Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | He Yeqing | + 3869 word(s) | 3869 | 2021-10-21 05:24:58 | | | |
| 2 | Conner Chen | Meta information modification | 3869 | 2021-11-03 09:50:29 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Yeqing, H. Association between Membrane Proteins and Disease. Encyclopedia. Available online: https://encyclopedia.pub/entry/15653 (accessed on 07 February 2026).
Yeqing H. Association between Membrane Proteins and Disease. Encyclopedia. Available at: https://encyclopedia.pub/entry/15653. Accessed February 07, 2026.
Yeqing, He. "Association between Membrane Proteins and Disease" Encyclopedia, https://encyclopedia.pub/entry/15653 (accessed February 07, 2026).
Yeqing, H. (2021, November 03). Association between Membrane Proteins and Disease. In Encyclopedia. https://encyclopedia.pub/entry/15653
Yeqing, He. "Association between Membrane Proteins and Disease." Encyclopedia. Web. 03 November, 2021.
Copy Citation
Cell membranes, including membrane carrier proteins, membrane channel proteins and ATP drive pumps, are the main transporters. Membrane transporters have wide, but specific tissue distributions. They can impact on multiple endogenous and xenobiotic processes. Transport proteins constitute approximately 10% of most proteomes and play vital roles in the translocation of solutes across the membranes of all organisms. The receptor proteins on the cell membrane are also important structures involved in substrate transport and signal communication. The obstacles of cell transport-related proteins directly lead to the lack or excess of certain substances in cells.
membrane transporters
target site recognition
targeted drug design
treatment of disease
1. Membrane Transporters
1.1. Carrier Proteins
Membrane carrier proteins are multiple transmembrane proteins and exist in almost all types of biofilms. Each carrier protein can transport a specific solute molecule through a series of conformations that mediate the transport of solute molecules across membranes and can participate in active and passive transportation. The plasma membrane of the cell has carrier proteins for the input of nutrients (sugars, amino acids, nucleotides, etc.). The mitochondrial intima contains carrier proteins that can input pyruvate and ADP and output ATP. Carrier proteins are highly selective in substrate transport, and usually only one class of molecules is transported. The transport of carrier proteins is similar to the process by which enzymes bind to substrates and the process becomes saturated. Some carrier proteins are pH dependent [1]. The properties of common biofilm carrier proteins are shown in Table 1.
Table 1. Summary of the properties of common carrier proteins in animal biofilms.
| Carrier Protein | Typical Positioning | Energy | Function | References |
|---|---|---|---|---|
| Na+-Glucose Pump | The apical plasma membrane of intestinal and renal cells | Na+ | Glucose active transport | [2] |
| Na+-K+ Pump | The plasma membrane of most animal cells | ATP | Na+ active pumping and K+ active pumping | [3][4] |
| Na +-H + Pump | The plasma membrane of animal cells | ATP | H+ active pumping | [5][6] |
| Na+ dependent neutral amino acid transporter | Absorbent epithelial cells | ATP | Amino acid pumping and downstream signal regulation of amino acid receptors | [7][8] |
| Na+ depends on a centralized carrier | Absorbent epithelial cells | ATP | Active transport of nucleosides | [9] |
| Glucose Carrier | The plasma membrane of most animal cells | — | Passive transport of glucose | [10] |
1.2. Channel Proteins
There are three types of channel proteins: ion channels, porins and aquaporins. Most of the channel proteins identified so far are ion channels [11]. Ion channels include electric pressure gated channels, ligand-gated channels and pressure activation channels [12]. The diameter and shape of the channel directly determine the selectivity of the ion passage, which does not involve the binding of the solute molecules. At present, more than 100 channel proteins have been discovered that are ubiquitous in the plasma membrane and the intima of various eukaryotic cells. The main classification of these is shown in Table 2. Porins are found in the outer membrane of Gram-negative bacteria and the outer membrane of mitochondria and chloroplasts. Porins have low selectivity and can pass through larger molecules.
Table 2. Classification of membrane channel proteins.
| Species | Distribution | Somatotype | Function | References |
|---|---|---|---|---|
| Aquaporins | Brain; membranes; kidneys; testis; liver; nasopharynx; lungs; intestines; eyes; etc. | AQP0; AQP1; AQP2; AQP3; AQP4; AQP5; AQP6; AQP7; AQP8; AQP9; AQP10; AQP11; AQP12 | Formation of various body fluids, reabsorption of water by tissues | [13][14] |
| Channel Protein | Chondriosome | MPTP | Apoptosis and necrosis | [15] |
| Ion Channel Protein | Various organizations | HCN; Slack; KcsA; TRPV; TRPM family; PKD1/2; PIEZO1/2; ENaC; TPCs; VDAC; SLC family; ASICs |
Signal transduction, excitement transfer, substance synthesis, energy metabolism, osmotic pressure balance, nutrition induction, substance transport | [16][17] |
1.3. ATP-Driven Pumps
ATP-driven pumps are a family of proteins that rely on the energy released by ATP hydrolysis to transport substances across membranes, including the P-type ion pump, F-type proton pump, V-type proton pump and the ATP-binding cassette (ABC) transporter [18]. The characteristics of the four ATP-driven pumps are shown in Table 3. The p-type pump is located in the plasma membrane, and each P-type pump has two α subunits and two β subunits. The α subunit plays the transport function, and at least one β subunit participates in the phosphorylation process. This acts to regulate the pump activity, assist the transport of Na+, K+, H+, Ca2+, and jointly maintain the homeostasis of ions inside and outside the plasma membrane. Type F is mostly found in the inner membrane of the mitochondria, the chloroplast capsule membrane and the bacterial plasma membrane. It plays an important role in energy conversion and converts ADP into ATP by proton dynamic potential, and so is also called ATP synthase [19]. The V-type proton pump has a complex structure with multiple subunits and is involved in H+ transport. The transport process does not involve phosphorylation intermediates, and is involved in maintaining renal acid balance, regulating apoptosis and cell cycle, nerve signal transmission and other physiological functions in animals. The structure, function and mechanism of the ABC protein have always been the focus of research [20]. ABC transporters are composed of two highly hydrophobic transmembrane domains and two intracellular nucleotide binding domains. They bind together through hydrophobic interactions and are widely distributed in various organisms from bacteria to humans. The transport substrates include sugars, ions, amino acids, phospholipids, peptides, polysaccharides and even proteins [21].
