1.1. Soil Microbial Processes Involved in Po Mobilization
In organic-input-amended-soils and P-depleted environments, there is generally a proliferation of free rhizosphere microbiome and symbiotic associations with mycorrhizal fungi which have the potential to mobilize and mineralize different forms of available and unavailable Po [
18,
82] (
Table 5). Phyla involved in Po mineralization include the dominant phyla
Proteobacteria followed by
Acidobacteria and
Actinobacteria [
83,
84]. The order
Xanthomonadales of the
Xanthomonadaceae family is known to contain several Po mineralizing genes. To obtain P from Po compounds,
Streptomyces uses an extracellular alkaline phosphatase encoded by the
phoA gene. Other alkaline phosphatase genes,
phoD and the
phoC, were initially described in the
Streptomyces avermitilis and
Streptomyces coelicolor genomes, respectively [
82].
Stenotrophomonas spp. have been shown to be important contributors to Po solubilization. Controlled experiments using plants inoculated with rhizosphere microbiome have provided further evidence for microbially mediated Po bioavailability to plants. Richardson et al. [
85] showed that both grasses and legumes exhibited an improved ability to utilize IHP-P when inoculated with bacteria isolates with high phytase activity.
Bacillus spp. and
Trichoderma spp. may be able to increase the use of IHP as a P source by plants due to the production of organic anions and phytase [
86,
87]. Nevertheless, there is little evidence of how these rhizosphere microbiomes may act in the presence of soil minerals that can immobilize both Po and hydrolytic enzymes. Soil minerals such as Fe, Al oxides, and clay minerals are known to considerably reduce the efficiency of applied P. It is assumed that adsorbed Po on Fe and Al oxides is protected from enzymatic hydrolysis leading to its accumulation in soil [
21] and its decreased use as a P source by plants. However, García-López et al. [
88] showed recently that
Bacillus subtilis improved the hydrolytic activity of myo-IHP even in the presence of high Fe oxide concentrations. This led to the conclusion that the rhizosphere microbiome could contribute to an increased hydrolyzing capacity in soil with high Po sorption capacity (
Table 5). The currently available research is inadequate to explain the extent to which rhizosphere microbiomes are able to access sorbed forms of P. Future work should be conducted by inoculating microorganisms with sorbed P complexes on major soil minerals such as goethite, gibssite, and major clays, under laboratory conditions and in the field. As most of the legacy P is poorly available to plants, especially the important component corresponding to Po pools [
89], understanding the impact of rhizosphere microbiomes in mobilizing this legacy P is crucial to reduce dependence on mined P fertilizers [
90]. Moreover, it is important to note that the hydrolysis of adsorbed Po depends to some extent on the release of organic anions or citrate by plant and rhizosphere microbiomes; this promotes the desorption and dissolution of Po making it available for hydrolysis [
91].
Table 5. Possible root traits and microbial activity involved in Po mobilization (solubilization and mineralization).
Different enzymes released by the rhizosphere microbiome that are involved in Po hydrolysis, such as phytases and alkaline and acid phosphatases, act specifically on particular Po substrates [
84]. Several studies using soil-specific enzyme additions have been published over the past decades [
23,
92]. Their results show that Po mineralization can be explained by the specificity of enzymatic activities on Po forms. For example, Scyllo-IHP was found to be most resistant to phytase activity [
93], while IHP hydrolysis has been reported for bacterial acid phosphomonoesterases [
94]. Other phytases from
Aspergillus spp. (EC 3.1.3.8) have also been reported to hydrolyze IHP, simple monoesters (G6P, GLY), and phosphoanhydrides, but their ability to hydrolyze diester bonds in nucleic acids [
23,
95] is contradicted by the results of various studies [
96]. Monoesterase enzymes have been found to hydrolyze diester phosphates; however, the release of phosphates from DNA is generally very low. This could be because monoesterases hydrolyze only the 5’ and 3’ phosphate residues of DNA [
97], while the other phosphate groups are not accessible [
95]. It has previously been shown that acid and alkaline phosphatase and phytase are not active on nucleotide pyrophosphate that contains nucleotide pyrophosphate bonds, nor on RNA and DNA that contain phosphodiester bonds [
98]. In organic inputs, nucleotide pyrophosphatase, which hydrolyzes nucleotide pyrophosphate to nicotinamide mononucleotide and AMP, and a P1 nuclease, which cleaves RNA and DNA to produce 5-phospho-monoesters, were used to access Po forms. The result showed that both enzymes (nucleotide pyrophosphatase and P1 nuclease) acted only on their own substrates (nucleotide pyrophosphate, or RNA and DNA, respectively). Therefore, it has been suggested that these enzymes could be used to release specific forms of phosphorus when present in the soil [
98].
