2012, 112, 347–356.
- Francioli, D.; Schulz, E.; Lentendu, G.; Wubet, T.; Buscot, F.; Reitz, T. Mineral vs. Organic Amendments: Microbial Community Structure, Activity and Abundance of Agriculturally Relevant Microbes Are Driven by Long-Term Fertilization Strategies. Front. Microbiol. 2016, 7, 1446.
- Li, W.; Feng, J.; Kwon, K.D.; Kubicki, J.D.; Phillips, B.L. Surface Speciation of Phosphate on Boehmite (γ-AlOOH) Determined from NMR Spectroscopy. Langmuir 2010, 26, 4753–4761.
- Li, G.; Li, H.; Leffelaar, P.A.; Shen, J.; Zhang, F. Characterization of Phosphorus in Animal Manures Collected from Three (Dairy, Swine, and Broiler) Farms in China. PLoS ONE 2014, 9, e102698.
- Liang, J.-L.; Liu, J.; Jia, P.; Yang, T.; Zeng, Q.; Zhang, S.; Liao, B.; Shu, W.; Li, J. Novel Phosphate-Solubilizing Bacteria Enhance Soil Phosphorus Cycling Following Ecological Restoration of Land Degraded by Mining. ISME J. 2020, 14, 1600–1613.
- Simpson, R.J.; Oberson, A.; Culvenor, R.A.; Ryan, M.H.; Veneklaas, E.J.; Lambers, H.; Lynch, J.P.; Ryan, P.R.; Delhaize, E.; Smith, F.A.; et al. Strategies and Agronomic Interventions to Improve the Phosphorus-Use Efficiency of Farming Systems. Plant Soil 2011, 349, 89–120.
- Robles-Aguilar, A.A.; Pang, J.; Postma, J.A.; Schrey, S.D.; Lambers, H.; Jablonowski, N.D. The Effect of PH on Morphological and Physiological Root Traits of Lupinus Angustifolius Treated with Struvite as a Recycled Phosphorus Source. Plant Soil 2019, 434, 65–78.
- White, P.J.; Hammond, J.P. Phosphorus nutrition of terrestrial plants. In The Ecophysiology of Plant-Phosphorus Interactions; Springer: Berlin/Heidelberg, Germany, 2008; pp. 51–81.
- Fang, D.; Wei, S.; Xu, Y.; Xiong, J.; Tan, W. Impact of Low-Molecular Weight Organic Acids on Selenite Immobilization by Goethite: Understanding a Competitive-Synergistic Coupling Effect and Speciation Transformation. Sci. Total Environ. 2019, 684, 694–704.
- Haling, R.E.; Brown, L.K.; Stefanski, A.; Kidd, D.R.; Ryan, M.H.; Sandral, G.A.; George, T.S.; Lambers, H.; Simpson, R.J. Differences in Nutrient Foraging among Trifolium Subterraneum Cultivars Deliver Improved P-Acquisition Efficiency. Plant Soil 2018, 424, 539–554.
- Ge, Z.; Rubio, G.; Lynch, J.P. The Importance of Root Gravitropism for Inter-Root Competition and Phosphorus Acquisition Efficiency: Results from a Geometric Simulation Model. Plant Soil 2000, 218, 159–171.
- Rubio, G.; Liao, H.; Yan, X.; Lynch, J.P. Topsoil Foraging and Its Role in Plant Competitiveness for Phosphorus in Common Bean. Crop Sci. 2003, 43, 598–607.
- Honvault, N.; Houben, D.; Nobile, C.; Firmin, S.; Lambers, H.; Faucon, M.-P. Tradeoffs among Phosphorus-Acquisition Root Traits of Crop Species for Agroecological Intensification. Plant Soil 2020, 461, 137–150.
- Hammond, J.P.; Broadley, M.R.; White, P.J.; King, G.J.; Bowen, H.C.; Hayden, R.; Meacham, M.C.; Mead, A.; Overs, T.; Spracklen, W.P. Shoot Yield Drives Phosphorus Use Efficiency in Brassica Oleracea and Correlates with Root Architecture Traits. J. Exp. Bot. 2009, 60, 1953–1968.
- Lynch, J.P. Root Phenes That Reduce the Metabolic Costs of Soil Exploration: Opportunities for 21st Century Agriculture: New Roots for Agriculture. Plant Cell Environ. 2015, 38, 1775–1784.
- Wen, Z.; Li, H.; Shen, Q.; Tang, X.; Xiong, C.; Li, H.; Pang, J.; Ryan, M.H.; Lambers, H.; Shen, J. Tradeoffs among Root Morphology, Exudation and Mycorrhizal Symbioses for Phosphorus-acquisition Strategies of 16 Crop Species. New Phytol. 2019, 223, 882–895.