Table 3. ATP-driven pump classification.
| Kind | Constitute | Distribution | Function | References |
|---|---|---|---|---|
| P type ion Pump | 2α subunits (transport), 2β subunits (regulatory) |
Plasma membrane; endoplasmic reticulum | Na+, K+, H+ and Ca2+are transported across membranes | [22] |
| Type F ion Pump | Multiple subunits, transmembrane domain F0 and cytoplasmic domain F1 | Mitochondrial inner membrane | ATP synthesis | [23] |
| V type ion Pump | Multiple subunits, transmembrane domain V0 and cytoplasmic domain V1 | Intracellular bodies; lysosomal membranes; osteoclasts | H+ transport | [24] |
| ABC Transporter Superfamily | Two transmembrane domains, two intracellular ATP-binding domains | All kinds of organisms | Amino acids, sugars, lipids, peptides, protein transport, macromolecule transport | [25][26] |
1.4. Functions of Membrane Transporters
1.4.1. Function of Carrier Protein
Carrier proteins are important proteins in the cell membrane as they form channels for the transport of nutrients such as sugars, amino acids, nucleotides and other small molecules to enter and exit the cell. Amino acid carriers in mammalian cells can be divided into neutral, acidic, alkaline transporters, and Na+ dependent and Na+ independent carriers, which are involved in amino acid transport in cells [27]. Amino acid transporters also participate in the regulation of intracellular signaling pathways, including the mechanistic target of rapamycin complex 1 (mTORC1) and general control nonderepressible 2 (GCN2), and participate in physiological homeostasis and nutritional health regulation [28]. Glucose uptake in mammalian cells is assisted by glucose transporter proteins. There are at least five such transporters that have different characteristics: the glucose transporter type 1 (GLUT1), GLUT2, GLUT3, GLUT4 and GLUT5. GLUT2 and GLUT4 are of great significance in diabetes. Sodium-glucose co-transporter (SGLT) is also the focus of research and plays a key role in intestinal glucose absorption [29].
1.4.2. Function of Channel Proteins
AQPs exist in the form of a tetramer, and in which each monomer is involved in water transport. AQPs can participate in the absorption and secretion of water to maintain a balance of body water, electrolyte metabolism, cerebrospinal fluid secretion, urine dilution and intestinal water [30]. AQP is also involved in tumor growth, invasion, and metastasis, gastrointestinal disease, and kidney disease [31][32]. The functions of ion channels include protein kinase activation and gene expression regulation, the regulation of cell excitability and muscle activity, and maintaining cell volume. Ion channels play an important physiological role in the body that is directly related to homeostasis.
2. Membrane Receptor Proteins
2.1. Types of Membrane Receptor Proteins
2.1.1. Ion Channel-Coupled Receptors
Ion channel-coupled receptors are a class of macromolecules that are ion channels and have ligand-binding sites, which can act as ion channels and signal transducers. Different from ion channels controlled by potential and chemically modified ion channels, the opening and closing of these ion channels are directly controlled by ligands, which are mainly neurotransmitters [33]. Ion-channel receptors can be cation channels, such as those for acetylcholine, glutamate, and serotonin, or anionic channels, such as those for glycine and gamma-aminobutyric acid [34]. Taking the N-methyl-D-Aspartic Acid receptor (NMDA) as an example, ion channels can be blocked by different channel blockers. The NMDA receptors can be blocked by endogenous magnesium ions, MK-801, and noncompetitive antagonists, such as memantine and ketamine [35]. The known subtype-selective NMDA receptor antagonists are zinc ions that bind to the N-terminal of the NR2 subunit (acting on the NMDA receptor containing the NR2A subunit) and Benz phenols that selectively block the NMDA receptor containing the NR2B subunit (Figure 2).
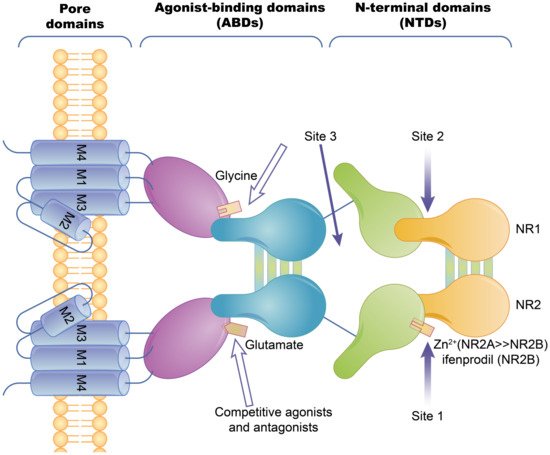
Figure 2. The basic structure of the NMDA ion-channel receptor. The NMDA receptor is composed of two NR1 subunits and two NR2 subunits in a dimer combination. Only one of the NR1/NR2 heterodimers is shown. The extracellular region of each subunit has two projections, the N-terminal and the agonist binding region, where the polymerization of subunits occurs at both the agonist binding region and the N-terminus. Agonist binding sites with glutamic acid are present in the NR2 subunit and glycine or serine binding sites are present in the NR1 subunit. Hollow arrows indicate the binding sites of competitive agonists or antagonists, and solid arrows indicate the regulatory sites for allosteric conditioning (e.g., zinc ions).