Soil properties may influence the conversion of Po into Pi by the rhizosphere microbiome. Among the soil properties, soil pH is an especially important factor that affects the efficacy and biochemical availability of enzymes that hydrolyze Po forms in the soil. For instance, at a pH of 7.5,
Aspergillus niger phytases remained in solution, but at a pH of 5.5, they were unavailable [
95]. However, the optimal pH varies according to the soil microorganism species and the associated plant. Fungal phytases, such as those of
Aspergillus fumigatus, require a pH between 4.5 and 6.5, in which 80% of activity takes place [
99]. Species such as
Rhizoctonia sp. and
Fusarium verticillioides can produce phytases at optimal pH of 4.0 and 5.0, respectively [
100], while in bacterial phytases the maximum activity was observed at a pH of 6.0–8.0, as in
Bacillus sp. [
101]. Most of the phytases are acidic and have an optimal pH between 4.5 and 6.0 [
102], whereas alkaline phytases in legume seeds [
103], lily pollen and cattail pollen, have been reported to have an optimal pH of 8.0 [
104]. For example, at pH 7.5, phytases from
Aspergillus niger remain available in solution, but at pH 5.5 they are not available [
95]. It is evident that phosphatases produced by rhizosphere microbiomes are more sensitive to pH. This pH dependence may be even greater than that of plant phosphatases. However, the optimal pH for Po mobilization varies among rhizosphere microbiome species. It is also important to understand how microorganisms could facilitate P mobilization from organic inputs or soil organic matter within a given pH range. We conclude that the determination of the optimal pH for Po mobilization by microbes and root traits requires careful assessment in future research.
In addition to pH, soil temperature has a strong effect on Po availability though studies have shown contradictory results on the influence of temperature on P solubilization by microbes. White et al. [
105] found 20–25 °C to be the optimal temperature for maximum microbial solubilization of P while 28 °C was reported by Chauhan et al. [
106] and Alori et al. [
107]. Others have reported 30 °C as the optimal temperature for solubilization and mineralization of Po [
101,
108]. Nautiyal et al. [
109] reported solubilization and hydrolysis of P at an extreme temperature of 45 °C in desert soil, while Johri et al. [
110] reported a low temperature of 10 °C. The optimum temperature for phytate-degrading enzymes ranges from 35 to 77 °C. In general, plant phytases, such as those from cereals, show maximum activity at lower temperatures than microbial phytases [
102]. The phytase from
Fusarium verticillioides showed an optimal temperature of 50 °C and stability up to 60 °C [
104]. The optimal temperature for phytase activity towards magnesium phytate (Mg-IHP) has been reported to reach 40 °C without and 50 °C with 5 mM Ca
2+ [
111] Most plant phytases have an optimal temperature of 45–60 °C, as reported by Johnson et al. [
112]. The lowest temperature has been reported to be 10 °C [
110]. However, it is generally assumed that a higher temperature (>30 °C) has a better effect on Po solubilization and availability as shown by the higher Po solubilization by
Bacillus megaterium at 36 °C than at 21 °C. As with pH, phosphatases produced by rhizosphere microbiomes are thermostable. Therefore, changes in the interactions between microbes and root traits as a result of temperature variations and how this could affect Po mobilization processes must be considered in the soil-plant system. Furthermore, understanding the optimal activity of microorganisms as a function of soil temperature is an important challenge for improving biofertilizer management practices and their positive effects on Po hydrolysis and P availability. However, despite the challenge of controlling soil temperature, it is nonetheless possible to identify the optimal dates and seasons to apply biofertilizer to maximize its effect. The effectiveness of microbial enzymatic activity is also influenced by different cations and other constituents in the soil solution. Modelling studies have shown that three classes of phytases, histidine acid phosphatases, β-propellant phytases, and purple acid phosphatases, would be unable to hydrolyze Al
3+, Fe
2+, Fe
3+, Cu
2+ salts of IHP, but would be able to hydrolyze Ca
2+, Mg
2+, and Mn
2+ salts [
113,
114]. This implies that P mobilization will depend on the nature of the cation that precipitates Po. Thus, the accessibility of precipitated Po by enzymes and its mobilization for plants will differ considerably depending on the nature and concentration of electrolytes in the soil.
