
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Ali Mirzaei | + 5595 word(s) | 5595 | 2021-10-14 04:44:59 | | | |
| 2 | Mehrdad Shahbaz | -1 word(s) | 5594 | 2021-10-26 13:42:23 | | | | |
| 3 | Camila Xu | Meta information modification | 5594 | 2021-10-27 03:03:20 | | |
Video Upload Options
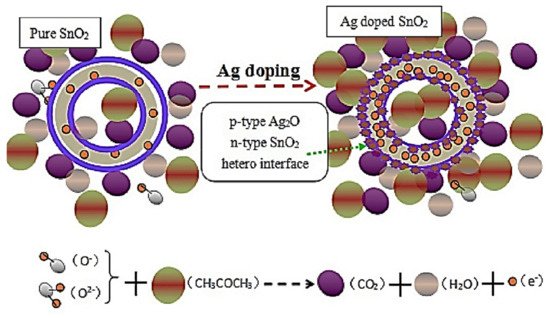
Ag is one of the cheapest noble metals which is extensively used in the decoration or doping of semiconducting metal oxides (SMOs) to boost the overall gas-sensing performances of nanostructured SMOs. This is due to the electronic and chemical sensitization of Ag nanoparticles.
1. Ag-Decorated/Loaded Acetone (CH3COCH3) Gas Sensors
2. Ag-Decorated/Loaded Chlorine (Cl2) Gas Sensors
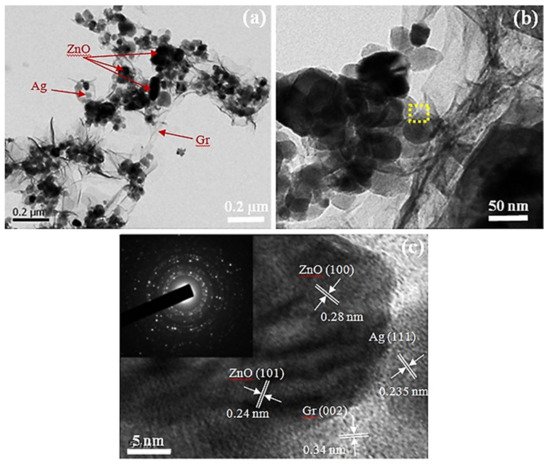
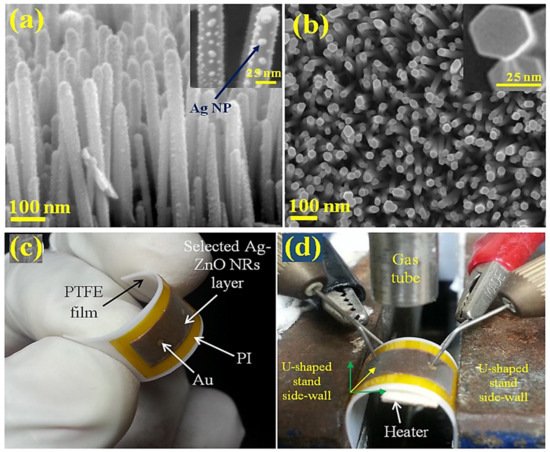
3. Ag-Decorated/Loaded Acetylene (C2H2) Gas Sensors
4. Ag-Decorated/Loaded Triethylamine (TEA) Gas Sensors
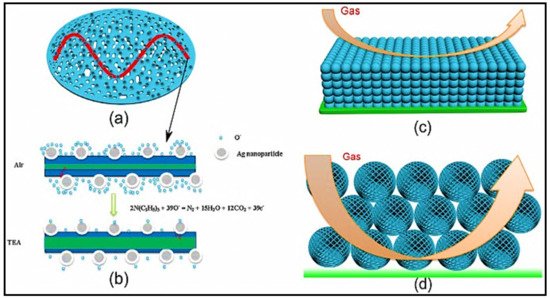
5. Ag-Decorated/Loaded Formaldehyde (HCHO) Gas Sensors
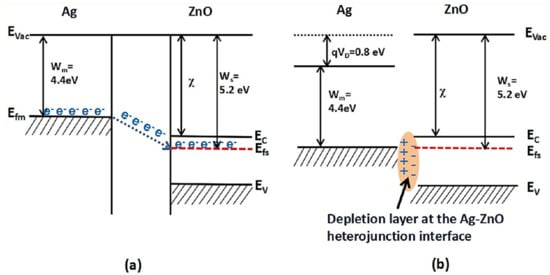
6. Ag-Decorated/Loaded Carbon Monoxide (CO) Gas Sensors
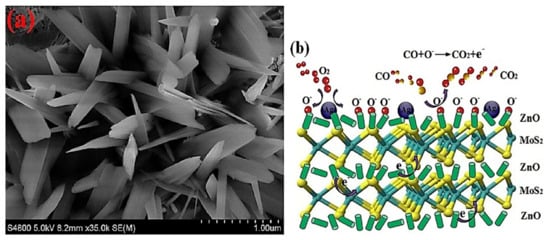
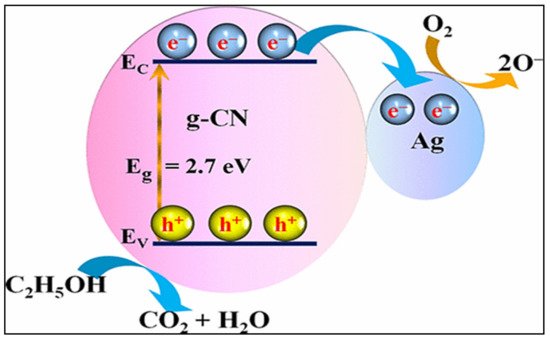
7. Ag-Decorated/Loaded Ethanol (C2H5OH) Gas Sensors
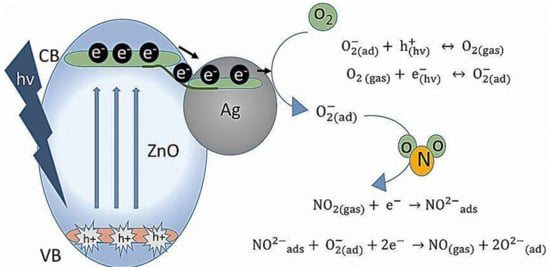
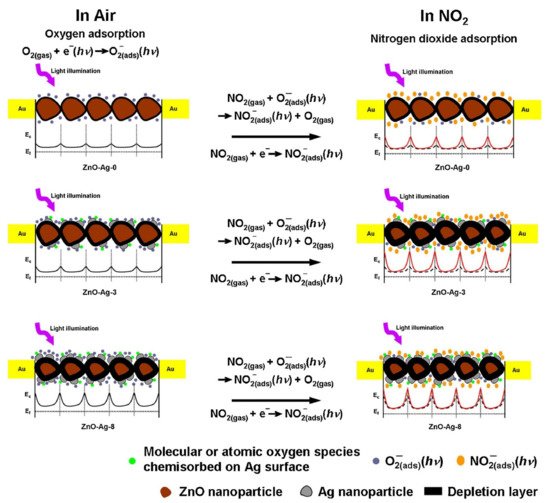
8. Ag-Decorated/Loaded Nitrogen Dioxide (NO2) Gas Sensors
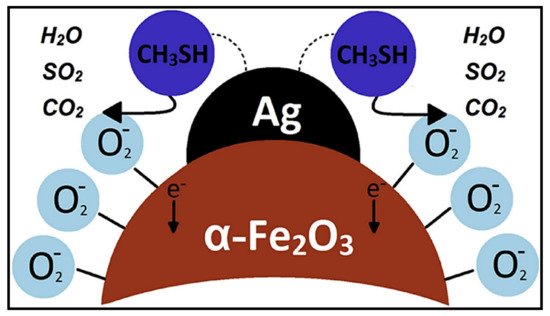
9. Ag-Decorated/Loaded Methyl Mercaptan (CH3SH) Gas Sensors
