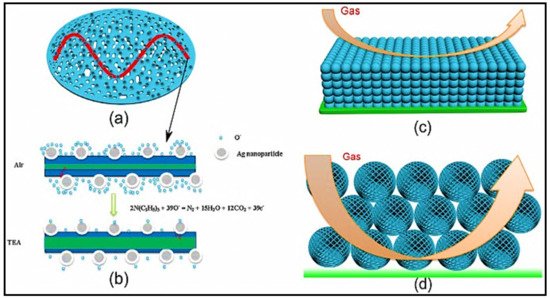
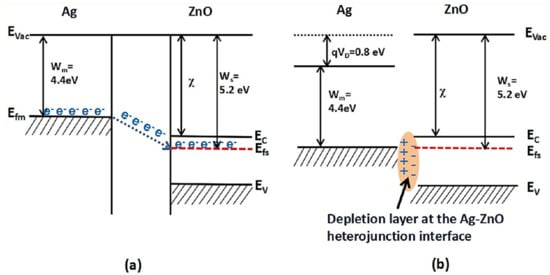
CH
3COCH
3 belongs to the family of volatile organic compounds (VOCs) that are employed as solvents in various industries. CH
3COCH
3 at higher levels may have a negative impact on the central nervous system and may be harmful to the eyes and nose. Therefore, the occupational threshold limit value for CH
3COCH
3 was set at 250 ppm considering a time-weighted average of 8 h [
47]. Accordingly, the development of sensitive CH
3COCH
3-sensing devices is required from the perspective of safety. In this regard, some attempts have been made to establish selective CH
3COCH
3 sensors by adding Ag into SMOs. For instance, Xu et al. [
48] described the synthesis of Ag-decorated SnO
2 hollow nanofibers (NFs) with large surface areas and their CH
3COCH
3 sensing properties. Typically, noble metals have higher electrical conductivities that enable the rapid transfer of electrons and catalysis of the oxidation of reducing gas molecules such as VOCs [
45,
46,
47,
48]. Thus, noble metal-incorporated sensing materials can be suitable candidates for detecting VOCs. The developed Ag-decorated SnO
2 sensor showed an excellent response and higher selectivity for CH
3COCH
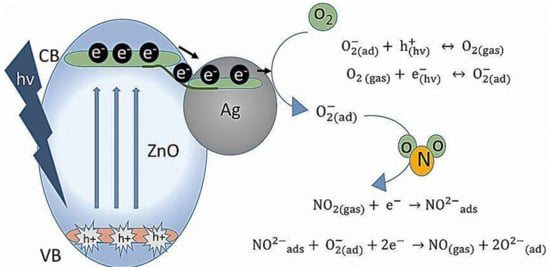
3 at 160 °C. A schematic of the sensing interactions between the CH
3COCH
3 gas molecules and pure and Ag-decorated SnO
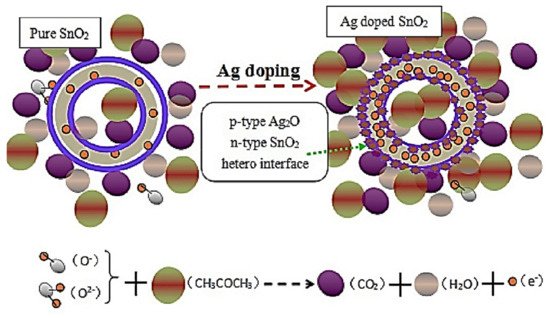
2 is shown in
Figure 1. Owing to the presence of p-type Ag
2O crystals, p-n heterojunction interfaces formed between p-type Ag
2O crystals and n-type SnO
2, generating a broader depletion layer on the SnO
2 side, thereby increasing the initial resistance of the sensing material. Upon exposure to CH
3COCH
3, significant modulation of the heterojunctions occurred and contributed to the sensor response. The Ag NPs produced Ag
2O in air, which has facilitated the development of highly electron-depleted layers while removing electrons from SnO
2. This process has increased the total resistance and the area adjacent to the Ag–SnO
2 interfaces, thus increasing the sensitivity of SnO
2 to CH
3COCH
3. Furthermore, one-dimensional (1D) SnO
2 hollow nanostructures fabricated via electrospinning considerably facilitated the diffusion and transport of electrons in CH
3COCH
3 molecules, thereby rapidly and substantially changing the sensor resistance. Additionally, SnO
2 hollow NFs with both outer and inner surfaces exhibited high surface areas and eventually contributed to high CH
3COCH
3 responses. In summary, the significantly high CH
3COCH
3 sensing performances of Ag/SnO
2 composites are attributed to the Ag dopant and 1D NF structures of SnO
2. Ag with high electrical conductivity enabled the rapid transfer of electrons and catalyzed the oxidation of the reducing CH
3COCH
3 gas molecules. Moreover, the SnO
2 NFs with higher surface areas led to an effective dispersion of catalyst particles and possessed a porous tube-like structure that promoted rapid gas flow and a superior ability to store and release oxygen ions.
In another study, Kilic et al. [
49] reported the CH
3COCH
3 sensing characteristics of Ag-loaded TiO
2 nanorods (NRs). They used a seed-mediated hydrothermal approach to grow TiO
2 NRs and subsequently loaded Ag onto TiO
2 NRs by thermally evaporating metallic Ag at different times of 30, 45, and 90 s. The authors demonstrated that the enhancement in the CH
3COCH
3 detection performance of the Ag-loaded TiO
2 (45 s) sensor was due to the catalytic activity of Ag. Actually, Ag loading increased the number of adsorption sites on the surface of TiO
2 and accelerated the rate of electron exchange between the TiO
2 surface and CH
3COCH
3 molecules. Under a CH
3COCH
3 atmosphere, the bonds between CH
3COCH
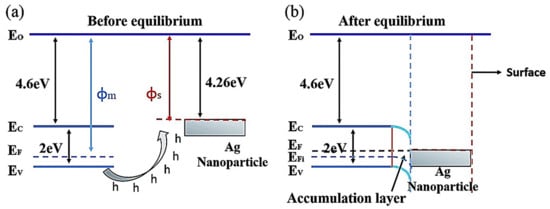
3 molecules were easily dissociated by Ag, allowing these molecules to quickly interact with the chemisorbed oxygen species. Moreover, the decoration of Ag NPs onto TiO
2 NRs pinned the Fermi level of TiO
2 because of the transfer of electrons from TiO
2 to Ag. This led to surface band bending and induced a more pronounced electron–hole separation effect, thereby enhancing the sensitivity of the sensor to CH
3COCH
3. The lower CH
3COCH
3 response of the Ag-loaded TiO
2 (90 s) sensor was associated with the accumulation of Ag clusters on the TiO
2 surface, which impeded O
2 diffusion within TiO
2 and reduced the catalytic activity of Ag.