Cell membranes, including membrane carrier proteins, membrane channel proteins and ATP drive pumps, are the main transporters. Membrane transporters have wide, but specific tissue distributions. They can impact on multiple endogenous and xenobiotic processes. Transport proteins constitute approximately 10% of most proteomes and play vital roles in the translocation of solutes across the membranes of all organisms. The receptor proteins on the cell membrane are also important structures involved in substrate transport and signal communication. The obstacles of cell transport-related proteins directly lead to the lack or excess of certain substances in cells.
- membrane transporters
- target site recognition
- targeted drug design
- treatment of disease
1. Membrane Transporters
1.1. Carrier Proteins
| Carrier Protein | Typical Positioning | Energy | Function | References |
|---|---|---|---|---|
| Na+-Glucose Pump | The apical plasma membrane of intestinal and renal cells | Na+ | Glucose active transport | [2] |
| Na+-K+ Pump | The plasma membrane of most animal cells | ATP | Na+ active pumping and K+ active pumping | [3][4] |
| Na +-H + Pump | The plasma membrane of animal cells | ATP | H+ active pumping | [5][6] |
| Na+ dependent neutral amino acid transporter | Absorbent epithelial cells | ATP | Amino acid pumping and downstream signal regulation of amino acid receptors | [7][8] |
| Na+ depends on a centralized carrier | Absorbent epithelial cells | ATP | Active transport of nucleosides | [9] |
| Glucose Carrier | The plasma membrane of most animal cells | — | Passive transport of glucose | [10] |
1.2. Channel Proteins
| Species | Distribution | Somatotype | Function | References |
|---|---|---|---|---|
| Aquaporins | Brain; membranes; kidneys; testis; liver; nasopharynx; lungs; intestines; eyes; etc. | AQP0; AQP1; AQP2; AQP3; AQP4; AQP5; AQP6; AQP7; AQP8; AQP9; AQP10; AQP11; AQP12 | Formation of various body fluids, reabsorption of water by tissues | [13][14] |
| Channel Protein | Chondriosome | MPTP | Apoptosis and necrosis | [15] |
| Ion Channel Protein | Various organizations | HCN; Slack; KcsA; TRPV; TRPM family; PKD1/2; PIEZO1/2; ENaC; TPCs; VDAC; SLC family; ASICs |
Signal transduction, excitement transfer, substance synthesis, energy metabolism, osmotic pressure balance, nutrition induction, substance transport | [16][17] |
1.3. ATP-Driven Pumps
| Kind | Constitute | Distribution | Function | References |
|---|---|---|---|---|
| P type ion Pump | 2α subunits (transport), 2β subunits (regulatory) |
Plasma membrane; endoplasmic reticulum | Na+, K+, H+ and Ca2+are transported across membranes | [22] |
| Type F ion Pump | Multiple subunits, transmembrane domain F0 and cytoplasmic domain F1 | Mitochondrial inner membrane | ATP synthesis | [23] |