- Lambers, H.; Shane, M.W.; Cramer, M.D.; Pearse, S.J.; Veneklaas, E.J. Root Structure and Functioning for Efficient Acquisition of Phosphorus: Matching Morphological and Physiological Traits. Ann. Bot. 2006, 98, 693–713.
- Pearse, S.J.; Veneklaas, E.J.; Cawthray, G.; Bolland, M.D.A.; Lambers, H. Triticum aestivum Shows a Greater Biomass Response to a Supply of Aluminium Phosphate than Lupinus Albus, despite Releasing Fewer Carboxylates into the Rhizosphere. New Phytol. 2006, 169, 515–524.
- Wang, L.; Liao, H.; Yan, X.; Zhuang, B.; Dong, Y. Genetic Variability for Root Hair Traits as Related to Phosphorus Status in Soybean. Plant Soil 2004, 261, 77–84.
- Zhu, J.; Kaeppler, S.M.; Lynch, J.P. Topsoil Foraging and Phosphorus Acquisition Efficiency in Maize (Zea mays). Funct. Plant Biol. 2005, 32, 749.
- Lamont, B.B. Structure, Ecology and Physiology of Root Clusters—A Review. Plant Soil 2003, 248, 1–19.
- Trachsel, S.; Kaeppler, S.M.; Brown, K.M.; Lynch, J.P. Shovelomics: High Throughput Phenotyping of Maize (Zea mays L.) Root Architecture in the Field. Plant Soil 2011, 341, 75–87.
- Fort, F.; Freschet, G.T. Plant Ecological Indicator Values as Predictors of Fine-root Trait Variations. J. Ecol. 2020, 108, 1565–1577.
- Parker, J.S.; Cavell, A.C.; Dolan, L.; Roberts, K.; Grierson, C.S. Genetic Interactions during Root Hair Morphogenesis in Arabidopsis. Plant Cell 2000, 12, 1961–1974.
- Raghothama, K.G. Phosphate Acquisition. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1999, 50, 665–693.
- Smith, F.W. The phosphate uptake mechanism. In Food Security in Nutrient-Stressed Environments: Exploiting Plants’ Genetic Capabilities; Adu-Gyamfi, J.J., Ed.; Developments in Plant and Soil Sciences; Springer: Dordrecht, The Netherlands, 2002; pp. 235–244. ISBN 978-94-017-1570-6.
- Ma, Z.; Guo, D.; Xu, X.; Lu, M.; Bardgett, R.D.; Eissenstat, D.M.; McCormack, M.L.; Hedin, L.O. Evolutionary History Resolves Global Organization of Root Functional Traits. Nature 2018, 555, 94–97.
- Ryan, P.; Delhaize, E.; Jones, D. Function and mechanism of organic anion exudation from plant to roots. Annu. Rev. Plant. Physiol. Plant. Mol. Biol. 2001, 52, 527–560.
- Nobile, C.; Houben, D.; Michel, E.; Firmin, S.; Lambers, H.; Kandeler, E.; Faucon, M.-P. Phosphorus-Acquisition Strategies of Canola, Wheat and Barley in Soil Amended with Sewage Sludges. Sci. Rep. 2019, 9, 14878.
- Zogli, P.; Pingault, L.; Libault, M. Physiological and Molecular Mechanisms and Adaptation Strategies in Soybean (Glycine max) Under Phosphate Deficiency. In Legume Nitrogen Fixation in Soils with Low Phosphorus Availability: Adaptation and Regulatory Implication; Sulieman, S., Tran, L.-S.P., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 219–242. ISBN 978-3-319-55729-8.
- Weisskopf, L.; Abou-Mansour, E.; Fromin, N.; Tomasi, N.; Santelia, D.; Edelkott, I.; Neumann, G.; Aragno, M.; Tabacchi, R.; Martinoia, E. White Lupin Has Developed a Complex Strategy to Limit Microbial Degradation of Secreted Citrate Required for Phosphate Acquisition. Plant Cell Environ. 2006, 29, 919–927.
- Guppy, C.N.; Menzies, N.W.; Moody, P.W.; Blamey, F.P.C. Competitive Sorption Reactions between Phosphorus and Organic Matter in Soil: A Review. Soil Res. 2005, 43, 189.
- Carminati, A.; Vetterlein, D.; Koebernick, N.; Blaser, S.; Weller, U.; Vogel, H.-J. Do Roots Mind the Gap? Plant Soil 2013, 367, 651–661.
- Richardson, A.E. Utilization of Soil Organic Phosphorus by Higher Plants. Org. Phosphorus Environ. 2005, 139, 165–184.
- Li, S.M.; Li, L.; Zhang, F.S.; Tang, C. Acid Phosphatase Role in Chickpea/Maize Intercropping. Ann. Bot. 2004, 94, 297–303.
- Neumann, G.; Massonneau, A.; Martinoia, E.; Römheld, V. Physiological Adaptations to Phosphorus Deficiency during Proteoid Root Development in White Lupin. Planta 1999, 208, 373–382.
