Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Amedeo Amedei | + 3590 word(s) | 3590 | 2021-10-19 06:07:21 | | | |
| 2 | Peter Tang | Meta information modification | 3590 | 2021-10-20 11:11:53 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Amedei, A. Gut–Brain Axis in Neuropsychological Disease Model of Obesity. Encyclopedia. Available online: https://encyclopedia.pub/entry/15172 (accessed on 13 January 2026).
Amedei A. Gut–Brain Axis in Neuropsychological Disease Model of Obesity. Encyclopedia. Available at: https://encyclopedia.pub/entry/15172. Accessed January 13, 2026.
Amedei, Amedeo. "Gut–Brain Axis in Neuropsychological Disease Model of Obesity" Encyclopedia, https://encyclopedia.pub/entry/15172 (accessed January 13, 2026).
Amedei, A. (2021, October 20). Gut–Brain Axis in Neuropsychological Disease Model of Obesity. In Encyclopedia. https://encyclopedia.pub/entry/15172
Amedei, Amedeo. "Gut–Brain Axis in Neuropsychological Disease Model of Obesity." Encyclopedia. Web. 20 October, 2021.
Copy Citation
Obesity is a multifactorial disorder in which various elements (genetic, host, and environment), play a definite role, even if none of them satisfactorily explains its etiology. A number of neurological comorbidities, such as anxiety and depression, charges the global obesity burden, and evidence suggests the hypothesis that the brain could be the seat of the initial malfunction leading to obesity. The gut microbiome plays an important role in energy homeostasis regulating energy harvesting, fat deposition, as well as feeding behavior and appetite. Dietary patterns, like the Western diet, are known to be a major cause of the obesity epidemic, probably promoting a dysbiotic drift in the gut microbiota.
obesity
microbiota
gut–brain axis
neurological disorders
nervous system
inflammation
1. Introduction
Obesity is an abnormal or excessive fat mass accumulation that affects the health status. The worldwide epidemic of obesity has become an important public health issue, with serious psychological and social consequences since, worldwide, over 650 million adults and 340 million children and adolescents are obese [1]. Obese phenotypes can be associated with some genetic predispositions [2][3] and with sedentary lifestyle. However, these factors alone fail to accurately describe and explain the complexity of the phenomenon. Obesity is a multifactorial disorder which is a result of the interaction of host and environmental factors and its prevalence in high income and upper middle-income countries is more than double that of low and lower middle income countries [4]. Because of that, it constitutes a social problem, especially in those upper middle-income countries, not just affecting the welfare state, but also creating issues in terms of social relations and acceptance, and personal development. Moreover, obesity is considered, sometimes erroneously, as the consequence of an unbalanced and/or mistaken feeding conduct. On the contrary, recent long-term studies reveal a more complex scenario, including neuropsychological and neurobiological factors [5], that in turn involve not only a different categorization of the pathology itself but also suggest that obesity cannot be adequately treated through simple nutritional plans, associated with training and exercise [6]. By considering the complex nature of the pathology (from genetic factors to behavioral and social ones) and given the poor effectiveness of many pharmacological and nutritional approaches, some researchers suggest that “behavioral dimension” should not be neglected, in order to develop new approaches, both preventive and therapeutic, that include obesity within neuropsychological syndromes [5].
2. Why a Neuropsychological View of Obesity?
Obesity occurs when energy intake exceeds energy expenditure over time, and, traditionally, it is considered as the consequence of a sedentary lifestyle and the usual excessive food consumption. Excessive adiposity is a major risk factor for cardiovascular disease, cancer, type 2 diabetes, and mood-related disorders, with obese individuals often suffering social stigmatization [7][8]. Given its multifactoriality, the obesity is a complex disease in which both genetic and environmental factors play a role in its development. Anyway, none of them satisfactorily explains the etiology yet and many details of this pathological condition remain murky. Since differences in the brain could be both a consequence of, and/or an explanatory factor for obesity, recent attention has shifted towards its neurobiological features, in particular in the pathogenic processes and in the clinical-related neurological conditions.
3. The Microbiota–Gut–Brain Axis
The intestine and the brain are intimately connected by the gut–brain axis, a complex bidirectional system in which the central and enteric nervous system communicate involving endocrine, immune and neuronal pathways. Communication and functions of this axis are regulated by the GM at the point that the concept of microbiota–gut–brain (MGB) axis has been introduced to underline the pivotal role of GM in the development of metabolic and neurological diseases.
The microbiota represents the community of microbes (bacteria, archaea, viruses, and fungi) that reside in a particular habitat (e.g., the gut microbiota) and establish with the host a mutually beneficial relationship (while the “microbiome” represents the collective genomes of microorganisms) [9]. In particular, the microbiota offers benefits to the host maintaining the gut integrity [10], harvesting energy [11], providing protection against pathogens [12] and regulating the immune system [13]. The human gastrointestinal tract holds more than 1000 bacterial species, mainly located within distal ileum and colon, which belong prevalently from Bacteroidetes and Firmicutes phyla. The GM com-position is highly dynamic and susceptible to rapid changes in response to external factors as diet, stress, smoking, infections, or perturbation of the healthy state [14]. In turn, changes in GM com-position and function, named dysbiosis, can be responsible for the development of various diseases (e.g., colorectal cancer) [15][16], and, can contribute to the disruption of the molecular dialogue between gut and brain [17].
The MGB axis is composed of the CNS, the autonomous nervous system (ANS), the neurons of the enteric nervous system (ENS), the HPA-axis and the GM. In particular, signals from the brain influence the motor, sensory, and secretory modalities of the gastro-intestinal tract, regulate the inflammatory process and influence the GM structure [18]. In turn, visceral messages from the gastro-intestinal trait can influence brain function [18][19]. For instance, under stress conditions, the cortisol released following HPA axis activation alters the gut permeability and barrier function, thus affecting the GM composition [18].
Conversely, the gut microbiome influences the brain functions modulating the levels of various brain transmitters (i.e., serotonin) [20] and circulating cytokines, that can exceed the BBB [21][22].
Growing evidence, involving studies in germ-free (GF) animal models, which intestinal flora is missing from birth, and humans exposed to probiotic agents or antibiotics, suggests that several pathologic conditions may be affected by a MGB axis dysregulation, such as autism spectrum disorders [23][24][25], anxiety/depression [26], and obesity [27][28][29].
3.1. Microbiome and Energy Harvest
The intestinal microbiome plays a key role in digestion and absorption of nutrients, regulating energy homeostasis through different mechanisms as energy extraction from food and the modulation of fat storage by the short chain fatty acids (SCFAs) and monosaccharides absorption.
The first information about the role of bacterial flora in the obesity physiopathology was obtained in GF mouse models [30][31][32]. These mice were significantly leaner than controls, despite introducing more calories from food [33]. Moreover, GF mice showed modified plasma fatty metabolic markers and lower amount of leptin and ghrelin, suggesting an energy imbalance [34]. When GF mice were transplanted with gut bacterial flora obtained from conventionally raised mice, they showed an increase in insulin resistance and in body fat without an observed increment in nutrient intake [29]. This evidence has placed the gut microbiome at the center of a completely new research field, concerning the pathophysiology of obesity. Biochemical and metagenomics data analysis suggested that the “obese microbiota” [35] was able to harvest more energy from the diet and this ability was also transferable. Thus, the colonization of GF mice with an obese microbiota (human or murine) induced an increase in total body fat higher than that one obtained through colonization with a lean microbiota [36]. In addition, when eutrophic GF mice received fecal microbiota from obese women, metabolic complications associated to obesity have been observed [37]. This evidence indicates how rapid, transmissible and flexible can be the relation between food and commensal microorganisms in obesity and metabolic syndrome.
Animal obesity models and obese humans present a similar microbial phylum taxonomic rank dysbiosis. In particular, humans and mice share two main phylum: Firmicutes and Bacteroidetes; an alteration of Firmicutes/Bacteroidetes ratio was observed in several obesity studies in both mice [38] and humans [35]. Indeed, the Firmicutes could break down indigestible carbohydrates and converting them into absorbable energy products [39][40][41]. However, in a meta-analysis study the observed alteration in the ratio Firmicutes/Bacteroidetes seemed to be unrelated to weight differences [42]. Also, the reduction in microbial diversity and alteration of particular microbial families or species have been observed in obesity conditions [20], such as the increase of Proteobacteria [43].