2.1.2. G Protein-Coupled Receptors
Cell surface transmembrane proteins are mainly composed of G-protein-coupled receptors (GPCRs), ion channels and transporters, which play an important role in neuronal signal processing and plasticity in the brain. The function of GPCRs in cell motility, growth differentiation and gene expression is closely related to tumors. The GPCRs are the largest family of cell-surface receptors and are ubiquitous on the surfaces of various eukaryotic cells. Conjugated GPCR proteins also mediate different signaling pathways [36]. As shown in Figure 3, GPCRs include a variety of neurotransmitters, peptide hormones and C-X-C chemokine receptor (CXCR), and receptors that accept exogenous physical and chemical factors in the senses of taste, vision and smell. CXCR4 plays a key role in tumor invasion and metastasis. CXCR can induce neutrophils, lymphocytes, monocytes and fibroblasts to aggregate and activate the inflammatory sites and participate in tissue injury repair. CXCR is important member of the GPCR family, it mainly causes downstream signal transmission. G proteins regulate ion channels through second messengers, and the activity of many ion channels is influenced by specific GPCR activation. Phosphatidylinositol signaling pathway is an extracellular signal molecule that binds to GPCRs on the cell surface, activates phospholipase C on the plasma membrane, hydrolyzes phosphatidylinositol bisphosphate (PIP2) on the plasma membrane into the inositol 1,4,5-trisphosphate (IP3) and diacylglycerol (DG), and converts extracellular signals into intracellular signals. The phosphatidylinositol-3-kinase/protein kinase B/mammalian target of rapamycin (PI3K/Akt/mTOR) signaling pathway is considered to be involved in the regulation of cellular physiological processes through the activation of downstream effector factors, and is directly related to cell growth, proliferation, cancer and longevity. This pathway is involved in the occurrence of human diseases and can regulate many biological functions in the body [37]. cGMP activates cGMP-dependent protein kinase PKG and downstream MAPK pathways, resulting in tumor cell dryness and metastasis [38]. Structurally, these receptors are monomer proteins with the amino terminus on the outer surface of the cell and the carboxyl terminus on the inner membrane. The complete peptide chain crosses the membrane seven times and so this type of receptor is also called a sevenfold transmembrane receptor. As the peptide chain repeatedly crosses the membrane, several loop structures are formed on the outer and inner sides of the membrane. These are responsible for binding to ligands (chemical and physical signals) and intracellular signal transmission, respectively. The cytoplasmic portion interacts with a GTP-binding protein (G protein), which is the first signaling molecule in the pathway [39]. G protein-coupled receptors regulate a variety of intracellular signaling cascades including G protein-dependent and G protein-independent pathways [40].
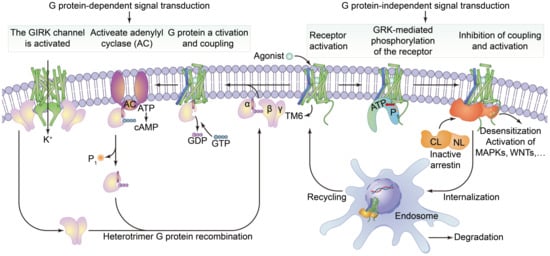
Figure 3. The structure of G protein-coupled receptors and downstream signal transduction pathways. It shows that the agonist binds to activate the receptor by inducing a conformational change in the transmembrane domain (TM6, blue). The activated receptor binds to a variety of intracellular signaling proteins, including G proteins (light purple), and GRKs (light orange), that are active (yellow) and inactive (dark orange). The coupling of heterotrimer G proteins to the receptor initiates nucleotide exchange, and then the G proteins dissociate into the Gα, Gβ and Gγ subunits. Both subunits regulate different downstream effector proteins. The GTP-bound Gα subunit regulates the activity of adenylate cyclase (AC, dark purple), whilst the Gβ and γ subunits interact with the g-protein-coupled internal rectifying potassium channel (GIRK, represented by cylindrical TM, green). The G protein-mediated signaling pathway is terminated by GTP hydrolysis and the recombination of Gα with Gβ and γ to form inactive heterotrimers. Activation of the receptor also leads to phosphorylation of GRKs and subsequent coupling of statin. Statin-coupled receptors lead to desensitization and statin-mediated activation of downstream effector proteins, such as mitogen-activated protein kinases (MAPKs) or SRC kinases. Statin activation also promotes receptor internalization into the endosome and subsequent degradation or circulation into the plasma membrane.
2.1.3. Enzyme-Linked Receptors
The receptors of many growth factors and cytokines have structures with single transmembrane glycoproteins, enzyme activities or involve the intracellular segment of the receptor associated with the enzyme. In contrast to the seven-fold transmembrane receptors (G protein-coupled-type receptors), these receptors are referred to as single-fold transmembrane receptors, and are also known as enzyme-linked receptors. The transmembrane regions of these receptors cross the membrane once, in contrast to the structure of seven-fold transmembrane receptors which have repeated transmembrane segments. Receptor tyrosine kinases are typical examples of enzyme-linked receptors [41]. Immunosorbent assay (Cell-ELISA) analysis of native and recombinant glutamate receptors, including growth factors and cytokines, such as growth hormone receptor, interleukin receptor, and tumor necrosis factor receptor, etc., (Figure 4).