| V type ion Pump | Multiple subunits, transmembrane domain V0 and cytoplasmic domain V1 | Intracellular bodies; lysosomal membranes; osteoclasts | H+ transport | [24] |
| ABC Transporter Superfamily | Two transmembrane domains, two intracellular ATP-binding domains | All kinds of organisms | Amino acids, sugars, lipids, peptides, protein transport, macromolecule transport | [25][26] |
1.4. Functions of Membrane Transporters
1.4.1. Function of Carrier Protein
1.4.2. Function of Channel Proteins
2. Membrane Receptor Proteins
2.1. Types of Membrane Receptor Proteins
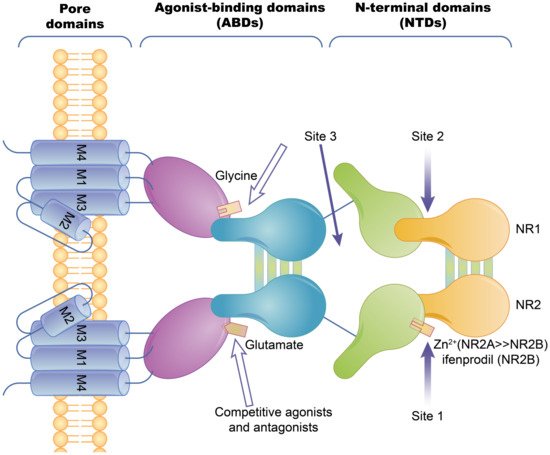
2.1.1. Ion Channel-Coupled Receptors
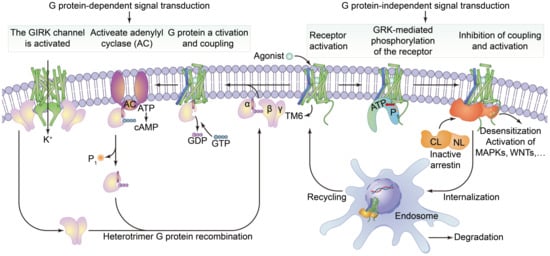
2.1.2. G Protein-Coupled Receptors
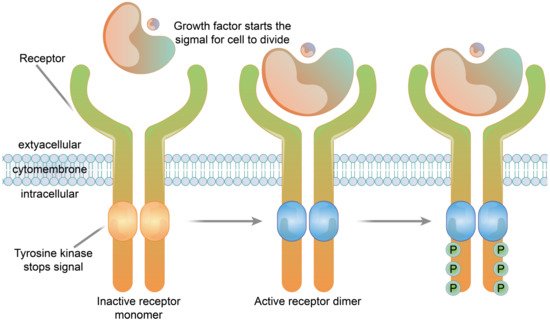
2.1.3. Enzyme-Linked Receptors
2.2. Function of Membrane Receptor Proteins
2.2.1. Ion Channel Receptor Functions
2.2.2. Function of G Protein-Coupled Receptors
2.2.3. Enzyme-Linked Receptor Functions
3. Association between Membrane Proteins and Disease
3.1. Abnormal Ion Channels Induce Cancer
3.2. Substrate Transport Disorders, Induced Metabolic Disorders of the Type of Disease
3.3. Membrane Receptors and Pathogen Invasion
This entry is adapted from the peer-reviewed paper 10.3390/membranes11100736
References
- Taru, S.G.; Nath, A.; Prasad, S.; Singhal, S.; Chandra, V.; Saikumar, G. Expression pattern of GLUT 1, 5, 8 and citrate synthase transcripts in buffalo (Bubalus bubalis) preimplantation embryos produced in vitro and derived in vivo. Reprod. Domest. Anim. 2020, 55, 1362–1370.
- Arow, M.; Waldman, M.; Yadin, D.; Nudelman, V.; Shainberg, A.; Abraham, N.G.; Freimark, D.; Kornowski, R.; Aravot, D.; Hochhauser, E.; et al. Sodium-glucose cotransporter 2 inhibitor Dapagliflozin attenuates diabetic cardiomyopathy. Cardiovasc. Diabetol. 2020, 19, 7.
- Felippe, G.C.; Ribeiro, S.A.; Ignácio, D.S.C.; Caire, C.H.; Burth, P. Na/K Pump and Beyond: Na/K-ATPase as a Modulator of Apoptosis and Autophagy. Molecules 2017, 22, 578.
- Askari, A. The other functions of the sodium pump. Cell Calcium 2019, 84, 102105.
- Packer, M. Activation and Inhibition of Sodium-Hydrogen Exchanger Is a Mechanism That Links the Pathophysiology and Treatment of Diabetes Mellitus with That of Heart Failure. Circulation 2017, 136, 1548–1559.
- Conrad, K.P. Might proton pump or sodium-hydrogen exchanger inhibitors be of value to ameliorate SARs-CoV-2 pathophysiology? Physiol. Rep 2021, 8, e14649.
- Magi, S.; Piccirillo, S.; Amoroso, S.; Lariccia, V. Excitatory Amino Acid Transporters (EAATs): Glutamate Transport and Beyond. Int. J. Mol. Sci. 2019, 20, 5674.
- Singh, S.; Arthur, S.; Sundaram, U. Mechanisms of Regulation of Transporters of Amino Acid Absorption in Inflammatory Bowel Diseases. Compr. Physiol. 2020, 10, 673–686.