In summary, the hydrolysis of Po and its release by enzymatic activity is generally affected by the biochemical nature of Po and its ability to interact with soil properties and rhizosphere microbiomes. A wide variety of bacteria, fungi, and endophytes can solubilize Po through the production of organic acids [
115]. This solubilization is very important because most forms of Po are high molecular weight compounds that are generally resistant to chemical hydrolysis. However, the mechanisms associated with the transformation of Po to Pi are poorly understood, and further work is needed, especially under field conditions. In most studies, the experimental conditions have suppressed interactions between system components. Therefore, these studies generally only indicate what is possible, but they do not necessarily indicate what is likely [
116,
117]. Indeed, knowledge of Po transformations is a prerequisite for understanding the potential contribution of lesser-known forms of Po in the organic-input-soil-plant system. In any case, it is evident that Po mineralization occurs in the rhizosphere and could contribute significantly to the requirements for plant growth. Factors affecting the rhizosphere microbiome are likely to influence the lability and stability of their enzymes. Some enzymes become stable through interactions with soil minerals and humic substances and retain some enzyme activity [
118]. In general, microbial activity is affected by biological (e.g., the amount and type of substrate, concentration of enzyme, etc.) and physicochemical processes (e.g., interactions with soil constituents pH, temperature, etc.). The former cause changes in enzyme production rates and microbial community composition, while the latter cause changes in adsorption/desorption reactions, substrate diffusion, and enzyme degradation rates [
119]. Critical factors affecting microbes and their enzyme activities include the amount and type of Po [
120], interactions with soil constituents, pH, temperature, and the concentrations of enzyme and product [
121]. In addition, because Po adsorbs rapidly and strongly onto soil particles, the binding processes involved also play a crucial role in the activity of rhizosphere microbiomes [
122,
123]. To date, there are relatively few studies that explore the enzymatic hydrolysis of adsorbed Po. It has been reported that mineral surfaces protect the majority of adsorbed phosphate esters from enzymatic hydrolysis, but whether this is a general finding remains open [
38]. Furthermore, the mechanism of this process is largely unknown in the case where the enzyme can access the adsorbed Po forms. The results of Olsson et al. [
124] on the hydrolysis of G1P on α-FeOOH surfaces showed the role of interactions at mineral surfaces with respect to the stabilization of Po molecules in soils [
125]. These authors provided a mechanistic explanation of how P can be mobilized via enzymatic activity despite strong interactions with soil minerals. This shed light on previous results showing that microbial stimulation and the resulting enzymatic activity can mobilize adsorbed Po from soil minerals [
113]. However, a question is whether the enzyme acts only on the soluble fraction that is reconstituted by desorption of the substrate or whether the hydrolysis reaction occurs at the interface between the aqueous solution and solid particles. We therefore recommend future studies on these issues to better understand the effect of rhizosphere microbiomes on Po dynamics.