- Damon, P.M.; Bowden, B.; Rose, T.; Rengel, Z. Crop Residue Contributions to Phosphorus Pools in Agricultural Soils: A Review. Soil Biol. Biochem. 2014, 74, 127–137.
- Aziz, T.; Rahmatullah; Maqsood, M.A.; Sabir, M.; Kanwal, S. Categorization of brassica cultivars for phosphorus acquisition from phosphate rock on basis of growth and ionic parameters. J. Plant Nutr. 2011, 34, 522–533.
- Oburger, E.; Jones, D.L.; Wenzel, W.W. Phosphorus Saturation and PH Differentially Regulate the Efficiency of Organic Acid Anion-Mediated P Solubilization Mechanisms in Soil. Plant Soil 2011, 341, 363–382.
- Ryan, J.; Curtin, D.; Cheema, M.A. Significance of Iron Oxides and Calcium Carbonate Particle Size in Phosphate Sorption by Calcareous Soils. Soil Sci. Soc. Am. J. 1985, 49, 74–76.
- Courty, P.-E.; Franc, A.; Garbaye, J. Temporal and Functional Pattern of Secreted Enzyme Activities in an Ectomycorrhizal Community. Soil Biol. Biochem. 2010, 42, 2022–2025.
- Kirk, G.J.D.; Santos, E.E.; Santos, M.B. Phosphate Solubilization by Organic Anion Excretion from Rice Growing in Aerobic Soil: Rates of Excretion and Decomposition, Effects on Rhizosphere PH and Effects on Phosphate Solubility and Uptake. New Phytol. 1999, 142, 185–200.
- Hallama, M.; Pekrun, C.; Lambers, H.; Kandeler, E. Hidden Miners—The Roles of Cover Crops and Soil Microorganisms in Phosphorus Cycling through Agroecosystems. Plant Soil 2019, 434, 7–45.
- Hayes, J.; Simpson, R.; Richardson, A. The Growth and Phosphorus Utilisation of Plants in Sterile Media When Supplied with Inositol Hexaphosphate, Glucose 1-Phosphate or Inorganic Phosphate. Plant Soil 2000, 220, 165–174.
- Darch, T.; Giles, C.D.; Blackwell, M.S.A.; George, T.S.; Brown, L.K.; Menezes-Blackburn, D.; Shand, C.A.; Stutter, M.I.; Lumsdon, D.G.; Mezeli, M.M.; et al. Inter- and Intra-Species Intercropping of Barley Cultivars and Legume Species, as Affected by Soil Phosphorus Availability. Plant Soil 2018, 427, 125–138.
- Pang, J.; Bansal, R.; Zhao, H.; Bohuon, E.; Lambers, H.; Ryan, M.H.; Ranathunge, K.; Siddique, K.H.M. The Carboxylate-Releasing Phosphorus-Mobilizing Strategy Can Be Proxied by Foliar Manganese Concentration in a Large Set of Chickpea Germplasm under Low Phosphorus Supply. New Phytol. 2018, 219, 518–529.
- Richardson, A.E.; Simpson, R.J. Soil Microorganisms Mediating Phosphorus Availability: Phosphorus plant physiology. Plant Physiol. 2011, 156, 989–996.
- Tinker, P.B. The role of microorganisms in mediating and facilitating the uptake of plant nutrients from soil. In Biológical Processes and Soil Fertility; Springer: Berlin/Heidelberg, Germany, 1984; pp. 77–91.
- Gashaw Deressa, T.; Schenk, M.K. Contribution of Roots and Hyphae to Phosphorus Uptake of Mycorrhizal Onion (Allium cepa L.)-A Mechanistic Modeling Approach. Z. Pflanzenernähr. Bodenk. 2008, 171, 810–820.
- Graham, J.H.; Eissenstat, D.M. Host Genotype and the Formation and Function of VA Mycorrhizae. Plant Soil 1994, 159, 179–185.
- Sattelmacher, B.; Horst, W.J.; Becker, H.C. Factors that contribute to genetic variation for nutrient efficiency of crop plants. Z. Pflanzenernaehr. Bodenk. 1994, 157, 215–224.
- Smith, S.E.; Gianinazzi-Pearson, V. Physiological Interactions Between Symbionts in Vesicular-Arbuscular Mycorrhizal Plants. Annu. Rev. Plant. Physiol. Plant. Mol. Biol. 1988, 39, 221–244.
- Gerke, J. Phytate (Inositol Hexakisphosphate) in Soil and Phosphate Acquisition from Inositol Phosphates by Higher Plants. A Review. Plants 2015, 4, 253–266.
More
Information
Subjects:
Area Studies
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.2K
Entry Collection:
Environmental Sciences
Revisions:
2 times
(View History)
Update Date:
22 Nov 2021
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