The complex interplay between host genetics, gut microbiome and environmental factors is crucial for the obesity pathophysiology (for instance, monozygotic twins showed a more similar GM profile than did dizygotic twins) [44]. Dietary pattern could also affect the bacterial structure, for example, westernized diets increase the abundance of Clostridia (Firmicutes phylum) populations that could extract more energy from the diet, consenting higher energy utilization [45]. The unused extra energy is then accumulated as fat deposits.
While different processes, by which an ‘‘obese microbiota’’ can affect body weight balance [33][46][47] have been indicated, the increased energy harvest via colonic fermentation and SCFAs’ production is the most direct [35]. Bacterial enzymes (specific glycoside hydrolases) metabolize otherwise not digested by humans food components, like vegetable fibers (such as resistant starch, cellulose, and inulin) that cannot be metabolized by human enzymes. The final product of this process are energy-rich substrates, such as SCFAs [48]. SCFAs can provide ≤10% of total daily caloric intake [49]. Obese individuals show significantly increased levels of SCFAs such as acetate, propionate, and butyrate [40][50]. Most of the bacterial SCFAs (in particular butyrate) are derived from the fermentation process of Clostridia cluster [51]. SCFAs not only operate as energy substrates for host tissues and bacteria but also act as signaling molecules in the host metabolism, showing a relevant role in mediation of gut motility, regulation of fat storage and appetite [11]. Indeed, dysbiosis induced by the type of diet has been correlated with an acetate increase that promotes hyperinsulinemia [52]. Furthermore, SCFAs can influence other obesity-associated conditions such as insulin resistance and hyperglycaemia [53].
Among the SCFAs, the propionate can be utilized locally through conversion into glucose by intestinal gluconeogenesis or diffuse into the portal vein to be utilized as a substrate for hepatic gluconeogenesis, preventing high SCFAs concentrations in blood [54]. In addition, propionate decreases human lipogenesis and serum cholesterol (in hepatic and non hepatic tissues) [55], and also reduce the fasting blood glucose and hepatic cholesterol in obese rats [56].
In addition, several studies shows acetate benefits on metabolism. Acetate could bind to the receptor GPR43 in several target organs. In adipose tissue, the GPR43 activation inhibits insulin signaling and suppresses fat accumulation, while systemically, it improves insulin sensitivity [57]. Mice deficient in the acetate receptor GPR43 become obese when fed a normal diet, whereas mice who overexpress GPR43 remain lean even when fed an obesogenic diet [57]. In the liver, acetate reduces lipid accumulation and improves liver function and mitochondrial efficiency. In adipose tissue, acetate inhibits fat breakdown but induces the browning of white adipose tissue and metabolic improvements, leading to a reduction in body fat [58]. Finally, prebiotics such as inulin, increased acetate production that crosses the blood–brain barrier of rats and results in reduced grehlin production and so, inducing a decrease of body weight, food intake, and fat mass [59]. Moreover prebiotic fructooligosaccharides increase acetate production, reducing body weight and fat mass because it favors a lower food intake in mice [60].
Microbial metabolites can also regulate the composition of bile acid species. A reduced bile acid amount in the intestine has been associated with inflammation and microbiota overgrowth [61]. Some intestinal bacteria are able to extract energy from the metabolization of bile acids, inducing the activation of bile acid receptors farnesoid X receptor (FXR) and Takeda G-protein-coupled receptor 5 (TGR5). These two receptors maintain insulin sensitivity and glucose tolerance in both liver and intestine [62][63]. In addition, other mechanisms have been proposed to account for the increased microbiota capacity to extract energy from the diet intake [64]:
-
GM influences energy homeostasis by regulating gene expression via complex mechanisms started by SCFAs and monosaccharides [65]. In particular, the commensal microorganisms stimulate monosaccharide cellular uptake [66] and induce lipogenesis by activating the transcription factors carbohydrate response element binding protein (ChREBP) and sterol response element binding protein (SREBP) [64]. Triacylglycerols, produced trought hepatic lipogenesis, are thus sent from the liver to the blood in the form of very low-density lipoprotein and chylomicrons.
-
HF diet triggers an increased absorption of bacterial lipopolysaccharide (LPS) (an endotoxin in the cell wall of Gram-negative bacteria) from the gut lumen to the bloodstream inducing low-grade inflammation, by activating B cells or dendritic cells activating and cytokine production [67]. The inflammation could also be stimulated by endotoxemia condition [66]; moreover, also the damaged gut barrier might contribute to this metabolic endotoxaemia [68].
-
GM induces a suppression of angiopoietin-like protein 4 (ANGPTL4) in the intestinal epithelium. ANGPTL4 is a circulating enzyme produced by liver, gut and adipose tissue that inhibits LPL; its suppression provokes an increase of triacylglycerol storage in the adipose tissue [33].
Of note, relevant evidence suggests that both the consumption of fermentable carbohydrates and the supplementation of SCFAs result in positive effects on host physiology and energy homeostasis. The resistant starch (RS) is a fermentable dietary fiber used as a carbohydrate source in food. Obanda and colleagues used obesity-prone and obesity-resistant rats to examine how weight gain and fat accretion relate to fermentation levels and GM after feeding RS [69]. Obese-prone rats fed with RS at 20% of the weight of the diet did not gain more body fat than the same type of rats fed the same diet except without the RS [69]. It could be possible that fermentation of prebiotics increased energy expenditure but with contemporary greater energy absorption, and so without net gain in weight and body fat. The authors hypothesized that dietary RS decreases body fat accumulation through stimulating endogenous GLP-1 and PYY production [70]. However, the majority of these recent researches have investigated the effect of SCFAs on animal models or in particular tissue or metabolic process. Since SCFAs have different and parallel metabolic processes that affect energy homeostasis, more studies are needed to bring these effects together in order to elucidate the real impact of SCFAs [46].
3.2. Microbiome and the Brain
As previously reported, the gut microbiome affects the host’s CNS functions (as cognitive and vegetative activities) through the MGB axis. CNS functions, vice versa, may influence the structure of the microbiota that inhabits the intestinal lumen [18]. Several studies suggest that this mutual interplay has a pivotal role in the occurrence of metabolic disorders, such as diabetes and obesity [71], but also in the development of eating and stress-related neuropsychiatric disorders, including [72] anxiety and depression.
Currently, researchers are focusing on whether the microbiome can have effects on the CNS process and on the hedonic and homeostatic control of dietary intake [73] (Figure 1).