10. Conclusions
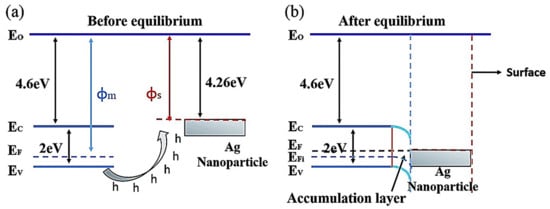
The gas-sensing performances of chemiresistive sensors, such as SMOs and related composites, can be enhanced by Ag doping owing to the catalytic activity and electronic/chemical sensitization effects of Ag. As an effective catalyst, Ag can attract abundant O2 molecules from the air and transfer them to the surfaces of SMOs, accordingly promoting the capture of electrons from SMOs by O2 molecules. Moreover, the higher electrical conductivity of Ag NPs facilitated rapid electron transfer and thereby improved the sensor response. Therefore, Ag may be an excellent choice as a sensitizer to improve the sensing performances of chemiresistive sensors as it offers additional active adsorption sites and charge transfer pathways to enhance surface reactions. Generally, the introduction of Ag in an optimal amount into the sensing material can lead to the best sensing properties, and a bell-shaped relationship typically exists between the sensor response and the addition amount of Ag. Due to the low cost of Ag than that of other noble metals, the incorporation of Ag into gas sensors is a highly promising strategy to not only reduce the overall price of these sensors but also enhance their sensing properties.
References
- Amiri, V.; Roshan, H.; Mirzaei, A.; Neri, G.; Ayesh, A.I. Nanostructured metal oxide-based acetone gas sensors: A review. Sensors 2020, 20, 3096.