- Takenaka, R.; Yasujima, T.; Furukawa, J.; Hishikawa, Y.; Yamashiro, T.; Ohta, K.; Inoue, K.; Yuasa, H. Functional Analysis of the Role of Equilibrative Nucleobase Transporter 1 (ENBT1/SLC43A3) in Adenine Transport in HepG2 Cells. J. Pharm. Sci. 2020, 109, 2622–2628.
- Yan, N. A Glimpse of Membrane Transport through Structures-Advances in the Structural Biology of the GLUT Glucose Transporters. J. Mol. Biol. 2017, 429, 2710–2725.
- Cosme, D.; Estevinho, M.M.; Rieder, F.; Magro, F. Potassium channels in intestinal epithelial cells and their pharmacological modulation: A systematic review. Am. J. Physiol. Cell Physiol. 2021, 320, C520–C546.
- Cheng, Y.R.; Jiang, B.Y.; Chen, C.C. Acid-sensing ion channels: Dual function proteins for chemo-sensing and mechano-sensing. J. Biomed. Sci. 2018, 25, 46.
- Li, C.; Wang, W. Molecular Biology of Aquaporins. Adv. Exp. Med. Biol. 2017, 969, 1–34.
- Dajani, S.; Saripalli, A.; Sharma-Walia, N. Water transport proteins-aquaporins (AQPs) in cancer biology. Oncotarget 2018, 9, 36392–36405.
- Bauer, T.M.; Murphy, E. Role of Mitochondrial Calcium and the Permeability Transition Pore in Regulating Cell Death. Circ. Res. 2020, 126, 280–293.
- Rivolta, I.; Binda, A.; Masi, A.; DiFrancesco, J.C. Cardiac and neuronal HCN channelopathies. Pflugers Arch. 2020, 472, 931–951.
- Ali, S.R.; Malone, T.J.; Zhang, Y.; Prechova, M.; Kaczmarek, L.K. Phactr1 regulates Slack (KCNT1) channels via protein phosphatase 1 (PP1). FASEB J. 2020, 34, 1591–1601.
- Schwiebert, E.M. ABC transporter-facilitated ATP conductive transport. Am. J. Physiol. 1999, 276, C1–C8.
- Hejzlarová, K.; Mráček, T.; Vrbacký, M.; Kaplanová, V.; Karbanová, V.; Nůsková, H.; Pecina, P.; Houštěk, J. Nuclear genetic defects of mitochondrial ATP synthase. Physiol. Res. 2014, 63, S57–S71.
- He, G.; Tian, W.; Qin, L.; Meng, L.; Wu, D.; Huang, Y.; Li, D.; Zhao, D.; He, T. Identification of novel heavy metal detoxification proteins in Solanum tuberosum: Insights to improve food security protection from metal ion stress. Sci. Total Environ. 2021, 779, 146197.
- Wu, C.; Chakrabarty, S.; Jin, M.; Liu, K.; Xiao, Y. Insect ATP-Binding Cassette (ABC) Transporters: Roles in Xenobiotic Detoxification and Bt Insecticidal Activity. Int. J. Mol. Sci. 2019, 20, 2829.
- El, R.N.; Lima, J.J.; Johnson, J.A. Proton pump inhibitors: From CYP2C19 pharmacogenetics to precision medicine. Expert Opin. Drug Metab. Toxicol. 2018, 14, 447–460.
- Anandakrishnan, R.; Zuckerman, D.M. Biophysical comparison of ATP-driven proton pumping mechanisms suggests a kinetic advantage for the rotary process depending on coupling ratio. PLoS ONE 2017, 12, e173500.
- Ueno, H.; Suzuki, K.; Murata, T. Structure and dynamics of rotary V(1) motor. Cell Mol. Life Sci. 2018, 75, 1789–1802.
- Rempel, S.; Gati, C.; Nijland, M.; Thangaratnarajah, C.; Karyolaimos, A.; de Gier, J.W.; Guskov, A.; Slotboom, D.J. A mycobacterial ABC transporter mediates the uptake of hydrophilic compounds. Nature 2020, 580, 409–412.