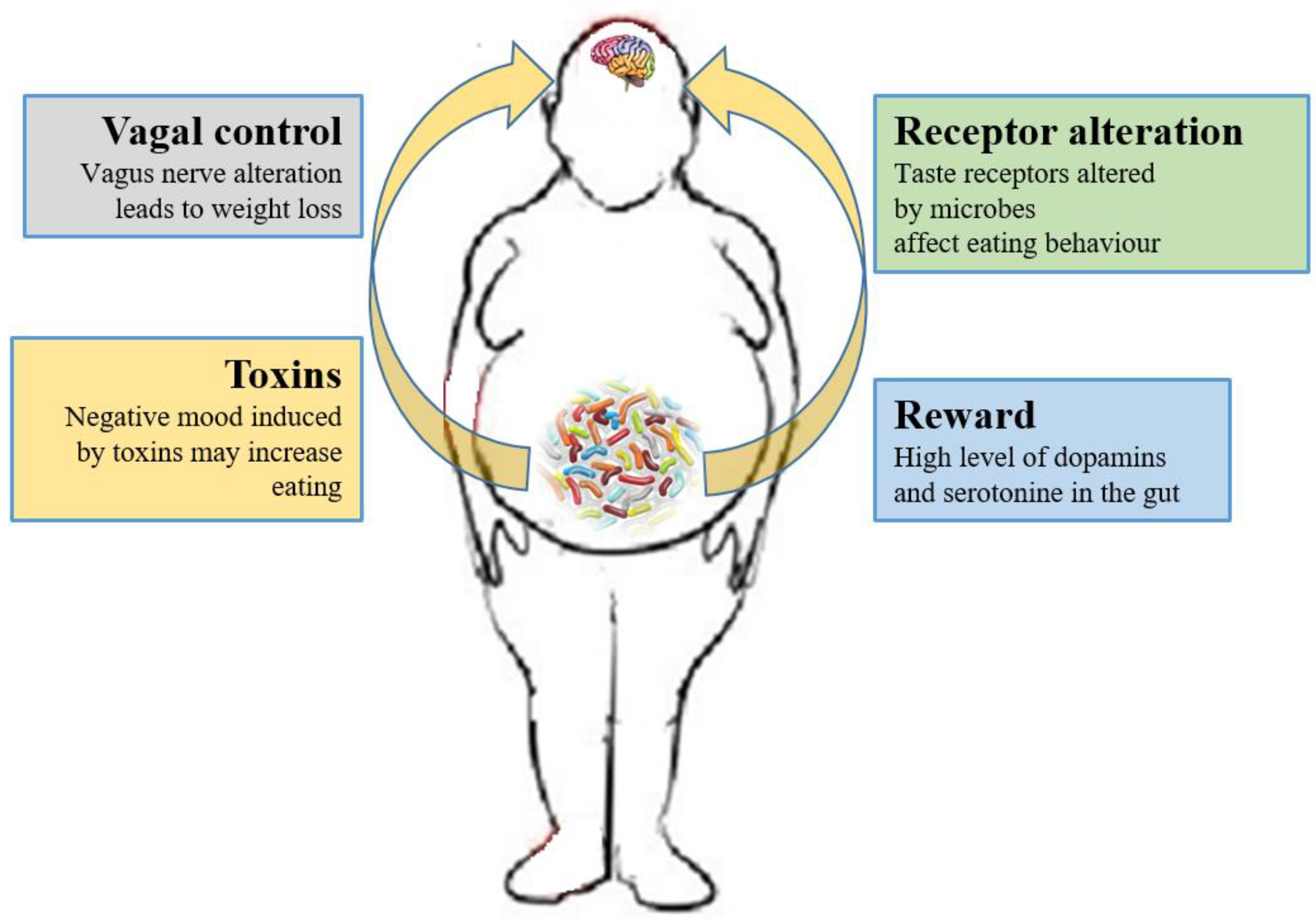
Figure 1. Relations between gut microbiota and eating behavior. The gut microbiota controls the eating behavior by several mechanisms, including changes to receptors such as taste receptors, regulation of reward pathways, production of toxins that alter mood, and deviating neurotransmission via the vagus nerve.
As previously described, the SCFAs produced by the microbiota have systemic effects, but they can also directly signal to the (enteral and central) nervous system, via stimulation of the vagus nerve, or indirectly through immune-neuroendocrine processes [67].
The role of the vagus nerve is important in the MGB axis because it connects the 100 million neurons of the enteric nervous system to the “nucleus tractus solitaries” [18]. The information inserted in this communication axis is then delivered to the hypothalamus, which modulates energy balance, appetite and dietary intake [74]. This information also includes signals from commensal microorganisms, linking the cognitive and emotional nucleus of the CNS with peripheral gut activity, finally leading to host eating control. Experiments showed that transection or blockade of the vagus nerve could induce a dramatic weight loss [75]. On the other hand, vagus nerve functions appear to drive extreme eating behavior in satiated animals when they are treated with norepinephrine [76]. The parasympathetic vagal activity was linked with weight loss also in anorexia nervosa [77], indicating that vagal signaling, involved in the modulation of body weight, can lead to pathological anorexia, and other CNS disorders as anxiety-depressive behaviors and autism [78][79].
The vagus nerve could be stimulated by enteroendocrine cell hormones as gut peptide YY (PYY) and glucagon-like peptide 1 (GLP-1). The satiety hormone PYY inhibits gut motility, increases gut transit time, and reduces appetite [80], while GLP1 decreases appetite and improves insulin sensitivity [81]. The bacterial SCFAs could alter the release of those hormones into systemic circulation binding to their specific enteroendocrine G-protein coupled receptors (GPRs) [82][83]. Through the GPRs activation, SCFAs induce leptin expression, which produces the suppression of the appetite and GLP-1 production [84]. The increased plasma GLP-1 and PYY levels inhibit ghrelin secretion [84] and regulate appetite by releasing it into the blood stream [85]. Acetate, the main SFCA produced by the microbiome, has a direct effect in the suppressing of appetite via central hypothalamic process [59]. The increased acetate production, due to an altered gut microbiome, induces the stimulation of the parasympathetic nervous system with improved secretion of ghrelin, obesity and hyperphagia [52]. Lactate, another bacterial metabolite produced by Enterobacteriaceae, Lactobacilli and Bifidobacteria, is the favorite substrate for neuronal cells and could prolong the postprandial satiety [86].
Furthermore, the microbiome can affect the central control of appetite by producing neuroactive metabolites as tryptophan, serotonin, gamma-aminobutyric acid, endocannabinoid ligands, and ghrelin. These bacterial metabolites are the exact analogs of the mammalian hormones implicated in behavior and mood signaling [87]. More than half of the dopamine and the majority of the body’s serotonin are produced at gut level [88]. Indeed, components of bacterial flora, as Bacillus cereus, Escherichia coli [89], B. subtilis, B. mycoides, Serratia marcescens, Proteus vulgaris, and Staphylococcus aureus [90] can produce dopamine. The probiotic B. infantis 35624 improves blood levels of tryptophan [91], a precursor of serotonin that mediates appetite-suppressant function by the regulation of melanocorting neurons, which control body weight homeostasis [92][93]. Moreover, the lactic acid producing bacteria could secrete the neurochemicals histamine [94] and GABA [95] that is involved in the regulation of feeding and energy balance [96][97]. Interestingly, GABA stimulates the same neuroreceptors that are targeted by anti-anxiety drugs (benzodiazepines).
3.3. The Role of Microbiome-Driven Inflammation
As widely discussed over the review, the immune system plays a crucial role in the gut–brain axis communications since immune mediators are important messengers of this complex dialogue and, consequently, it mechanistically links the function’s impairments in both brain and gut, as shown by the association between chronic gut inflammation and psychological morbidity [98][99][100][101]. The immune system plays a key role in obesity and correlated pathologies, such as in the colorectal cancer [102]. In obese patients, a chronic low-grade inflammatory state is maintained [103][104] and the peripheral inflammation, with the activation of innate immune components (like TLRs) and the loss of intestinal barrier integrity, can lead to neuro-inflammation. Interestingly, recent studies have demonstrated that dysbiosis and inflammation may concur to the development of various diseases, including obesity and depression disorders [105]. In addition, numerous studies have now clearly confirmed that the gut microbiome can, qualitatively and quantitatively, shape the host immune responses, both in the gut and in systemic tissues. In this way, the GM influences the concentration and profile of cytokines present in any given individual and, in turn, differentially affects the brain function. For example, GF mice show numerous immune abnormalities, including impaired antibody responses, diminished numbers of T and B lymphocytes and a defective production of cytokines (such as IL-10, TNF-alfa, IL-6 and IL-1) [106][107]. Moreover, selective GM constituents shape specific aspects of adaptive and innate immunity, including the differentiation of particular effector T-cell lineages [108][109][110]. The obesity-associated dysbiosis is characterized by a remarkable inflammatory potential of microbiota [111][112], which is able to activate innate and adaptive immunity in the gut and beyond, increasing the inflammatory tone by TLRs activation and production of pro-inflammatory cytokines [113]. Sen and colleagues have demonstrated that a dysbiotic microbiota (high sugar diet-associated) alters the vagal gut–brain communication [114], producing an inflammatory state that increases gut permeability. The result is the passage of LPS and pro-inflammatory cytokines from the lumen to the lamina propria (triggering an inflammatory response) and so, microglia activation in the nodose ganglion and finally leading to vagal remodeling [111]. Moreover, a microbiota with enhanced pro-inflammatory activity has been demonstrated to be able to promote intestinal inflammation, inducing colitis and metabolic syndrome [35][113]. The loss of intestinal barrier integrity, seems to be a crucial step in the obesity pathogenesis and related diseases, including neurological disorders [106][115] (Figure 2). In fact, the leaky gut allows the translocation of Gram-negative bacteria’s components into the mesenteric lymph nodes and the circulation, boosting the release of pro-inflammatory cytokines (especially TNF-alpha), via TLR2/4 direct or indirect activation [115][116][117][118], and increasing the production of IgA and IgM [119][120]. In general the gut permeability can be considered the direct consequence of the dysbiotic microbiota-driven local gastrointestinal inflammation [121][122], and notably, in obese mice, the prebiotics’ supplementation can improve the gut integrity, reducing the weight gain [123]. The leaky gut and the associated-inflammation lead to peripheral insulin resistance and hyperglycemia, supporting the obesity establishment; moreover, the increased inflammatory cytokines in the peripheral system can affect the BBB integrity, contributing to the development of mood disorders [122]. Bruce-Keller and colleagues have linked obesity, microbiome, and neurologic dysfunction, demonstrating the ability of HF diet-dysbiotic microbiota to increase inflammatory gene expression in the medial prefrontal cortex associated with anxiety and memory impairment [124]. Moreover, the inflammation generated by HF diet-dysbiotic microbiota can activate the microglia [125], a process observed in various neurological disorders [125][126][127][128] and associated with weight gain and bacteria-driven hyperphagia [129].