- Xu, X.; Chen, Y.; Zhang, G.; Ma, S.; Lu, Y.; Bian, H.; Chen, Q. Highly sensitive VOCs-acetone sensor based on Ag-decorated SnO2 hollow nanofibers. J. Alloy. Compd. 2017, 703, 572–579.
- Li, M.; Zhu, H.; Wang, B.; Cheng, J.; Yan, W.; Xia, S.; Tang, Z. Ultrasensitive and highly selective detection of methoxy propanol based on Ag-decorated SnO2 hollow nanospheres. Sens. Actuators B Chem. 2016, 232, 545–556.
- Korotcenkov, G. Gas response control through structural and chemical modification of metal oxide films: State of the art and approaches. Sens. Actuators B Chem. 2005, 107, 209–232.
- Kılıç, A.; Alev, O.; Özdemir, O.; Arslan, L.Ç.; Büyükköse, S.; Öztürk, Z.Z. The effect of Ag loading on gas sensor properties of TiO2 nanorods. Thin Solid Film. 2021, 726, 138662.
- Li, Q.; Zhang, W.; Wang, C.; Ma, J.; Ning, L.; Fan, H. Ag modified bismuth ferrite nanospheres as a chlorine gas sensor. RSC Adv. 2018, 8, 33156–33163.
- Abideen, Z.U.; Kim, J.H.; Lee, J.H.; Kim, J.Y.; Mirzaei, A.; Kim, H.W.; Kim, S.S. Electrospun metal oxide composite nanofibers gas sensors: A review. J. Korean Ceram. Soc. 2017, 54, 366–379.
- Iftekhar Uddin, A.S.M.; Phan, D.-T.; Chung, G.-S. Low temperature acetylene gas sensor based on Ag nanoparticles-loaded ZnO-reduced graphene oxide hybrid. Sens. Actuators B Chem. 2015, 207, 362–369.
- Lee, K.-W.; Uddin, A.S.M.I.; Phan, D.-T.; Chung, G.-S. Fabrication of low-temperature acetylene gas sensor based on Ag nanoparticles-loaded hierarchical ZnO nanostructures. Electron. Lett. 2014, 51, 572–574.
- Gupta Chatterjee, S.; Chatterjee, S.; Ray, A.K.; Chakraborty, A.K. Graphene–metal oxide nanohybrids for toxic gas sensor: A review. Sens. Actuators B Chem. 2015, 221, 1170–1181.
- Uddin, A.S.M.I.; Yaqoob, U.; Phan, D.-T.; Chung, G.-S. A novel flexible acetylene gas sensor based on PI/PTFE-supported Ag-loaded vertical ZnO nanorods array. Sens. Actuators B Chem. 2015, 222, 536–543.
- Espinosa, E.H.; Ionescu, R.; Bittencourt, C.; Felten, A.; Erni, R.; Van Tendeloo, G.; Pireaux, J.J.; Llobet, E. Metal-decorated multi-wall carbon nanotubes for low temperature gas sensing. Thin Solid Film. 2007, 515, 8322–8327.
- Mirzaei, A.; Leonardi, S.; Neri, G. Detection of hazardous volatile organic compounds (VOCs) by metal oxide nanostructures-based gas sensors: A review. Ceram. Int. 2016, 42, 15119–15141.
- Ju, D.X.; Xu, H.Y.; Qiu, Z.W.; Zhang, Z.C.; Xu, Q.; Zhang, J.; Wang, J.Q.; Cao, B.Q. Near room temperature, fast-response, and highly sensitive triethylamine sensor assembled with Au-loaded ZnO/SnO2 core-shell nanorods on flat alumina substrates. ACS Appl. Mater. Interfaces 2015, 7, 19163–19171.
- Mitsubayashi, K.; Kubotera, Y.; Yano, K.; Hashimoto, Y.; Kon, T.; Nakakura, S.; Nishi, Y.; Endo, H. Trimethylamine biosensor with flavin-containing monooxygenase type 3 (FMO3) for fish-freshness analysis. Sens. Actuators B Chem. 2004, 103, 463–467.
- Li, W.; Xu, H.; Yu, H.; Zhai, T.; Xu, Q.; Yang, X.; Wang, J.; Cao, B. Different morphologies of ZnO and their triethylamine sensing properties. J. Alloy. Compd. 2017, 706, 461–469.
- Shen, Z.; Zhang, X.; Mi, R.; Liu, M.; Chen, Y.; Chen, C.; Ruan, S. On the high response towards TEA of gas sensors based on Ag-loaded 3D porous ZnO microspheres. Sens. Actuators B Chem. 2018, 270, 492–499.