- Amawi, H.; Sim, H.M.; Tiwari, A.K.; Ambudkar, S.V.; Shukla, S. ABC Transporter-Mediated Multidrug-Resistant Cancer. Adv. Exp. Med. Biol. 2019, 1141, 549–580.
- Seow, H.F.; Bröer, S.; Bröer, A.; Bailey, C.G.; Potter, S.J.; Cavanaugh, J.A.; Rasko, J.E. Hartnup disorder is caused by mutations in the gene encoding the neutral amino acid transporter SLC6A19. Nat. Genet. 2004, 36, 1003–1007.
- Fotiadis, D.; Kanai, Y.; Palacín, M. The SLC3 and SLC7 families of amino acid transporters. Mol. Aspects Med. 2013, 34, 139–158.
- Danne, T.; Garg, S.; Peters, A.L.; Buse, J.B.; Mathieu, C.; Pettus, J.H.; Alexander, C.M.; Battelino, T.; Ampudia-Blasco, F.J.; Bode, B.W.; et al. International Consensus on Risk Management of Diabetic Ketoacidosis in Patients with Type 1 Diabetes Treated with Sodium-Glucose Cotransporter (SGLT) Inhibitors. Diabetes Care 2019, 42, 1147–1154.
- Denker, B.M.; Smith, B.L.; Kuhajda, F.P.; Agre, P. Identification, purification, and partial characterization of a novel Mr 28,000 integral membrane protein from erythrocytes and renal tubules. J. Biol. Chem. 1988, 263, 15634–15642.
- Camilleri, M.; Carlson, P.; Chedid, V.; Vijayvargiya, P.; Burton, D.; Busciglio, I. Aquaporin Expression in Colonic Mucosal Biopsies From Irritable Bowel Syndrome with Diarrhea. Clin. Transl. Gastroenterol. 2019, 10, e19.
- Verkman, A.S.; Hara-Chikuma, M.; Papadopoulos, M.C. Aquaporins--new players in cancer biology. J. Mol. Med. 2008, 86, 523–529.
- Mussina, K.; Toktarkhanova, D.; Filchakova, O. Nicotinic Acetylcholine Receptors of PC12 Cells. Cell Mol. Neurobiol. 2021, 41, 17–29.
- Vibholm, A.K.; Landau, A.M.; Møller, A.; Jacobsen, J.; Vang, K.; Munk, O.L.; Orlowski, D.; Sørensen, J.C.; Brooks, D.J. NMDA receptor ion channel activation detected in vivo with GE-179 PET after electrical stimulation of rat hippocampus. J. Cereb. Blood Flow Metab. 2021, 41, 1301–1312.
- Paoletti, P.; Neyton, J. NMDA receptor subunits: Function and pharmacology. Curr. Opin. Pharmacol. 2007, 7, 39–47.
- Kofuji, P.; Araque, A. G-Protein-Coupled Receptors in Astrocyte-Neuron Communication. Neuroscience 2021, 456, 71–84.
- Duan, Y.; Haybaeck, J.; Yang, Z. Therapeutic Potential of PI3K/AKT/mTOR Pathway in Gastrointestinal Stromal Tumors: Rationale and Progress. Cancers 2020, 12, 2972.
- Lv, Y.; Wang, X.; Li, X.; Xu, G.; Bai, Y.; Wu, J.; Piao, Y.; Shi, Y.; Xiang, R.; Wang, L. Nucleotide de novo synthesis increases breast cancer stemness and metastasis via cGMP-PKG-MAPK signaling pathway. PLoS Biol. 2020, 18, e3000872.
- Hilger, D.; Masureel, M.; Kobilka, B.K. Structure and dynamics of GPCR signaling complexes. Nat. Struct. Mol. Biol. 2018, 25, 4–12.
- Weis, W.I.; Kobilka, B.K. The Molecular Basis of G Protein-Coupled Receptor Activation. Annu. Rev. Biochem. 2018, 87, 897–919.
- Spiess, K.; Fares, S.; Sparre-Ulrich, A.H.; Hilgenberg, E.; Jarvis, M.A.; Ehlers, B.; Rosenkilde, M.M. Identification and functional comparison of seven-transmembrane G-protein-coupled BILF1 receptors in recently discovered nonhuman primate lymphocryptoviruses. J. Virol. 2015, 89, 2253–2267.