Apart from the rhizosphere microbiome, organic-inputs-derived microorganisms also play a major role in the mobilization of Po. Organic inputs imply the addition of carbon sources and often even contain their own microbiota, equivalent to inoculation of microbes, and this is a very important issue that needs more study. Amendment to organic inputs generally increases the diversity of rhizosphere microbiomes and their enzymatic activities in the soil [
126]. These positive reactions highlight the role of organic-inputs-derived microorganisms in Po availability and, on the other hand, their high content of organic matter [
127] which is the main substrate of most microorganisms [
65]. However, complex questions remain about how the addition of organic inputs alters the soil microbial community and especially how this relates to soil Po mineralization. To successfully manage organic inputs, there is a need to develop a consistent procedure to quantitatively compare the potential of these different microorganisms to release orthophosphate from different sources of Po. As the organic-input/soil-plant continuum consists of various forms of Po with different chemical properties, their solubilization and hydrolysis rates would be strongly related to the diversity of soil microbial communities. Therefore, it is crucial to develop and utilize more advanced approaches to support the roles soil microbes, especially via phosphate-solubilizing microorganism-derived enzymes, play in releasing free Pi from Po forms in the soil [
107,
128]. In sum, the main microbial processes involved in P dynamics that are synthesized and highlighted in this section, and the factors that influence them, greatly affect soil P mobilization processes. They could, therefore, if well understood, contribute to increasing the P use efficiency of organic wastes and those accumulated in agricultural soils. Furthermore, it is known that plant roots inoculated with commercial microorganisms can express synergistic effects to solubilize Pi in the soil. In contrast, little is known about their effect on Po pools. Therefore, further research is needed to evaluate the application and efficacy of commercial microorganisms on various crops with contrasting root traits and fertilized with different P sources under field conditions.
Current cropping models often focus on understanding competition for light and the effects of N or P fertilization, but do not consider interactions with the rhizosphere microbiome and how it affects soil Po forms. Thus, the development of cropping models that consider Po dynamics is needed to determine the efficiency of Po use in multi-species cropping systems and to manage P sustainably in the agroecosystem. Furthermore, it is important to note that current decision support tools do not consider Po sorption, its mineralization kinetics, and the effect of root trait and rhizosphere microbiome interactions on its dynamics. Therefore, in future research aimed at assisting farmers in organic input management, these parameters that govern Po dynamics, should be studied further and integrated into decision support tools. This will not only improve the decision support tools but also make them more focused on Po mobilization in the organic-input/soil-plant system.
Another important factor is the effect of rhizosphere microbial populations on Po mineralization. Some studies have shown that soils taken from the rhizosphere slowed the sorption of phytate or phytase into the rhizosphere, suggesting that rhizosphere soils alter the adsorption of phytase and thus the release of orthophosphate from soils [
114]. This is an important observation because Po dynamics are altered in the rhizosphere, so it is possible that Po from organic inputs is more available around plant roots. Therefore, we strongly suggest that, along with the mobilization process by soil microbes, root mechanisms/traits should be exploited to facilitate soil Po mobilization at the field scale.
The hydrolysis of Po and its release by enzymatic activity is influenced by the biochemical nature of Po and its ability to interact with soil properties and rhizosphere microbiomes. However, current work is insufficient to understand the extent to which rhizosphere microbiomes can access sorbed forms of P from both soil constituents and organic matter. Furthermore, investigations into the potential of microbes to mobilize Po have been conducted on cultivable microbes, yet most root-associated rhizosphere microbiomes are not cultivable. We recommend future investigations to screen rhizosphere bacteria in the presence of different forms of Po and different soil properties, to identify those that are effective in Po mobilization.