- Castro-Hurtado, I.; Mandayo, G.G.; Castaño, E. Conductometric formaldehyde gas sensors. A review: From conventional films to nanostructured materials. Thin Solid Film. 2013, 548, 665–676.
- Xiong, J.; Zhang, P.; Huang, S.; Zhang, Y. Comprehensive influence of environmental factors on the emission rate of formaldehyde and VOCs in building materials: Correlation development and exposure assessment. Environ. Res. 2016, 151, 734–741.
- Wang, J.; Yunus, R.; Li, J.; Li, P.; Zhang, P.; Kim, J. In situ synthesis of manganese oxides on polyester fiber for formaldehyde decomposition at room temperature. Appl. Surf. Sci. 2015, 357, 787–794.
- Dong, C.; Liu, X.; Han, B.; Deng, S.; Xiao, X.; Wang, Y. Nonaqueous synthesis of Ag-functionalized In2O3/ZnO nanocomposites for highly sensitive formaldehyde sensor. Sens. Actuators B Chem. 2016, 224, 193–200.
- Xing, X.; Xiao, X.; Wang, L.; Wang, Y. Highly sensitive formaldehyde gas sensor based on hierarchically porous Ag-loaded ZnO heterojunction nanocomposites. Sens. Actuators B Chem. 2017, 247, 797–806.
- Wang, S.; Xiao, B.; Yang, T.; Wang, P.; Xiao, C.; Li, Z.; Zhao, R.; Zhang, M. Enhanced HCHO gas sensing properties by Ag-loaded sunflower-like In2O3 hierarchical nanostructures. J. Mater. Chem. A 2014, 2, 6598–6604.
- Nakate, U.T.; Patil, P.; Na, S.-I.; Yu, Y.T.; Suh, E.-k.; Hahn, Y.-B. Fabrication and enhanced carbon monoxide gas sensing performance of p-CuO/n-TiO2 heterojunction device. Colloids Surf. A Physicochem. Eng. 2020, 612, 125962.
- Niakan, H.; Zhang, C.; Hu, Y.; Szpunar, J.A.; Yang, Q. Thermal stability of diamond-like carbon–MoS2 thin films in different environments. Thin Solid Film 2014, 562, 244–249.
- Zhang, D.; Sun, Y.E.; Jiang, C.; Yao, Y.; Wang, D.; Zhang, Y. Room-temperature highly sensitive CO gas sensor based on Ag-loaded zinc oxide/molybdenum disulfide ternary nanocomposite and its sensing properties. Sens. Actuators B Chem. 2017, 253, 1120–1128.
- Wang, Y.; Cui, Y.; Meng, X.; Zhang, Z.; Cao, J. A gas sensor based on Ag-modified ZnO flower-like microspheres: Temperature-modulated dual selectivity to CO and CH4. Surf. Interfaces 2021, 24, 101110.
- Mirzaei, A.; Janghorban, K.; Hashemi, B.; Bonyani, M.; Leonardi, S.G.; Neri, G. Highly stable and selective ethanol sensor based on α-Fe2O3 nanoparticles prepared by Pechini sol–gel method. Ceram. Inter. 2016, 42, 6136–6144.
- Tomer, V.K.; Malik, R.; Kailasam, K. Near-room-temperature ethanol detection using Ag-loaded mesoporous carbon nitrides. ACS Omega 2017, 2, 3658–3668.
- Zhang, Q.; Zang, P.; Hu, W.; Li, J.; Liu, Y.; Liu, Y.; Yu, F.; Zhang, C.; Xu, M. Performance degradation mechanism of the light-activated room temperature NO2 gas sensor based on Ag-ZnO nanoparticles. Appl. Surf. Sci. 2021, 541, 148418.
- Wang, Y.; Cui, X.; Yang, Q.; Liu, J.; Gao, Y.; Sun, P.; Lu, G. Preparation of Ag-loaded mesoporous WO3 and its enhanced NO2 sensing performance. Sens. Actuators B Chem. 2016, 225, 544–552.
- Espid, E.; Taghipour, F. Facile synthesis and UV-activated gas sensing performance of Ag: ZnO nano-ellipsoids. ECS J. Solid State Sci. Technol. 2018, 7, 3089.
- Zhang, Q.; Xie, G.; Xu, M.; Yu, S.; Tai, H.; Du, H.; Jiang, Y. Visible light-assisted room temperature gas sensing with ZnO-Ag heterostructure nanoparticles. Sens. Actuators B Chem. 2018, 259, 269–281.
- Garcia, D.; Picasso, G.; Hidalgo, P.; Peres, H.E.M.; Sun Kou, R.; Gonçalves, J.M. Sensors based on Ag-loaded hematite (α-Fe2O3) nanoparticles for methyl mercaptan detection at room temperature. Anal. Chem. Res. 2016, 12, 74–81.