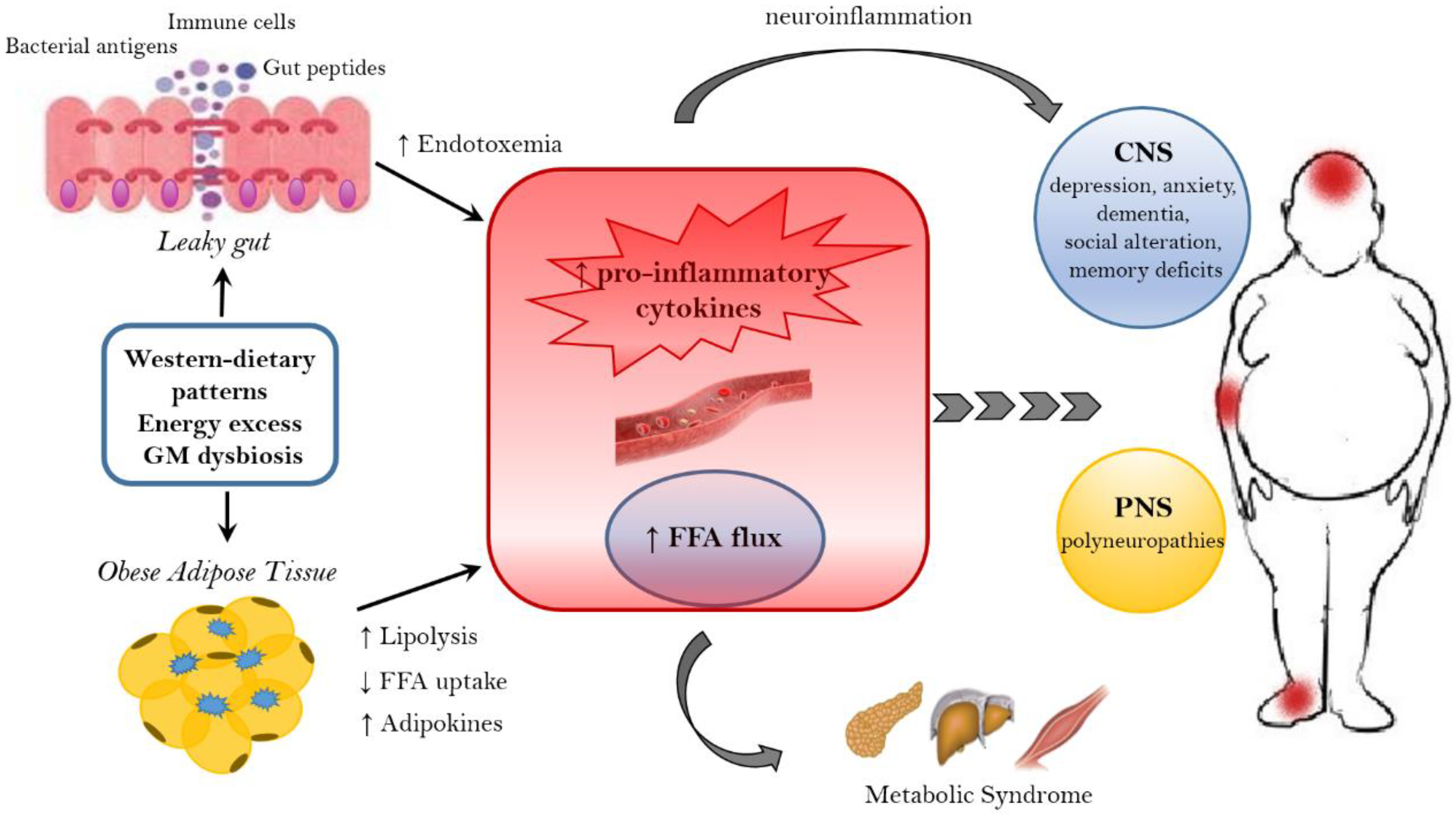
Figure 2. Mechanisms linking obesity to neurological comorbidities. Western-dietary patterns, rich in saturated fat and simple sugars, excessive food intake, and gut microbiota (GM) dysbiosis are related to obesity and its neurological comorbidities through the establishment of an inflammatory state. A dysbiotic microbiota contributes to the leaky gut syndrome, allowing the translocation of gut peptides and bacterial products that increase the peripheral inflammatory tone inducing neuroinflammation. In addition, the dysfunctional obese adipose tissue lead to the increased circulation of inflammatory cytokines, adipokines and FFA. FFA, beside the action on peripheral tissue, where they contribute to the establishment of a metabolic syndrome, have a detrimental effect on both the CNS and PNS. In the CNS, neuroinflammation and lipotoxic FFA can lead to dementia, cognitive impairment, anxiety, and depression, whereas in the PNS the end result are peripheral neuropathies. FFA = free fatty acids. CNS = Central nervous system. PNS = peripheral nervous system.
Finally, expanding the knowledge of the mechanisms underlying the triggers of such inflammatory responses in obesity could offer unique opportunities for intervention strategies reducing the risk of related neurological conditions and supporting personalized treatments [130].
References
- World Health Organisation Website. Available online: http://www.who.int/news-room/factsheets/detail/obesity-and-overweight (accessed on 16 February 2018).
- Bessesen, D.H. Update on obesity. J. Clin. Endocrinol. Metab. 2008, 93, 2027–2034.
- Loos, R.J.F.; Bouchard, C. FTO: The first gene contributing to common forms of human obesity. Obes. Rev. 2008, 9, 246–250.
- Rennie, K.L.; Jebb, S.A. Prevalence of obesity in Great Britain. Obes. Rev. 2005, 6, 11–12.
- Rennie, K.L.; Johnson, L.; Jebb, S.A. Behavioural determinants of obesity. Best Pract. Res. Clin. Endocrinol. Metab. 2005, 19, 343–358.
- Jauch-Chara, K.; Oltmanns, K.M. Obesity—A neuropsychological disease? Systematic review and neuropsychological model. Prog. Neurobiol. 2014, 114, 84–101.
- Bean, M.K.; Stewart, K.; Olbrisch, M.E. Obesity in America: Implications for clinical and health psychologists. J. Clin. Psychol. Med. Settings 2008, 15, 214–224.
- Luppino, F.S.; de Wit, L.M.; Bouvy, P.F.; Stijnen, T.; Cuijpers, P.; Penninx, B.W.; Zitman, F.G. Overweight, obesity, and depression: A systematic review and meta-analysis of longitudinal studies. Arch. Gen. Psychiatry 2010, 67, 220–229.
- Archie, E.A.; Theis, K.R. Animal behavior meets microbial ecology. Anim. Behav. 2011, 82, 425–436.
- Natividad, J.M.M.; Verdu, E.F. Modulation of intestinal barrier by intestinal microbiota: Pathological and therapeutic implications. Pharmacol. Res. 2013, 69, 42–51.
- den Besten, G.; van Eunen, K.; Groen, A.K.; Venema, K.; Reijngoud, D.J.; Bakker, B.M. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J. Lipid Res. 2013, 54, 2325–2340.