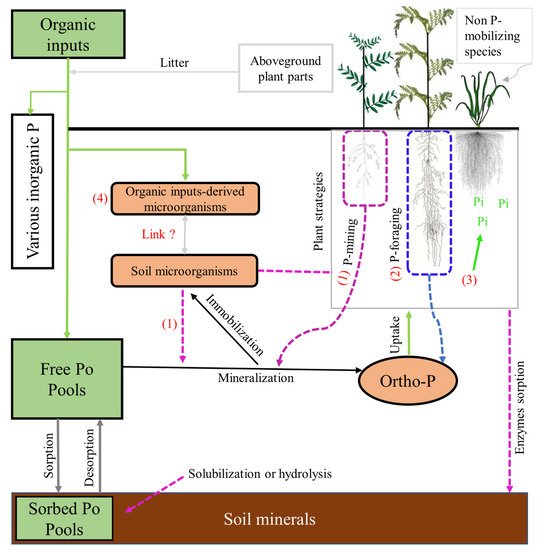
1.2. Root Mechanisms Involved in the Fate of Po Forms
The contribution of plants to Po availability has been known for many decades. Most plants have developed strategies to increase P acquisition in P-deficient soil or to specifically access different forms of Po and Pi in the soil [
143]. These strategies cover a wide range of morphological, architectural, and physiological traits [
144]. In general, these traits could be categorized into three types of P acquisition strategies:
foraging, mining, and collective
microbial-root strategy (
Figure 1 and
Table 5).
The plant P-
foraging strategy relates to the acquisition of P in the soil solution through morphological and architectural traits by maximizing soil exploration. Through this strategy, plants can induce a diffusion gradient which in turn favours the desorption process of the different adsorbed Po forms [
145,
146]. The phosphorus-
foraging strategy would also improve the efficiency of P uptake by slowing the rate at which Po moves from the free or moderately adsorbed form to the strongly adsorbed form on soil compounds [
143].
Foraging strategy involves morphological traits such as specific root length, diameter and radius, and architectural traits, including root length density, root biomass, root hairs, and the formation of clustered roots or arbuscular mycorrhizal symbioses that allow plants to increase their
foraging capacity [
147]. These traits alter the C cost of soil exploration by regulating the extent of competition within and between root systems [
148,
149]. Among morphological traits, root radius is considered important in the efficiency of P use. Plants with finer/thinner roots can explore and contact a greater volume of soil per unit root area. Gahoonia et Nielsen [
78] reported that plant species with finer roots may be more effective in mobilizing Po and absorption of Pi from the soil. Very recently, trade-offs between thicker and thinner roots have been observed by Honvault et al. [
150]. Thicker roots are reported to have greater carboxylate release and phosphatase activity in the rhizosphere, affecting the desorption and mineralization of Po [
150]. In contrast, thinner roots exhibit the morphological traits (
foraging) that favour the exploration and contact of a larger volume of soil to permit P mobilization process [
151,
152]. These observations are consistent with other results showing that species with finer fibrous roots express higher levels of morphological traits to access more Po in the soil [
147,
153,
154]. However, since fine roots (i.e., root hairs with small root radius) tend to renew faster than large roots [
148], the cost of C to produce fine roots could be higher, since they would also need to be replaced more frequently [
149]. In addition to root radius, the formation of root groups, such as proteoid and dauciform root groups, commonly found in plant species belonging to the families
Proteinaceae and
Fabaceae [
154] and others, is very strongly related to the dynamics of Po in soil. These roots provide a very dense mat of root hairs and also specialize in the efficient synthesis and secretion of citrate and malate (organic anions) and phosphatases, which help solubilize insoluble Po resources and hydrolyze Po for plant uptake [
155]. Various effects of root growth and variation in root hair length on Po dynamics and contribution to P uptake have been reported in several species, including maize, wheat, barley, beans, soybean, and white clover [
156,
157]. Higher root length density in the upper soil layers was shown to be the most important root trait of wheat for Po mobilisation in response to organic input [
158]. Moreover, variation in root growth angle and root hairs may have a significant impact on the total mobilisation of P in the soil [
159]. In closely related maize genotypes, the effects of root growth and root hair length variation reportedly led to a 100% increase in total mobilized P and, in other genotypes, to as much as a 600% increase in P mobilization [
157]. Such increases occur since root growth maximizes soil exploration and can induce a diffusion gradient and modify soil properties to promote P desorption. Root hairs are smaller in diameter than roots and grow perpendicular to the root axis, forming up to 77% of the root surface [
160] of soil/field crops [
161,
162]. The presence of root hairs can be very important for the effectiveness of Po mobilization through a considerable increase of root area in the soil. It has been reported that root hairs can contribute up to 70% of Po uptake [
78,
163]. Root hair length and density are highly controlled by P bioavailability. Geometric modeling indicates that root hair responses to P availability interact synergistically to enhance Po and Pi acquisition [
164]. Root hairs also aid in the dispersal of root exudates such as organic acids into the rhizosphere, which improves Po bioavailability in many soils [
165]. Root morphological traits show a much more significant influence on Po acquisition generally in winter wheat genotypes than biochemical transformation by acid phosphatases [
156,
166]. These biochemical and morphological changes can vary considerably between and within plant species [
18,
20]. The potential ability of plants root traits to utilize poorly available Po sources is, however, greatly influenced by genetic makeup.