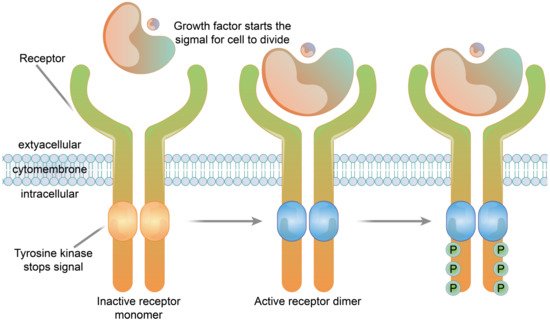
Figure 4. The structure of a receptor tyrosine kinase. In the absence of ligand binding, the receptor is combined with two monomers and is not active. The signaling molecule and the extracellular receptor combine to form dimers on the membrane. The tail end of the two receptors and the intracellular structure domain interact to activate the protein kinase function, which causes the phosphorylation of tail tyrosine residues. Phosphorylation causes the tail of the receptor’s intracellular domain to assemble into a signaling complex. The phosphorylated tyrosine site becomes the binding site for intracellular signaling proteins. Ten to twenty different intracellular signaling proteins can bind to the phosphorylated site of the receptor tail and are activated. Signal complexes amplify the information and activate a series of biochemical reactions in cells through several different signal transduction pathways, or combine different pieces of information to produce a comprehensive response.
2.2. Function of Membrane Receptor Proteins
2.2.1. Ion Channel Receptor Functions
The ultimate role of the ion-channel receptor signal transduction is to cause changes in the cell membrane potential. Ion-channel receptors affect the function of cells by transforming chemical signals into electrical signals [42]. The rapid conduction of electrical signals is transformed into chemical signal transmission, and then into electrical signals or cellular reactions to realize the rapid transformation of external signals [43]. Ion channel receptors are closely related to neural activity and muscle movement, and directly affect physiological activity in the body.
2.2.2. Function of G Protein-Coupled Receptors
GPCRs are the largest family of cell signal transducers in the human body. GPCRs are involved in almost all physiological life activities and are responsible for regulating the responses of cells to light, odors, hormones, neurotransmitters and chemokines [44][45]. The extracellular regions of the GPCRs undergo conformational changes after binding to excitatory signaling molecules (e.g., odors, hormones, neurotransmitters, chemokines), leading to transmembrane helix movements, especially in the intracellular portion of the sixth transmembrane helix that turns outwards [46]. At this time, the intracellular region of the activated receptor can recruit and bind downstream effector proteins (such as G protein and B-Arrestin, etc.), that regulate physiological activities in vivo through the cyclic adenosine monophosphate (cAMP) signaling, phosphatidylinositol signaling and calcium ion signaling pathways [47].
2.2.3. Enzyme-Linked Receptor Functions
Enzyme-linked receptor proteins are both receptors and enzymes. Once activated by ligands, the receptors have enzyme activities and amplify signals, and so they are also called catalytic receptors. This type of receptor transduction signal is usually related to cell growth, reproduction, differentiation and survival [48]. Receptor tyrosine kinases are typical enzyme-linked receptors. Extracellular ligands are soluble or membrane-bound peptides or hormones that include a variety of growth factors such as insulin, etc. The main function of enzyme-linked receptor proteins is to control cell growth and differentiation rather than regulating intermediate metabolism. Receptor tyrosine kinases are widely expressed in a variety of mammalian tissues and play important biological functions in the nervous, immune, hematopoietic and urinary systems [49].
3. Association between Membrane Proteins and Disease
3.1. Abnormal Ion Channels Induce Cancer
Ion-channel proteins are composed of protein complexes that allow suitably sized ions to pass along the concentration gradient. Studies have found that the K+ channel plays an important role in cell conduction, which is closely related to many diseases and plays an important role in the study of tumors and cancer [50]. The K+ channel protein promotes Ca2+ influx and G1 phase progression by influencing tumor cell membrane potential, or by changing cell volume, the concentration of intracellular substances related to DNA synthesis and cell cycle regulation can be affected, thus causing the rapid proliferation of tumor cells [51][52]. In addition, K+ channel protein expression is also associated with tumor metastasis, for example, overexpression of the G protein-gated inwardly rectifying potassium (GIRK) is associated with lymph node metastasis of breast cancer [53]. Inhibition of K+ channel activity by chemical reagents can inhibit the proliferation of cancer cells, indicating that the K+ channel protein can be used as a drug target.
The Na+ channel is a kind of transmembrane glycoprotein located in the plasma membrane of the cell, its main function is to maintain cell excitability and conduction. The selective Na+ permeability of voltage-gated Na+ channels is the basis of action potential generation in excitatory cells such as neurons. Bennett et al. found that prostate cancer cells with a high expression level of voltage-gate sodium channel and strong activity had better aggressiveness [54]. In breast cancer, cervical cancer and melanoma, it has been found that upregulation of the Na+ channel can promote tumor metastasis.
The Ca2+ channel is involved in intracellular regulation of almost all biological functions of the body, such as heart and muscle contraction, nerve information transmission, cell proliferation and apoptosis, cell division and differentiation, etc. [55]. Studies have found that the store-operated Ca2+ entry (SOCE), as an important pathway to regulate intracellular calcium homeostasis, is closely related to tumorigenesis and plays an important role in maintaining calcium homeostasis in angiogenesis and tumor immunogenicity changes [56].
Na+/K+-atPase (NKA) utilizes the hydrolysis of ATP to actively transport Na+ and K+ across the membrane, against the concentration gradient to transport Na+ to the extracellular and K+ to the intracellular, which can maintain cell membrane localization and make the cell present an excited state [57].
3.2. Substrate Transport Disorders, Induced Metabolic Disorders of the Type of Disease
Membrane transporters have different structures and physiological functions. Gene mutations usually produce defective proteins that cause abnormal transport channels. This normally results from the loss of normal function due to functional enhancement, functional loss and dominant and negative effects. Any kind of membrane transport-related protein disorder may cause abnormal physiological states in cells, resulting in cellular substrate transport disorders and cell body diseases. The cytoplasmic membrane has selective permeability, which plays an important role in cell substrate metabolism and osmotic pressure maintenance. Carrier proteins are transmembrane proteins with multiple cyclotron folds that bind specifically to the molecule being delivered to allow it to cross the plasma membrane. Their functional mutations or deletions can lead to a series of diseases related to material transport, as summarized below.