- Bäumler, A.J.; Sperandio, V. Interactions between the microbiota and pathogenic bacteria in the gut. Nature 2016, 535, 85–93.
- Gensollen, T.; Iyer, S.S.; Kasper, D.L.; Blumberg, R.S. How colonization by microbiota in early life shapes the immune system. Science 2016, 352, 539–544.
- Ochoa-Repáraz, J.; Kasper, L.H. Gutmicrobiome and the risk factors in central nervous system autoimmunity. FEBS Lett. 2014, 588, 4214–4222.
- Albenberg, L.G.; Wu, G.D. Diet and the intestinal microbiome: Associations, functions, and implications for health and disease. Gastroenterology 2014, 146, 1564–1572.
- Russo, E.; Bacci, G.; Chiellini, C.; Fagorzi, C.; Niccolai, E.; Taddei, A.; Ricci, F.; Ringressi, M.N.; Borrelli, R.; Melli, F.; et al. Preliminary Comparison of Oral and Intestinal Human Microbiota in Patients with Colorectal Cancer: A Pilot Study. Front. Microbiol. 2018, 8, 2699.
- Carding, S.; Verbeke, K.; Vipond, D.T.; Corfe, B.M.; Owen, L.J. Dysbiosis of the gut mi-crobiota in disease. Microb. Ecol. Health Dis. 2015, 26, 26191.
- Mayer, E.A. Gut feelings: The emerging biology of gut-brain communication. Nat. Rev. Neurosci. 2011, 12, 453–466.
- O’Mahony, S.M.; Hyland, N.P.; Dinan, T.G.; Cryan, J.F. Maternal separation as a model of brain-gut axis dysfunction. Psychopharmacology 2011, 214, 71–88.
- Desbonnet, L.; Garrett, L.; Clarke, G.; Bienenstock, J.; Dinan, T.G. The probiotic Bifidobacteria infantis: Anassessmentofpotential antidepressantpropertiesinthe rat. J. Psychiatr. Res. 2009, 43, 164–174.
- Forsythe, P.; Bienenstock, J. Immunomodulationby commensalandprobioticbacteria. Immunol. Investig. 2010, 39, 429–448.
- Duerkop, B.A.; Vaishnava, S.; Hooper, L.V. Immune responsestothemicrobiotaatthe intestinalmucosalsur-face. Immunity 2009, 31, 368–376.
- Adams, J.B.; Johansen, L.J.; Powell, L.D.; Quig, D.; Rubin, R.A. Gastrointestinalflora and gastrointestinalsta-tusinchil-drenwithautism–comparisons to typical children and correlation with autism severity. BMC Gastroenterol. 2011, 11, 22.
- Thomas, R.H.; Meeking, M.M.; Mepham, J.R.; Tichenoff, L.; Possmayer, F.; Liu, S.; MacFabe, D.F. The entericbacterialme-tabolitepropi-onic acidaltersbrainandplasma phospholipid molecularspecies:fur-ther developmentofarodentmodel of autismspectrumdisorders. J. Neuroinflamm. 2012, 9, 153.
- Grimaldi, R.; Gibson, G.R.; Vulevic, J.; Giallourou, N.; Castro-Mejía, J.L.; Hansen, L.H.; Gibson, E.L.; Nielsen, D.S.; Costabile, A. A prebiotic intervention study in children with autism spectrum disorders (ASDs). BMC Microb. 2018, 6, 133.
- Foster, J.A.; Mc Vey Neufeld, K.A. Gut–brain axis: How the microbiome influences anxiety and depression. Trend Neurosci. 2013, 36, 305–312.
- Manco, M. Gut microbiota and developmental programming of the brain: From evidence in behavioral endophenotypes to novel perspective in obesity. Front. Cell. Infect. Microbiol. 2012, 2, 109.
- Davey, K.J.; O’Mahony, S.M.; Schellekens, H.; O’Sullivan, O.; Bienenstock, J.; Cotter, P.D.; Dinan, T.G.; Cryan, J.F. Gender-dependent consequences of chronic olanzapine in the rat: Effects on body weight, inflammatory, metabolic and microbiota parameters. Psychopharmacology 2012, 221, 155–169.
- Turnbaugh, P.J.; Gordon, J.I. The core gut microbiome, energy balance and obesity. J. Physiol. 2009, 587, 4153–4158.
- Thompson, G.R.; Trexler, P.C. Gastrointestinal structure and function in germ-free or gnotobiotic animals. Gut 1971, 12, 230–235.
- Wostmann, B.S. Germ Free and Gnotobiotic Animal Models: Background and Applications; CRC Press: Boca Raton, FL, USA, 1996; pp. 1–188.
- McCracken, V.; Lorenz, R. The gastrointestinal ecosystem: A precarious alliance among epithelium, immunity and microbiota. Cell. Microbiol. 2001, 3, 1–11.
- Bäckhed, F.; Ding, H.; Wang, T.; Hooper, L.V.; Koh, G.Y.; Nagy, A.; Semenkovich, C.F.; Gordon, J.I. The gut microbiota as an environmental factor that regulates fat storage. Proc. Natl. Acad. Sci. USA 2004, 101, 15718–15723.
- Duca, F.A.; Swartz, T.D.; Sakar, Y.; Covasa, M. Increased oral detection, but decreased intestinal signaling for fats in mice lacking gut microbiota. PLoS ONE 2012, 7, e39748.
- Turnbaugh, P.J.; Ley, R.E.; Mahowald, M.A.; Magrini, V.; Mardis, E.R.; Gordon, J.I. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 2006, 444, 1027–1031.
- Ridaura, V.K.; Faith, J.J.; Rey, F.E.; Cheng, J.; Duncan, A.E.; Kau, A.L.; Griffin, N.W.; Lombard, V.; Henrissat, B.; Bain, J.R.; et al. Gut microbiota from twins discordant for obesity modulate metabolism in mice. Science 2013, 341, 1241214.
- Tremaroli, V.; Karlsson, F.; Werling, M.; Ståhlman, M.; Kovatcheva-Datchary, P.; Olbers, T.; Fändriks, L.; le Roux, C.W.; Nielsen, J.; Bäckhed, F. Roux-en-Y gastric bypass and vertical banded gastroplasty induce long-term changes on the human gut microbiome contributing to fat mass regulation. Cell. Metab. 2015, 22, 228–238.
- Turnbaugh, P.J.; Hamady, M.; Yatsunenko, T.; Cantarel, B.L.; Duncan, A.; Ley, R.E.; Sogin, M.L.; Jones, W.J.; Roe, B.A.; Affourtit, J.P.; et al. A core gut microbiome in obese and lean twins. Nature 2009, 457, 480–484.
- Turnbaugh, P.J.; Ridaura, V.K.; Faith, J.J.; Rey, F.E.; Knight, R.; Gordon, J.I. The effect of diet on the human gut microbiome: A metagenomic analysis in humanized gnotobiotic mice. Sci. Transl. Med. 2009, 1, 6–14.
- Brinkworth, G.D.; Noakes, M.; Clifton, P.M.; Bird, A.R. Comparative effects of very low-carbohydrate, high-fat and high-carbohydrate, low-fat weight-loss diets on bowel habit and faecal short-chain fatty acids and bacterial populations. Br. J. Nutr. 2009, 101, 1493–1502.
- de La Serre, C.B.; Ellis, C.L.; Lee, J.; Hartman, A.L.; Rutledge, J.C.; Raybould, H.E. Propensity to high-fat diet-induced obesity in rats is associ-ated with changes in the gut microbiota and gut inflammation. Am. J. Physiol. Gastrointest. Liver Physiol. 2010, 299, 440–448.
- Angelakis, E.; Armougom, F.; Million, M.; Raoult, D. The relationship between gut microbiota and weight gain in humans. Future Microbiol. 2012, 7, 91–109.
- Ravussin, Y.; Koren, O.; Spor, A.; LeDuc, C.; Gutman, R.; Stombaugh, J.; Knight, R.; Ley, R.E.; Leibel, R.L. Responses of gut microbiota to diet composition and weight loss in lean and obese mice. Obesity 2012, 20, 738–747.
- Goodrich, J.K.; Waters, J.L.; Poole, A.C.; Sutter, J.L.; Koren, O.; Blekhman, R.; Beaumont, M.; Van Treuren, W.; Knight, R.; Bell, J.T.; et al. Human genetics shape the gut microbiome. Cell 2014, 159, 789–799.