The plant P
mining strategy, relates to the acquisition of P in the soil solution through physiological traits involving release of substances into the soil from the roots, including carbohydrates, organic and amino acids, phenolic compounds, proteins, fatty acids, sterols, enzymes, polysaccharides and phospholipids [
167,
168]. Among these compounds, carboxylic acids, PME activity, phenolic and mucilage compounds, and protons are the main physiological traits involved in P
mining strategies for Po mobilization [
169]. Through this strategy, plants increase the turnover of poorly available Po pools [
34,
154] by desorption, solubilization, and mineralization processes. Before any hydrolysis process by the enzymes, Po, if not free, must first be desorbed or dissociated from soil minerals or organic matter. The secretion of organic acids by P-mobilizing species improves the availability of Po forms by promoting their desorption from soil constituents to the soil solution, in which they are subsequently mineralized by phosphatases [
170]. Organic acids are generally predicted to be strongly linked to Po mobilization in the soil-plant system [
171]. Plant roots have the ability to produce organic acids, particularly short-chain organic acids, such as lactate, acetate, oxalate, succinate, fumarate, malate, citrate, isocitrate, and aconitate, which can mobilize both Po and Pi [
172]. Among the organic acids, malic and citric acids are the most widespread and abundantly detected in root exudates [
173]. Given the highest affinities between Po forms and soil components, chelation between organic acids in root exudates and soil constituents is the main mechanism for Po solubilization by organic acids in soil [
174]. Citrate has been shown to chelate Fe and Al oxyhydroxide and Mn and Ca carbonates, which can actively displace adsorbed Po into free forms [
175]. Low molecular weight organic acids generally carry one or more negative charges. By complexolysis, negatively charged organic acids can release Po from insoluble forms of Po. These reactions lead to the solubilization of insoluble forms of Po such as Ca-Po, Na-Po etc. [
176]. The impact of direct chelation in the solubilization of Po by citrate exudation has, for instance, been demonstrated in rice [
177]. In general, citrate and oxalate have a higher potential for mobilization of Po compared to other organic anions [
165,
171]. After Po is desorbed from soil minerals by organic acids, phosphatases and phytases released by roots can contribute to its efficient use by plants [
178,
179]. Chickpea, which appears to produce both phosphomonoesterases and diesterases, is thought to improve P nutrition, probably through
mining and mineralization of Po forms in soil [
180,
181]. Various studies have demonstrated that plants have a limited ability to access P in the form of IHP (the main form of Po) due to its low availability in soil solution and low levels of extracellular phosphatase or phytase [
31,
95]. It has also been shown that wheat and many other species are able to utilize P from G6P, GLY, and phosphodiesters (DNA and RNA), due to their
mining capacity, but are limited to acquiring P directly from myo-IHP, although it is abundant in many soils [
92]. This suggests that the biological importance of the different forms of Po will be driven by their turnover rates. Therefore, it was considered that plants with optimal
mining strategies for phytase release could potentially be used to improve the efficiency of inositol phosphate mobilization. The challenges are to understand the functioning of root-derived phytase activities on Po forms, and the chemical nature, soil properties, and root traits of crop species, to increase Po desorption and hydrolysis.