GLUT1 deficiency is a metabolic disease of the brain that is caused by mutations in the SLC2A1 gene. Up to now, more than 140 different SLC2A1 variants have been found, all of which can lead to decreased expression or the complete loss of GLUT1 function, impaired glucose transport in the brain, and insufficient energy supply [58][59]. Mutations of the GLUT1 protein lead to misfolding and polymerization resulting in impaired ACTIVITY of GLUT1 [60]. Cystinuria is a disease characterized by specific defects in the transport of cystine, lysine, arginine and ornithine in hereditary proximal renal tubular epithelial cells and the jejunal mucosa. Cystinuria results in the excessive excretion of these four amino acids in urine. Lysine, arginine, and ornithine are easily soluble in water, whilst cystine is less soluble in water, cystine has low solubility in urine and can crystallize to form stones [61]. AQP maintains renal urine concentration through the cell transport of water and low molecular solutes. Mutations or functional defects in AQP genes may lead to severe nephrogenic diabetes insipidus. A large number of studies have shown that AQPs are associated with acute kidney injury and various chronic kidney diseases [62][63].
The dysfunction of the ATP-binding cassette (ABC) transporter proteins can lead to several human diseases. More than 20 ABC transporters are known to be associated with human diseases, ABCA4 is linked to Stargardt disease. ABCA7 is linked to Alzheimer’s disease, two-thirds of ABCB lesions lead to immune deficiency and the lesions of ABCC7 can lead to cystic fibrosis [64][65]. P-glycoprotein (P-gp), Multidrug Resistance protein-1 (MRP1) and ABC superfamily G member 2 (ABCG2) transporters are expressed at abnormally high levels in the cell membranes of tumor cells [66]. P-gp plays an important role in the exogenous defense system, which can transport organic cations, carbohydrates, antibiotics and anticancer drugs as substrates [67]. Studies have shown that MRP1 is resistant to some cancer drugs, such as anthracyclines, vinblastine alkaloids, and campinetin, which is a major challenge in the clinical treatment of neuroblastoma [68]. Sodium-glucose co-transporter-2 inhibitors (SGLT-2i) mediated glycosuria decreases with a decrease in blood glucose concentration and renal glucose threshold, and the risk of hypoglycemia does not occur with medication alone. Therefore, due to its unique mechanism of action, SGLT-2i has become an important new target drug for the treatment of the type 2 diabetes mellitus (T2DM) and diabetic nephropathy (DN) [69]. ABCG2, known as breast cancer resistance protein (BCRP), can make cancer cells resistant to a variety of drugs, such as topotecan, mitoxantrone and daunorubicin [70].
3.3. Membrane Receptors and Pathogen Invasion
Pathogens invade cells by combining with specific receptors on the cell surface. Under normal circumstances, receptors on the cell surface can distinguish between pathogens and normal substances within the body. When encountering pathogens, cells stimulate autoimmunity and kill the pathogens. However, some pathogens can camouflage their surface structures, and evade cell recognition through the endocytosis pathway.
In the case of HIV, HIV requires CD4 receptors to enter cells. It also requires co-receptors such as cinnamoyl-COA reductase (CCR) or CXCR. Macrophages and dendritic cells have CD4 and CCR5 receptors, and CD4T lymphocytes have CD4 and CXCR4 receptors. The entry of HIV into human cells requires the participation of CD4, CCR5 or CXCR4 receptors, otherwise it cannot cause infection [71]. Studies have shown that people who are resistant to HIV have a deletion mutation in their CCR5 receptor gene, that results in a 32-base pair loss. In these cases, the normal CCR5 receptor protein cannot be encoded and so macrophages and dendritic cells lacking the CCR5 cannot be infected by the virus and individuals are resistant to AIDS [72]. Since the outbreak of the novel coronavirus, the mechanism of the invasion of human cells by COVID-19 has become clear. To invade human cells, the novel coronavirus must catch the angiotensin-converting enzyme 2 (ACE2) protein on the surface of human cell membrane with the help of the S protein on its surface. The virus binds to these proteins before entering human cells and causing infection [73].
References
- Taru, S.G.; Nath, A.; Prasad, S.; Singhal, S.; Chandra, V.; Saikumar, G. Expression pattern of GLUT 1, 5, 8 and citrate synthase transcripts in buffalo (Bubalus bubalis) preimplantation embryos produced in vitro and derived in vivo. Reprod. Domest. Anim. 2020, 55, 1362–1370.
- Arow, M.; Waldman, M.; Yadin, D.; Nudelman, V.; Shainberg, A.; Abraham, N.G.; Freimark, D.; Kornowski, R.; Aravot, D.; Hochhauser, E.; et al. Sodium-glucose cotransporter 2 inhibitor Dapagliflozin attenuates diabetic cardiomyopathy. Cardiovasc. Diabetol. 2020, 19, 7.
- Felippe, G.C.; Ribeiro, S.A.; Ignácio, D.S.C.; Caire, C.H.; Burth, P. Na/K Pump and Beyond: Na/K-ATPase as a Modulator of Apoptosis and Autophagy. Molecules 2017, 22, 578.
- Askari, A. The other functions of the sodium pump. Cell Calcium 2019, 84, 102105.
- Packer, M. Activation and Inhibition of Sodium-Hydrogen Exchanger Is a Mechanism That Links the Pathophysiology and Treatment of Diabetes Mellitus with That of Heart Failure. Circulation 2017, 136, 1548–1559.