- Magnusson, K.R.; Hauck, L.; Jeffrey, B.M.; Elias, V.; Humphrey, A.; Nath, R.; Perrone, A.; Bermudez, L.E. Relationships between diet-related changes in the gut microbiome and cognitive flexibility. Neuroscience 2015, 300, 128–140.
- Byrne, C.S.; Chambers, E.S.; Morrison, D.J.; Frost, G. The role of short chain fatty acids in appetite regulation and energy homeostasis. Int. J. Obes. 2015, 39, 1331–1338.
- Cani, P.D.; Amar, J.; Iglesias, M.A.; Poggi, M.; Knauf, C.; Bastelica, D.; Neyrinck, A.M.; Fava, F.; Tuohy, K.M.; Chabo, C.; et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes 2007, 56, 1761–1772.
- Flint, H.J.; Bayer, E.A.; Rincon, M.T.; Lamed, R.; White, B.A. Polysaccharide utilization by gut bacteria: Potential for new insights from ge-nomic analysis. Nat. Rev. Microbiol. 2008, 6, 121–131.
- Sudo, N.; Chida, Y.; Aiba, Y.; Sonoda, J.; Oyama, N.; Yu, X.N.; Kubo, C.; Koga, Y. Postnatal microbial colonization programs the hypo-thalamic-pituitary-adrenal system for stress response in mice. J. Physiol. 2004, 558, 263–275.
- Schwiertz, A.; Taras, D.; Schafer, K.; Beijer, S.; Bos, N.A.; Donus, C.; Hardt, P.D. Microbiota and SCFA in lean and overweight healthy subjects. Obesity 2010, 18, 190–195.
- Louis, P.; Young, P.; Holtrop, G.; Flint, H.J. Diversity of human colonic butyrate-producing bacteria revealed by analysis of the butyryl-CoA: Acetate CoA-transferase gene. Environ. Microbiol. 2010, 12, 304–314.
- Perry, R.J.; Peng, L.; Barry, N.A.; Cline, G.W.; Zhang, D.; Cardone, R.L.; Petersen, K.F.; Kibbey, R.G.; Goodman, A.L.; Shulman, G.I. Acetate mediates a microbiome-brain-β cell axis promoting metabolic syndrome. Nature 2016, 534, 213–217.
- Boulange, C.L.; Neves, A.L.; Chilloux, J.; Nicholson, J.K.; Dumas, M.E. Impact of the gut microbiota on inflammation, obesity, and metabolic disease. Genome Med. 2016, 8, 42.
- den Besten, G.; Lange, K.; Havinga, R.; van Dijk, T.H.; Gerding, A.; van Eunen, K.; Müller, M.; Groen, A.K.; Hooiveld, G.J.; Bakker, B.M.; et al. Gut-derived short-chain fatty acids are vividly assimilated into host carbohydrates and lipids. Am. J. Physiol. Gastrointest. Liver Physiol. 2013, 305, G900–G910.
- Hosseini, E.; Grootaert, C.; Verstraete, W.; Van de Wiele, T. Propionate as a health-promoting microbial metabolite in the human gut. Nutr. Rev. 2011, 69, 245–258.
- Berggren, A.M.; Nyman, E.M.; Lundquist, I.; Björck, I.M. Influence of orally and rectally administered propionate on cholesterol and glucose metabolism in obese rats. Br. J. Nutr. 1996, 76, 287–294.
- Kimura, I.; Ozawa, K.; Inoue, D.; Imamura, T.; Kimura, K.; Maeda, T.; Terasawa, K.; Kashihara, D.; Hirano, K.; Tani, T.; et al. The gut microbiota suppresses insulin-mediated fat accumulation via the short-chain fatty acid receptor GPR43. Nat. Commun. 2013, 4, 1829.
- Sahuri-Arisoylu, M.; Brody, L.P.; Parkinson, J.R.; Parkes, H.; Navaratnam, N.; Miller, A.D.; Thomas, E.L.; Frost, G.; Bell, J.D. Reprogramming of hepatic fat accumulation and ‘browning’ of adipose tissue by the short-chain fatty acid acetate. Int. J. Obes. 2016, 40, 955–963.
- Frost, G.; Sleeth, M.L.; Sahuri-Arisoylu, M.; Lizarbe, B.; Cerdan, S.; Brody, L.; Anastasovska, J.; Ghourab, S.; Hankir, M.; Zhang, S.; et al. The short-chain fatty acid acetate reduces appetite via a central homeostatic mechanism. Nat. Commun. 2014, 5, 3611.
- Everard, A.; Lazarevic, V.; Gaïa, N.; Johansson, M.; Ståhlman, M.; Backhed, F.; Delzenne, N.M.; Schrenzel, J.; François, P.; Cani, P.D. Microbiome of prebiotic-treated mice reveals novel targets involved in host response during obesity. ISME J. 2014, 8, 2116–2130.
- Ridlon, J.M.; Kang, D.J.; Hylemon, P.B.; Bajaj, J.S. Bile acids and the gut microbiome. Curr. Opin. Gastroenterol. 2014, 30, 332–338.
- Schmoller, A.; Hass, T.; Strugovshchikova, O.; Melchert, U.H.; Scholand-Engler, H.G.; Peters, A.; Schweiger, U.; Hohagen, F.; Oltmanns, K.M. Evidence for a relationship between body mass and energy metabolism in the human brain. J. Cereb. Blood Flow Metab. 2010, 30, 1403–1410.
- Jauch-Chara, K.; Friedrich, A.; Rezmer, M.; Melchert, U.H.; Scholand-Engler, H.G.; Hallschmid, M.; Oltmanns, K.M. Intranasal insulin suppresses food intake via enhancement of brain energy levels in humans. Diabetes 2012, 61, 2261–2268.
- DiBaise, J.K.; Zhang, H.; Crowell, M.D.; Krajmalnik-Brown, R.; Decker, G.A.; Rittmann, B.E. Gut microbiota and its possible relationship with obesity. Mayo Clin. Proc. 2008, 83, 460–469.
- Xu, J.; Bjursell, M.K.; Himrod, J.; Deng, S.; Carmichael, L.K.; Chiang, H.C.; Hooper, L.V.; Gordon, J.I. A genomic view of the human-Bacteroides thetaiotaomicron symbiosis. Science 2003, 299, 2074–2076.
- Bäckhed, F.; Ley, R.E.; Sonnenburg, J.L.; Peterson, D.A.; Gordon, J.I. Host-bacterial mutualism in the human intestine. Science 2005, 307, 1915–1920.
- Torres-Fuentes, C.; Schellekens, H.; Dinan, T.G.; Cryan, J.F. The microbiota-gut-brain axis in obesity. Lancet Gastroenterol. Hepatol. 2017, 2, 747–756.
- Delzenne, N.M.; Neyrinck, A.M.; Backhed, F.; Cani, P.D. Targeting gut microbiota in obesity: Effects of prebiotics and probiotics. Nat. Rev. Endocrinol. 2011, 7, 639–646.
- Obanda, D.; Page, R.; Guice, J.; Raggio, A.M.; Husseneder, C.; Marx, B.; Stout, R.W.; Welsh, D.A.; Taylor, C.M.; Luo, M. CD Obesity-Prone Rats, but not Obesity-Resistant Rats, Robustly Ferment Resistant Starch Without Increased Weight or Fat Accretion. Obesity 2018, 2, 570–577.
- Zhou, J.; Martin, R.J.; Raggio, A.M.; Shen, L.; McCutcheon, K.; Keenan, M.J. The importance of GLP-1 and PYY in resistant starch’s effect on body fat in mice. Mol. Nutr. Food Res. 2015, 59, 1000–1003.
- Patterson, E.; Ryan, P.M.; Cryan, J.F.; Dinan, T.G.; Ross, R.P.; Fitzgerald, G.F.; Stanton, C. Gut microbiota, obesity and diabetes. Postgrad. Med. J. 2016, 92, 286–300.
- Dinan, T.G.; Cryan, J.F. Mood by microbe: Towards clinical translation. Genome Med. 2016, 8, 36.