The collective
microbial-root strategy refers to the investment of resources by plants to interact with the microbial community to access Po in soil. Many plants have developed a symbiotic association with vesicular arbuscular mycorrhizal fungi (AMF) that grow out from the root into the surrounding soil, extending the capacity of the root to mineralize Po and take up Pi in soil solution [
182]. The association of roots with arbuscular mycorrhizae is thought to be much more related to the mobilization of total P than root hairs and root length activity [
183]. The symbiotic association of plant roots with mycorrhizae is reported to extend further from the roots than root hairs, and is also active in areas where P forms are adsorbed onto soil components [
184,
185]. A significant contribution of AMF to the uptake of P by plants has been reported, particularly in soils with high Po binding capacity. It is also accepted that AMF store Po in their vacuoles, which can be hydrolyzed and transported as Pi in the host plant [
186]. In addition, AMF also hydrolyze Po by releasing acid phosphatase in the soil. However, the relative contribution of root-derived extracellular phosphatases in the use of Po is still unclear, as the number and activity of bacteria and fungi are higher in the rhizosphere than in the soil in general [
34].
Overall, the roles of P
mining, foraging and collective
microbial-root strategies are understood and their indirect, and sometimes direct, effects on Po dynamics/mobilization have been demonstrated [
164,
166]. The processes differ according to the nature of Po forms (e.g., phosphate, mono, or diester), the structure and function of soil microbial communities, and the physicochemical properties of the soil and climate. For instance, the availability of IHP, G6P, GLY, and phosphodiesters (DNA and RNA), as a direct effect of P
mining,
foraging and collective
microbial-root strategies, remains unclear. Although the release of organic acids can make G6P, GLY, and phosphodiesters (DNA and RNA) available to plants, it is less efficient at solubilizing IHP [
187], probably due to it binding strongly to soil. Interactions between plant traits in the mobilization of P have been studied but remain poorly understood and unconfirmed. To this end, when developing new crop varieties or cultivars, selection should be based on crops with high Po use efficiency to promote greater P availability. Thus, in future crop breeding programs, traits involved in Po use efficiency should be identified and recorded. Specifically, efficient cultivars with genes and traits that produce strong phosphatase/phytase activities should be identified for better mobilization of the major Po form (Myo-IHP). There may also be trade-offs between physiological and morphological traits [
150,
154]. These trade-offs have been examined very recently in different crop families and species [
150]. Trade-offs between thicker and thinner roots were observed [
150], with thicker roots showing greater carboxylate release or phosphatase activity in the rhizosphere. Trade-offs and coordination between traits were strongly influenced by soil type. However, their effect on the availability of Po forms still needs to be elucidated [
153]. Thus, we suggest that these strategies can be exploited using combinations of species with contrasting strategies, or by using a single species to better understand their actual effects on Po forms in agroecosystems.
Both plant functional traits and organic input characteristics strongly interact to modulate Po dynamics. However, it is still unclear to what extent these contribute to the mobilization of sparingly available Po forms. Research efforts should focus on understanding the relationships between plant functional traits, Po nature, and organic input properties in order to characterize the dynamics of Po, model its fate in the soil-plant system, and better understand its consequences for P availability. Further, crop models to estimate total soil Po reserves and, at minimum, its specific forms (IHP, G6P, GLY, etc.), in a multi-species cropping system can be developed based on plant traits (i.e., shoot and root morphological and chemical characteristics), including inter- and intraspecific trait variability and soil properties. This trait-based approach to modeling P mobilization can be developed and would be potentially useful in different climates.