- Conrad, K.P. Might proton pump or sodium-hydrogen exchanger inhibitors be of value to ameliorate SARs-CoV-2 pathophysiology? Physiol. Rep 2021, 8, e14649.
- Magi, S.; Piccirillo, S.; Amoroso, S.; Lariccia, V. Excitatory Amino Acid Transporters (EAATs): Glutamate Transport and Beyond. Int. J. Mol. Sci. 2019, 20, 5674.
- Singh, S.; Arthur, S.; Sundaram, U. Mechanisms of Regulation of Transporters of Amino Acid Absorption in Inflammatory Bowel Diseases. Compr. Physiol. 2020, 10, 673–686.
- Takenaka, R.; Yasujima, T.; Furukawa, J.; Hishikawa, Y.; Yamashiro, T.; Ohta, K.; Inoue, K.; Yuasa, H. Functional Analysis of the Role of Equilibrative Nucleobase Transporter 1 (ENBT1/SLC43A3) in Adenine Transport in HepG2 Cells. J. Pharm. Sci. 2020, 109, 2622–2628.
- Yan, N. A Glimpse of Membrane Transport through Structures-Advances in the Structural Biology of the GLUT Glucose Transporters. J. Mol. Biol. 2017, 429, 2710–2725.
- Cosme, D.; Estevinho, M.M.; Rieder, F.; Magro, F. Potassium channels in intestinal epithelial cells and their pharmacological modulation: A systematic review. Am. J. Physiol. Cell Physiol. 2021, 320, C520–C546.
- Cheng, Y.R.; Jiang, B.Y.; Chen, C.C. Acid-sensing ion channels: Dual function proteins for chemo-sensing and mechano-sensing. J. Biomed. Sci. 2018, 25, 46.
- Li, C.; Wang, W. Molecular Biology of Aquaporins. Adv. Exp. Med. Biol. 2017, 969, 1–34.
- Dajani, S.; Saripalli, A.; Sharma-Walia, N. Water transport proteins-aquaporins (AQPs) in cancer biology. Oncotarget 2018, 9, 36392–36405.
- Bauer, T.M.; Murphy, E. Role of Mitochondrial Calcium and the Permeability Transition Pore in Regulating Cell Death. Circ. Res. 2020, 126, 280–293.
- Rivolta, I.; Binda, A.; Masi, A.; DiFrancesco, J.C. Cardiac and neuronal HCN channelopathies. Pflugers Arch. 2020, 472, 931–951.
- Ali, S.R.; Malone, T.J.; Zhang, Y.; Prechova, M.; Kaczmarek, L.K. Phactr1 regulates Slack (KCNT1) channels via protein phosphatase 1 (PP1). FASEB J. 2020, 34, 1591–1601.
- Schwiebert, E.M. ABC transporter-facilitated ATP conductive transport. Am. J. Physiol. 1999, 276, C1–C8.
- Hejzlarová, K.; Mráček, T.; Vrbacký, M.; Kaplanová, V.; Karbanová, V.; Nůsková, H.; Pecina, P.; Houštěk, J. Nuclear genetic defects of mitochondrial ATP synthase. Physiol. Res. 2014, 63, S57–S71.
- He, G.; Tian, W.; Qin, L.; Meng, L.; Wu, D.; Huang, Y.; Li, D.; Zhao, D.; He, T. Identification of novel heavy metal detoxification proteins in Solanum tuberosum: Insights to improve food security protection from metal ion stress. Sci. Total Environ. 2021, 779, 146197.
- Wu, C.; Chakrabarty, S.; Jin, M.; Liu, K.; Xiao, Y. Insect ATP-Binding Cassette (ABC) Transporters: Roles in Xenobiotic Detoxification and Bt Insecticidal Activity. Int. J. Mol. Sci. 2019, 20, 2829.
- El, R.N.; Lima, J.J.; Johnson, J.A. Proton pump inhibitors: From CYP2C19 pharmacogenetics to precision medicine. Expert Opin. Drug Metab. Toxicol. 2018, 14, 447–460.
- Anandakrishnan, R.; Zuckerman, D.M. Biophysical comparison of ATP-driven proton pumping mechanisms suggests a kinetic advantage for the rotary process depending on coupling ratio. PLoS ONE 2017, 12, e173500.
- Ueno, H.; Suzuki, K.; Murata, T. Structure and dynamics of rotary V(1) motor. Cell Mol. Life Sci. 2018, 75, 1789–1802.
- Rempel, S.; Gati, C.; Nijland, M.; Thangaratnarajah, C.; Karyolaimos, A.; de Gier, J.W.; Guskov, A.; Slotboom, D.J. A mycobacterial ABC transporter mediates the uptake of hydrophilic compounds. Nature 2020, 580, 409–412.
- Amawi, H.; Sim, H.M.; Tiwari, A.K.; Ambudkar, S.V.; Shukla, S. ABC Transporter-Mediated Multidrug-Resistant Cancer. Adv. Exp. Med. Biol. 2019, 1141, 549–580.
- Seow, H.F.; Bröer, S.; Bröer, A.; Bailey, C.G.; Potter, S.J.; Cavanaugh, J.A.; Rasko, J.E. Hartnup disorder is caused by mutations in the gene encoding the neutral amino acid transporter SLC6A19. Nat. Genet. 2004, 36, 1003–1007.
- Fotiadis, D.; Kanai, Y.; Palacín, M. The SLC3 and SLC7 families of amino acid transporters. Mol. Aspects Med. 2013, 34, 139–158.
- Danne, T.; Garg, S.; Peters, A.L.; Buse, J.B.; Mathieu, C.; Pettus, J.H.; Alexander, C.M.; Battelino, T.; Ampudia-Blasco, F.J.; Bode, B.W.; et al. International Consensus on Risk Management of Diabetic Ketoacidosis in Patients with Type 1 Diabetes Treated with Sodium-Glucose Cotransporter (SGLT) Inhibitors. Diabetes Care 2019, 42, 1147–1154.