- Sandhu, K.V.; Sherwin, E.; Schellekens, H.; Stanton, C.; Dinan, T.G.; Cryan, J.F. Feeding the microbiota-gut-brain axis: Diet, microbiome, and neuropsychiatry. Transl. Res. 2017, 179, 223–244.
- Benarroch, E.E. Neural control of feeding behavior: Overview and clinical correlations. Neurology 2010, 74, 1643–1650.
- Camilleri, M.; Toouli, J.; Herrera, M.F.; Kulseng, B.; Kow, L.; Pantoja, J.P.; Marvik, R.; Johnsen, G.; Billington, C.J.; Moody, F.G.; et al. Intra-abdominal vagal blocking (VBLOC therapy): Clinical results with a new implantable medical device. Surgery 2008, 143, 723–731.
- Sawchenko, P.E.; Gold, R.M.; Leibowitz, S.F. Evidence for vagal involvement in the eating elicited by adrenergic stimulation of the paraventricular nucleus. Brain Res. 1981, 225, 249–269.
- Kollai, M.; Bonyhay, I.; Jokkel, G.; Szonyi, L. Cardiac vagal hyperactivity in adolescent anorexia nervosa. Eur. Heart J. 1994, 15, 1113–1118.
- Carabotti, M.; Scirocco, A.; Maselli, M.A.; Severi, C. The gut-brain axis: Interactions between enteric microbiota, central and en-teric nervous systems. Ann. Gastroenterol. 2015, 28, 203–209.
- Mayer, E.A.; Tillisch, K.; Gupta, A. Gut/brain axis and the microbiota. J. Clin. Investig. 2015, 125, 926–938.
- Samuel, B.S.; Shaito, A.; Motoike, T.; Rey, F.E.; Backhed, F.; Manchester, J.K.; Hammer, R.E.; Williams, S.C.; Crowley, J.; Yanagisawa, M.; et al. Effects of the gut microbiota on host adiposity are modulated by the short-chain fatty-acid binding G protein-coupled receptor, Gpr41. Proc. Natl. Acad. Sci. USA 2008, 105, 16767–16772.
- Shah, M.; Vella, A. Effects of GLP-1 on appetite and weight. Rev. Endocr. Metab. Disord. 2014, 15, 181–187.
- Brown, A.J.; Goldsworthy, S.M.; Barnes, A.A.; Eilert, M.M.; Tcheang, L.; Daniels, D.; Muir, A.I.; Wigglesworth, M.J.; Kinghorn, I.; Fraser, N.J.; et al. The Orphan G protein-coupled receptors GPR41 and GPR43 are activated by propionate and other short chain carboxylic acids. J. Biol. Chem. 2003, 278, 11312–11319.
- Xiong, Y.; Miyamoto, N.; Shibata, K.; Valasek, M.A.; Motoike, T.; Kedzierski, R.M.; Yanagisawa, M. Short-chain fatty acids stimulate leptin production in adipocytes through the G protein-coupled receptor GPR41. Proc. Natl. Acad. Sci. USA 2004, 101, 1045–1050.
- Nohr, M.K.; Pedersen, M.H.; Gille, A.; Egerod, K.L.; Engelstoft, M.S.; Husted, A.S.; Sichlau, R.M.; Grunddal, K.V.; Poulsen, S.S.; Han, S.; et al. GPR41/FFAR3 and GPR43/FFAR2 as cosensors for short-chain fatty acids in enteroendocrine cells vs FFAR3 in enteric neurons and FFAR2 in enteric leukocytes. Endocrinology 2013, 154, 3552–3564.
- Christiansen, C.B.; Gabe, M.B.N.; Svendsen, B.; Dragsted, L.O.; Rosenkilde, M.M.; Holst, J.J. The impact of short-chain fatty acids on GLP-1 and PYY secretion from the isolated perfused rat colon. Am. J. Physiol. Gastrointest. Liver Physiol. 2018, 315, G53–G65.
- Silberbauer, C.J.; Surina-Baumgartner, D.M.; Arnold, M.; Langhans, W. Prandial lactate infusion inhibits spontaneous feeding in rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2000, 278, R646–R653.
- Clarke, G.; Stilling, R.M.; Kennedy, P.J.; Stanton, C.; Cryan, J.F.; Dinan, T.G. Minireview: Gut microbiota: The neglected endocrine organ. Mol. Endocrinol. 2014, 28, 1221–1238.
- Eisenhofer, G.; Aneman, A.; Friberg, P.; Hooper, D.; Fåndriks, L.; Lonroth, H.; Hunyady, B.; Mezey, E. Substantial production of dopamine in the human gastrointestinal tract. J. Clin. Endocrinol. Metab. 1997, 82, 3864–3871.
- Lyte, M. Probiotics function mechanistically as delivery vehicles for neuroactive compounds: Microbial endocrinology in the design and use of probiotics. Bioessays 2011, 33, 574–581.
- Tsavkelova, E.A.; Klimova, Slu.; Cherdyntseva, T.A.; Netrusov, A.I. Hormones and hormone-like substances of microorganisms: A review. Prikl. Biokhim. Mikrobiol. 2006, 42, 261–268.
- Desbonnet, L.; Garrett, L.; Clarke, G.; Kiely, B.; Cryan, J.F.; Dinan, T.G. Effects of the probiotic Bifidobacterium infantis in the maternal separation model of depression. Neuroscience 2010, 170, 1179–1188.
- Heisler, L.K.; Jobst, E.E.; Sutton, G.M.; Zhou, L.; Borok, E.; Thornton-Jones, Z.; Liu, H.Y.; Zigman, J.M.; Balthasar, N.; Kishi, T.; et al. Serotonin reciprocally regulates melanocortin neurons to modulate food intake. Neuron 2006, 51, 239–249.
- Xu, Y.; Jones, J.E.; Kohno, D.; Williams, K.W.; Lee, C.E.; Choi, M.J.; Anderson, J.G.; Heisler, L.K.; Zigman, J.M.; Lowell, B.B.; et al. 5-HT2CRs expressed by pro-opiomelanocortin neurons regulate energy homeostasis. Neuron 2008, 60, 582–589.
- Thomas, C.M.; Hong, T.; van Pijkeren, J.P.; Hemarajata, P.; Trinh, D.V.; Hu, W.; Britton, R.A.; Kalkum, M.; Versalovic, J. Histamine derived from probiotic Lactobacillus reuteri suppresses TNF via modulation of PKA and ERK signaling. PLoS ONE 2012, 7, e31951.
- Lyte, M.; Vulchanova, L.; Brown, D.R. Stress at the intestinal surface: Catecholamines and mucosa-bacteria interactions. Cell Tissue Res. 2011, 343, 23–32.
- Delgado, T.C. Glutamate and GABA in appetite regulation. Front. Endocrinol. 2013, 4, 103.
- Bravo, J.A.; Forsythe, P.; Chew, M.V.; Escaravage, E.; Savignac, H.M.; Dinan, T.G.; Bienenstock, J.; Cryan, J.F. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc. Natl. Acad. Sci. USA 2011, 108, 16050–16055.
- Goodhand, J.R.; Wahed, M.; Mawdsley, J.E.; Farmer, A.D.; Aziz, Q.; Rampton, D.S. Mood disorders in inflammatory bowel disease: Relation to diagnosis, disease activity, perceived stress, and other factors. Inflamm. Bowel Dis. 2012, 18, 2301–2309.
- Addolorato, G.; Capristo, E.; Stefanini, G.F.; Gasbarrini, G. Inflammatory bowel disease: A study of the association between anxiety and depression, physical morbidity, and nutritional status. Scand. J. Gastroenterol. 1997, 32, 1013–1021.
- Kovacs, Z.; Kovacs, F. Depressive and anxiety symptoms, dysfunctional attitudes and social aspects in irritable bowel syndrome and inflammatory bowel disease. Int. J. Psychiatry Med. 2007, 37, 245–255.
- Mardini, H.E.; Kip, K.E.; Wilson, J.W. Crohn’s disease: A two-year prospective study of the association between psy-chological distress and disease activity. Dig. Dis. Sci. 2004, 49, 492–497.