- Holdorf, A.D.; Green, J.M.; Levin, S.D.; Denny, M.F.; Straus, D.B.; Link, V.; Changelian, P.S.; Allen, P.M.; Shaw, A.S. Proline residues in CD28 and the Src homology (SH)3 domain of Lck are required for T cell costimulation. J. Exp. Med. 1999, 190, 375–384.
- Pandey, S.; Zhang, W.; Assmann, S.M. Roles of ion channels and transporters in guard cell signal transduction. FEBS Lett. 2007, 581, 2325–2336.
- Venkatakrishnan, A.J.; Deupi, X.; Lebon, G.; Tate, C.G.; Schertler, G.F.; Babu, M.M. Molecular signatures of G-protein-coupled receptors. Nature 2013, 494, 185–194.
- Katritch, V.; Cherezov, V.; Stevens, R.C. Structure-function of the G protein-coupled receptor superfamily. Annu. Rev. Pharmacol. Toxicol. 2013, 53, 531–556.
- Molnár, E. Cell-Based Enzyme-Linked Immunosorbent Assay (Cell-ELISA) Analysis of Native and Recombinant Glutamate Receptors. Methods Mol. Biol. 2019, 1941, 47–54.
- Morishima, M.; Tahara, S.; Wang, Y.; Ono, K. Oxytocin Downregulates the Ca(V)1.2 L-Type Ca(2+) Channel via Gi/cAMP/PKA/CREB Signaling Pathway in Cardiomyocytes. Membranes 2021, 11, 234.
- Ji, R.; Meng, L.; Li, Q.; Lu, Q. TAM receptor deficiency affects adult hippocampal neurogenesis. Metab. Brain Dis. 2015, 30, 633–644.
- Lee, I.J.; Hilliard, B.A.; Ulas, M.; Yu, D.; Vangala, C.; Rao, S.; Lee, J.; Gadegbeku, C.A.; Cohen, P.L. Monocyte and plasma expression of TAM ligand and receptor in renal failure: Links to unregulated immunity and chronic inflammation. Clin. Immunol. 2015, 158, 231–241.
- Anderson, K.J.; Cormier, R.T.; Scott, P.M. Role of ion channels in gastrointestinal cancer. World J. Gastroenterol. 2019, 25, 5732–5772.
- Zhang, Y.; Feng, Y.; Chen, L.; Zhu, J. Effects of Intermediate-Conductance Ca(2+)-Activated K(+) Channels on Human Endometrial Carcinoma Cells. Cell Biochem. Biophys. 2015, 72, 515–525.
- Pardo, L.A.; Stühmer, W. The roles of K(+) channels in cancer. Nat. Rev. Cancer 2014, 14, 39–48.
- Luján, R.; Aguado, C. Localization and Targeting of GIRK Channels in Mammalian Central Neurons. Int. Rev. Neurobiol. 2015, 123, 161–200.
- Bennett, E.S.; Smith, B.A.; Harper, J.M. Voltage-gated Na+ channels confer invasive properties on human prostate cancer cells. Pflugers Arch. 2004, 447, 908–914.
- Cox, D.H. Ca(2+)-regulated ion channels. BMB Rep. 2011, 44, 635–646.
- Xie, J.; Pan, H.; Yao, J.; Zhou, Y.; Han, W. SOCE and cancer: Recent progress and new perspectives. Int. J. Cancer 2016, 138, 2067–2077.
- Aperia, A.; Akkuratov, E.E.; Fontana, J.M.; Brismar, H. Na+-K+-ATPase, a new class of plasma membrane receptors. Am. J. Physiol. Cell Physiol. 2016, 310, C491–C495.
- Krawczyk, M.A.; Kunc, M.; Styczewska, M.; Gabrych, A.; Karpinsky, G.; Izycka-Swieszewska, E.; Bien, E. High Expression of Solute Carrier Family 2 Member 1 (SLC2A1) in Cancer Cells Is an Independent Unfavorable Prognostic Factor in Pediatric Malignant Peripheral Nerve Sheath Tumor. Diagnostics 2021, 11, 598.