- Denker, B.M.; Smith, B.L.; Kuhajda, F.P.; Agre, P. Identification, purification, and partial characterization of a novel Mr 28,000 integral membrane protein from erythrocytes and renal tubules. J. Biol. Chem. 1988, 263, 15634–15642.
- Camilleri, M.; Carlson, P.; Chedid, V.; Vijayvargiya, P.; Burton, D.; Busciglio, I. Aquaporin Expression in Colonic Mucosal Biopsies From Irritable Bowel Syndrome with Diarrhea. Clin. Transl. Gastroenterol. 2019, 10, e19.
- Verkman, A.S.; Hara-Chikuma, M.; Papadopoulos, M.C. Aquaporins--new players in cancer biology. J. Mol. Med. 2008, 86, 523–529.
- Mussina, K.; Toktarkhanova, D.; Filchakova, O. Nicotinic Acetylcholine Receptors of PC12 Cells. Cell Mol. Neurobiol. 2021, 41, 17–29.
- Vibholm, A.K.; Landau, A.M.; Møller, A.; Jacobsen, J.; Vang, K.; Munk, O.L.; Orlowski, D.; Sørensen, J.C.; Brooks, D.J. NMDA receptor ion channel activation detected in vivo with GE-179 PET after electrical stimulation of rat hippocampus. J. Cereb. Blood Flow Metab. 2021, 41, 1301–1312.
- Paoletti, P.; Neyton, J. NMDA receptor subunits: Function and pharmacology. Curr. Opin. Pharmacol. 2007, 7, 39–47.
- Kofuji, P.; Araque, A. G-Protein-Coupled Receptors in Astrocyte-Neuron Communication. Neuroscience 2021, 456, 71–84.
- Duan, Y.; Haybaeck, J.; Yang, Z. Therapeutic Potential of PI3K/AKT/mTOR Pathway in Gastrointestinal Stromal Tumors: Rationale and Progress. Cancers 2020, 12, 2972.
- Lv, Y.; Wang, X.; Li, X.; Xu, G.; Bai, Y.; Wu, J.; Piao, Y.; Shi, Y.; Xiang, R.; Wang, L. Nucleotide de novo synthesis increases breast cancer stemness and metastasis via cGMP-PKG-MAPK signaling pathway. PLoS Biol. 2020, 18, e3000872.
- Hilger, D.; Masureel, M.; Kobilka, B.K. Structure and dynamics of GPCR signaling complexes. Nat. Struct. Mol. Biol. 2018, 25, 4–12.
- Weis, W.I.; Kobilka, B.K. The Molecular Basis of G Protein-Coupled Receptor Activation. Annu. Rev. Biochem. 2018, 87, 897–919.
- Spiess, K.; Fares, S.; Sparre-Ulrich, A.H.; Hilgenberg, E.; Jarvis, M.A.; Ehlers, B.; Rosenkilde, M.M. Identification and functional comparison of seven-transmembrane G-protein-coupled BILF1 receptors in recently discovered nonhuman primate lymphocryptoviruses. J. Virol. 2015, 89, 2253–2267.
- Holdorf, A.D.; Green, J.M.; Levin, S.D.; Denny, M.F.; Straus, D.B.; Link, V.; Changelian, P.S.; Allen, P.M.; Shaw, A.S. Proline residues in CD28 and the Src homology (SH)3 domain of Lck are required for T cell costimulation. J. Exp. Med. 1999, 190, 375–384.
- Pandey, S.; Zhang, W.; Assmann, S.M. Roles of ion channels and transporters in guard cell signal transduction. FEBS Lett. 2007, 581, 2325–2336.
- Venkatakrishnan, A.J.; Deupi, X.; Lebon, G.; Tate, C.G.; Schertler, G.F.; Babu, M.M. Molecular signatures of G-protein-coupled receptors. Nature 2013, 494, 185–194.
- Katritch, V.; Cherezov, V.; Stevens, R.C. Structure-function of the G protein-coupled receptor superfamily. Annu. Rev. Pharmacol. Toxicol. 2013, 53, 531–556.
- Molnár, E. Cell-Based Enzyme-Linked Immunosorbent Assay (Cell-ELISA) Analysis of Native and Recombinant Glutamate Receptors. Methods Mol. Biol. 2019, 1941, 47–54.
- Morishima, M.; Tahara, S.; Wang, Y.; Ono, K. Oxytocin Downregulates the Ca(V)1.2 L-Type Ca(2+) Channel via Gi/cAMP/PKA/CREB Signaling Pathway in Cardiomyocytes. Membranes 2021, 11, 234.
- Ji, R.; Meng, L.; Li, Q.; Lu, Q. TAM receptor deficiency affects adult hippocampal neurogenesis. Metab. Brain Dis. 2015, 30, 633–644.
- Lee, I.J.; Hilliard, B.A.; Ulas, M.; Yu, D.; Vangala, C.; Rao, S.; Lee, J.; Gadegbeku, C.A.; Cohen, P.L. Monocyte and plasma expression of TAM ligand and receptor in renal failure: Links to unregulated immunity and chronic inflammation. Clin. Immunol. 2015, 158, 231–241.
- Anderson, K.J.; Cormier, R.T.; Scott, P.M. Role of ion channels in gastrointestinal cancer. World J. Gastroenterol. 2019, 25, 5732–5772.
- Zhang, Y.; Feng, Y.; Chen, L.; Zhu, J. Effects of Intermediate-Conductance Ca(2+)-Activated K(+) Channels on Human Endometrial Carcinoma Cells. Cell Biochem. Biophys. 2015, 72, 515–525.