- Niccolai, E.; Ricci, F.; Russo, E.; Nannini, G.; Emmi, G.; Taddei, A.; Ringressi, M.N.; Melli, F.; Miloeva, M.; Cianchi, F.; et al. The Different Functional Distribution of “Not Effector” T Cells (Treg/Tnull) in Colorectal Cancer. Front. Immunol. 2017, 8, 1900.
- Gregor, M.F.; Hotamisligil, G.S. Inflammatory mechanisms in obesity. Annu. Rev. Immunol. 2011, 29, 415–445.
- Lumeng, C.N.; Saltiel, A.R. Inflammatory links between obesity and metabolic disease. J. Clin. Investig. 2011, 121, 2111–2117.
- Slyepchenko, A.; Maes, M.; Jacka, F.N.; Köhler, C.A.; Barichello, T.; McIntyre, R.S.; Berk, M.; Grande, I.; Foster, J.A.; Vieta, E.; et al. Gut microbiota, bacterial translocation, and interactions with diet: Pathophysiological links between major depressive disorder and non-communicable medical comorbidities. Psychother. Psychosom. 2017, 86, 31–46.
- Ikeda, M.; Hamada, K.; Sumitomo, N.; Okamoto, H.; Sakakibara, B. Serum amyloid A, cytokines, and corticosterone responses in germfree and conventional mice after lipopolysaccharide injection. Biosci. Biotechnol. Biochem. 1999, 63, 1006–1010.
- Souza, D.G.; Vieira, A.T.; Soares, A.C.; Pinho, V.; Nicoli, J.R.; Vieira, L.Q.; Teixeira, M.M. The essential role of the inte-stinal microbiota in facilitating acute inflammatory responses. J. Immunol. 2004, 173, 4137–4146.
- Atarashi, K.; Tanoue, T.; Ando, M.; Kamada, N.; Nagano, Y.; Narushima, S.; Suda, W.; Imaoka, A.; Setoyama, H.; Nagamori, T.; et al. Th17 cell induction by adhesion of microbes to intestinal epithelial cells. Cell 2015, 163, 367–380.
- Furusawa, Y.; Obata, Y.; Fukuda, S.; Endo, T.A.; Nakato, G.; Takahashi, D.; Nakanishi, Y.; Uetake, C.; Kato, K.; Kato, T.; et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature 2013, 504, 446–450.
- Seo, S.U.; Kamada, N.; Muñoz-Planillo, R.; Kim, Y.G.; Kim, D.; Koizumi, Y.; Hasegawa, M.; Himpsl, S.D.; Browne, H.P.; Lawley, T.D.; et al. Distinct commensals induce interleukin-1β via NLRP3 inflammasome in inflammatory mo-nocytes to promote intestinal inflammation in response to injury. Immunity 2015, 42, 744–755.
- Sen, T.; Cawthon, C.R.; Ihde, B.T.; Hajnal, A.; DiLorenzo, P.M.; de La Serre, C.B.; Czaja, K. Diet-driven microbiota dysbiosis is associated with vagal remodeling and obesity. Physiol. Behav. 2017, 173, 305–317.
- Chassaing, B.; Koren, O.; Goodrich, J.K.; Poole, A.C.; Srinivasan, S.; Ley, R.E.; Gewirtz, A.T. Dietary emulsifiers impact the mouse gut microbiota promoting colitis and metabolic syndrome. Nature 2015, 519, 92–96.
- Sanz, Y.; Moya-Perez, A. Microbiota, inflammation and obesity. Adv. Exp. Med. Biol. 2014, 817, 291–317.
- Rezzi, S.; Ramadan, Z.; Martin, F.P.; Fay, L.B.; van Bladeren, P.; Lindon, J.C.; Nicholson, J.K.; Kochhar, S. Human metabolic pheno-types link directly to specific dietary preferences in healthy individuals. J. Proteome Res. 2007, 6, 4469–4477.
- Maes, M.; Kubera, M.; Mihaylova, I.; Geffard, M.; Galecki, P.; Leunis, J.C.; Berk, M. Increased autoimmune responses against auto-epitopes modified by oxidative and nitrosative damage in depression: Implications for the pathways to chronic depression and neuroprogression. J. Affect. Disord. 2013, 149, 23–29.
- Chan, E.; Riches, D.W. Ifn-gamma + Lps induction of inos is modulated by Erk, Jnk/Sapk, and P38 Mapk in a mouse ma-crophage cell line. Am. J. Physiol. Cell Physiol. 2001, 280, C441–C450.
- Wischmeyer, P.E. Glutamine: Role in gut protection in critical illness. Curr. Opin. Clin. Nutr. Metab. Care 2006, 9, 607–612.
- Lucas, K.; Maes, M. Role of the toll like receptor (Tlr) radical cycle in chronic inflammation: Possible treat-ments targeting the Tlr4 pathway. Mol. Neurobiol. 2013, 48, 190–204.
- Maes, M.; Kubera, M.; Leunis, J.C. The gut-brain barrier in major depression: Intestinal mucosal dysfunction with an increased translocation of lps from gram negative enterobacteria (leaky gut) plays a role in the inflammatory pathophysiology of depression. Neuro Endocrinol. Lett. 2008, 29, 117–124.
- Maes, M.; Mihaylova, I.; Leunis, J.C. Increased serum iga and igm against lps of enterobacteria in chronic fatigue syndrome (Cfs): Indication for the involvement of gram-negative enterobacteria in the etiology of cfs and for the presence of an increased gut-intestinal permeability. J. Affect. Disord. 2007, 99, 237–240.
- Hamilton, M.K.; Boudry, G.; Lemay, D.G.; Raybould, H.E. Changes in intestinal barrier function and gut microbiota in high-fat diet-fed rats are dynamic and region dependent. Am. J. Physiol. Gastrointest. Liver Physiol. 2015, 308, G840–G851.
- deMelo, L.G.P.; Nunes, S.O.V.; Anderson, G.; Vargas, H.O.; Barbosa, D.S.; Galecki, P.; Carvalho, A.F.; Maes, M. Shared metabolic and immune-inflammatory, oxidative and nitrosative stress pathways in the metabolic syndrome and mood disorders. Prog. Neuropsychopharmacol. Biol. Psychiatry 2017, 78, 34–50.
- Cani, P.D.; Possemiers, S.; Van de Wiele, T.; Guiot, Y.; Everard, A.; Rottier, O.; Geurts, L.; Naslain, D.; Neyrinck, A.; Lambert, D.M.; et al. Changes in gut microbiota control inflammation in obese mice through a mechanism involving GLP-2-driven improvement of gut permeability. Gut 2009, 58, 1044–1055.
- Bruce-Keller, A.J.; Salbaum, J.M.; Luo, M.; Blanchard, E.; Taylor, C.M.; Welsh, D.A.; Berthoud, H.R. Obese-type gut microbiota induce neurobehavioral changes in the absence of obesity. Biol. Psychiatry 2015, 77, 607–615.
- Aguzzi, A.; Barres, B.A.; Bennett, M.L. Microglia: Scapegoat, saboteur, or something else? Science 2013, 339, 156–161.
- Perry, V.H.; Nicoll, J.A.; Holmes, C. Microglia in neurodegenerative disease. Nat. Rev. Neurol. 2010, 6, 193–201.
- Kettenmann, H.; Hanisch, U.K.; Noda, M.; Verkhratsky, A. Physiology of microglia. Physiol. Rev. 2011, 91, 461–553.
- Kingwell, K. Neurodegenerative disease: Microglia in early disease stages. Nat. Rev. Neurol. 2012, 8, 475.
- Vaughn, A.C.; Cooper, E.M.; DiLorenzo, P.M.; O’Loughlin, L.J.; Konkel, M.E.; Peters, J.H.; Hajnal, A.; Sen, T.; Lee, S.H.; de La Serre, C.B.; et al. Energy-dense diet triggers changes in gut microbiota, reorganization of gut-brain vagal communication and increases body fat accumulation. Acta Neurobiol. Exp. 2017, 77, 18–30.
- Amedei, A.; Boem, F. I’ve Gut A Feeling: Microbiota Impacting the Conceptual and Experimental Perspectives of Personalized Medicine. Int. J. Mol. Sci. 2018, 19, 3756.
More
Information
Subjects:
Nutrition & Dietetics
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.1K
Revisions:
2 times
(View History)
Update Date:
20 Oct 2021
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