- Di Vito, L.; Licchetta, L.; Pippucci, T.; Baldassari, S.; Stipa, C.; Mostacci, B.; Alvisi, L.; Tinuper, P.; Bisulli, F. Phenotype variability of GLUT1 deficiency syndrome: Description of a case series with novel SLC2A1 gene mutations. Epilepsy Behav. 2018, 79, 169–173.
- Raja, M.; Kinne, R. Mechanistic Insights into Protein Stability and Self-aggregation in GLUT1 Genetic Variants Causing GLUT1-Deficiency Syndrome. J. Membr. Biol. 2020, 253, 87–99.
- Sahota, A.; Tischfield, J.A.; Goldfarb, D.S.; Ward, M.D.; Hu, L. Cystinuria: Genetic aspects, mouse models, and a new approach to therapy. Urolithiasis 2019, 47, 57–66.
- King, L.S.; Yasui, M.; Agre, P. Aquaporins in health and disease. Mol. Med. Today 2000, 6, 60–65.
- He, J.; Yang, B. Aquaporins in Renal Diseases. Int J. Mol. Sci. 2019, 20, 366.
- Theodoulou, F.L.; Kerr, I.D. ABC transporter research: Going strong 40 years on. Biochem. Soc. Trans. 2015, 43, 1033–1040.
- Silverton, L.; Dean, M.; Moitra, K. Variation and evolution of the ABC transporter genes ABCB1, ABCC1, ABCG2, ABCG5 and ABCG8: Implication for pharmacogenetics and disease. Drug Metabol. Drug Interact. 2011, 26, 169–179.
- Ansermot, N.; Rebsamen, M.; Chabert, J.; Fathi, M.; Gex-Fabry, M.; Daali, Y.; Besson, M.; Rossier, M.; Rudaz, S.; Hochstrasser, D.; et al. Influence of ABCB1 gene polymorphisms and P-glycoprotein activity on cyclosporine pharmacokinetics in peripheral blood mononuclear cells in healthy volunteers. Drug Metab. Lett. 2008, 2, 76–82.
- Pajic, M.; Norris, M.D.; Cohn, S.L.; Haber, M. The role of the multidrug resistance-associated protein 1 gene in neuroblastoma biology and clinical outcome. Cancer Lett. 2005, 228, 241–246.
- Lu, J.F.; Pokharel, D.; Bebawy, M. MRP1 and its role in anticancer drug resistance. Drug Metab Rev. 2015, 47, 406–419.
- Ninčević, V.; Omanović, K.T.; Roguljić, H.; Kizivat, T.; Smolić, M.; Bilić, Ć.I. Renal Benefits of SGLT 2 Inhibitors and GLP-1 Receptor Agonists: Evidence Supporting a Paradigm Shift in the Medical Management of Type 2 Diabetes. Int. J. Mol. Sci. 2019, 20, 5831.
- Jonker, J.W.; Smit, J.W.; Brinkhuis, R.F.; Maliepaard, M.; Beijnen, J.H.; Schellens, J.H.; Schinkel, A.H. Role of breast cancer resistance protein in the bioavailability and fetal penetration of topotecan. J. Natl. Cancer Inst. 2000, 92, 1651–1656.
- Bruner, K.M.; Cohn, L.B. HIV-1 reservoir dynamics in CD4+ T cells. Curr. Opin. HIV AIDS 2019, 14, 108–114.
- Cao, Z.; Li, J.; Chen, H.; Song, C.; Shen, Z.; Zhou, X.; Lan, G.; Zhu, Q.; Liang, S.; Xing, H.; et al. Effects of HIV-1 genotype on baseline CD4+ cell count and mortality before and after antiretroviral therapy. Sci. Rep. 2020, 10, 15875.
- Barnes, C.O.; Jette, C.A.; Abernathy, M.E.; Dam, K.A.; Esswein, S.R.; Gristick, H.B.; Malyutin, A.G.; Sharaf, N.G.; Huey-Tubman, K.E.; Lee, Y.E.; et al. SARS-CoV-2 neutralizing antibody structures inform therapeutic strategies. Nature 2020, 588, 682–687.