- Pardo, L.A.; Stühmer, W. The roles of K(+) channels in cancer. Nat. Rev. Cancer 2014, 14, 39–48.
- Luján, R.; Aguado, C. Localization and Targeting of GIRK Channels in Mammalian Central Neurons. Int. Rev. Neurobiol. 2015, 123, 161–200.
- Bennett, E.S.; Smith, B.A.; Harper, J.M. Voltage-gated Na+ channels confer invasive properties on human prostate cancer cells. Pflugers Arch. 2004, 447, 908–914.
- Cox, D.H. Ca(2+)-regulated ion channels. BMB Rep. 2011, 44, 635–646.
- Xie, J.; Pan, H.; Yao, J.; Zhou, Y.; Han, W. SOCE and cancer: Recent progress and new perspectives. Int. J. Cancer 2016, 138, 2067–2077.
- Aperia, A.; Akkuratov, E.E.; Fontana, J.M.; Brismar, H. Na+-K+-ATPase, a new class of plasma membrane receptors. Am. J. Physiol. Cell Physiol. 2016, 310, C491–C495.
- Krawczyk, M.A.; Kunc, M.; Styczewska, M.; Gabrych, A.; Karpinsky, G.; Izycka-Swieszewska, E.; Bien, E. High Expression of Solute Carrier Family 2 Member 1 (SLC2A1) in Cancer Cells Is an Independent Unfavorable Prognostic Factor in Pediatric Malignant Peripheral Nerve Sheath Tumor. Diagnostics 2021, 11, 598.
- Di Vito, L.; Licchetta, L.; Pippucci, T.; Baldassari, S.; Stipa, C.; Mostacci, B.; Alvisi, L.; Tinuper, P.; Bisulli, F. Phenotype variability of GLUT1 deficiency syndrome: Description of a case series with novel SLC2A1 gene mutations. Epilepsy Behav. 2018, 79, 169–173.
- Raja, M.; Kinne, R. Mechanistic Insights into Protein Stability and Self-aggregation in GLUT1 Genetic Variants Causing GLUT1-Deficiency Syndrome. J. Membr. Biol. 2020, 253, 87–99.
- Sahota, A.; Tischfield, J.A.; Goldfarb, D.S.; Ward, M.D.; Hu, L. Cystinuria: Genetic aspects, mouse models, and a new approach to therapy. Urolithiasis 2019, 47, 57–66.
- King, L.S.; Yasui, M.; Agre, P. Aquaporins in health and disease. Mol. Med. Today 2000, 6, 60–65.
- He, J.; Yang, B. Aquaporins in Renal Diseases. Int J. Mol. Sci. 2019, 20, 366.
- Theodoulou, F.L.; Kerr, I.D. ABC transporter research: Going strong 40 years on. Biochem. Soc. Trans. 2015, 43, 1033–1040.
- Silverton, L.; Dean, M.; Moitra, K. Variation and evolution of the ABC transporter genes ABCB1, ABCC1, ABCG2, ABCG5 and ABCG8: Implication for pharmacogenetics and disease. Drug Metabol. Drug Interact. 2011, 26, 169–179.
- Ansermot, N.; Rebsamen, M.; Chabert, J.; Fathi, M.; Gex-Fabry, M.; Daali, Y.; Besson, M.; Rossier, M.; Rudaz, S.; Hochstrasser, D.; et al. Influence of ABCB1 gene polymorphisms and P-glycoprotein activity on cyclosporine pharmacokinetics in peripheral blood mononuclear cells in healthy volunteers. Drug Metab. Lett. 2008, 2, 76–82.
- Pajic, M.; Norris, M.D.; Cohn, S.L.; Haber, M. The role of the multidrug resistance-associated protein 1 gene in neuroblastoma biology and clinical outcome. Cancer Lett. 2005, 228, 241–246.
- Lu, J.F.; Pokharel, D.; Bebawy, M. MRP1 and its role in anticancer drug resistance. Drug Metab Rev. 2015, 47, 406–419.
- Ninčević, V.; Omanović, K.T.; Roguljić, H.; Kizivat, T.; Smolić, M.; Bilić, Ć.I. Renal Benefits of SGLT 2 Inhibitors and GLP-1 Receptor Agonists: Evidence Supporting a Paradigm Shift in the Medical Management of Type 2 Diabetes. Int. J. Mol. Sci. 2019, 20, 5831.
- Jonker, J.W.; Smit, J.W.; Brinkhuis, R.F.; Maliepaard, M.; Beijnen, J.H.; Schellens, J.H.; Schinkel, A.H. Role of breast cancer resistance protein in the bioavailability and fetal penetration of topotecan. J. Natl. Cancer Inst. 2000, 92, 1651–1656.
- Bruner, K.M.; Cohn, L.B. HIV-1 reservoir dynamics in CD4+ T cells. Curr. Opin. HIV AIDS 2019, 14, 108–114.
- Cao, Z.; Li, J.; Chen, H.; Song, C.; Shen, Z.; Zhou, X.; Lan, G.; Zhu, Q.; Liang, S.; Xing, H.; et al. Effects of HIV-1 genotype on baseline CD4+ cell count and mortality before and after antiretroviral therapy. Sci. Rep. 2020, 10, 15875.
- Barnes, C.O.; Jette, C.A.; Abernathy, M.E.; Dam, K.A.; Esswein, S.R.; Gristick, H.B.; Malyutin, A.G.; Sharaf, N.G.; Huey-Tubman, K.E.; Lee, Y.E.; et al. SARS-CoV-2 neutralizing antibody structures inform therapeutic strategies. Nature 2020, 588, 682–687.
More
Information
Subjects:
Cell Biology
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
3.3K
Entry Collection:
Tight Junction and Its Proteins
Revisions:
2 times
(View History)
Update Date:
04 Nov 2021
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




