
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Kamil Kayode Katibi | + 5516 word(s) | 5516 | 2021-09-27 05:01:10 | | | |
| 2 | Vivi Li | Meta information modification | 5516 | 2021-10-12 11:31:14 | | | | |
| 3 | Vivi Li | Meta information modification | 5516 | 2021-11-23 10:26:38 | | |
Video Upload Options
Over the years, the persistent occurrence of superfluous endocrine-disrupting compounds (EDCs) (sub µg L−1) in water has led to serious health disorders in human and aquatic lives, as well as undermined the water quality. At present, there are no generally accepted regulatory discharge limits for the EDCs to avert their possible negative impacts. Moreover, the conventional treatment processes have reportedly failed to remove the persistent EDC pollutants, and this has led researchers to develop alternative treatment methods. Comprehensive information on the recent advances in the existing novel treatment processes and their peculiar limitations is still lacking. In this regard, the various treatment methods for the removal of EDCs are critically studied and reported in this entry.
1. Introduction
2. Treatment Processes in Removing Endocrine Disrupting Compounds
Conventional Treatment Process
| Major Contaminants/Sources | Treatment Process | Treatment Factor | Brief Procedure | Major Findings | Limitations | References |
|---|---|---|---|---|---|---|
| TCS, NP2EO, IBF, DCF, TCS, BPA, KFN, NP, NP1EO, NPX/wastewater, and sewage sludge samples | Conventional treatment (mesophilic anaerobic sludge digestion) |
(HRT: 9 h; SRT: 8 d), (SRT: 17 d). (HRT: 23 h, SRT: 18 d). |
Wastewater samples collection. Sewage sludge samples were homogenized extractions of wastewater samples. |
The removal efficiency of DCF and IBF ranged between 39% and 100%, IBF and NPX were ˃80%. | Higher proportions of NP in digested sludge. Detection of TCS and NP in treated wastewater. Too many modular units. |
[30] |
| E1, E2, E3, EE2, BPA, and 4-NP/rural wastewater effluent |
Activated sludge. Micro-power biofilm reactor. Constructed wetland. Stabilization pond. |
Temp: 30 and 70 °C; HRT = 12–24; 24–120; 24–240; 10–16. |
Biological contact oxidation. Subsurface flow. Facultative pond. Anoxic oxidation. |
Percent removal of target EDCs > 70% in summer. | Unstable performance of decentralized processes. Pronounced impacts of effluent discharged on the quality of receiving water. Too many modular units. |
[31] |
| BPA, E1, E2, E3, EE2, and DES/effluent from a wastewater treatment plant | WWTP activated sludge treatment processes. Oxidation ditch reversed anaerobic and sequential batch reactor SBR. |
HRT: 7.6–35.31 h SRT: 5.8–31.9 days |
73.7% of BPA was removed. High removal rates of EDCs (i.e., > 85%). |
Some concentrations of EDCs were found in the effluents and can pose potential risks to ecosystems and human health. Longer HRT and SRT. |
[32] | |
| 59 EDC contaminants/ wastewater effluents | Fluidized powdered activated carbon (PAC) pilot (WWTP configuration). | SRT: 5–7 days; bed depth: 1–3 m. hydraulic velocity: 6–12 m/h; contact time: 10–20 min. |
Pre-primary and biological treatments. Pre-treatment (screening). Biofiltration system, Micropollutant analyses. | Removal of parabens and pesticides ranged between 50% and 95%, paracetamol, IBF, sulfamethoxazole 60–80% | Artificial sweeteners (1000–10,000 ng/L), BPA and NP (100–1000 ng/L) were detected in the effluent. | [21] |
3. Contemporary Techniques for the Removal of EDCs from Various Water Sources
3.1. Catalytic Degradation of EDCs
3.2. Photo-Catalytic Degradation of EDCs
| Major Contaminants/Source | Treatment Process | Treatment Factor | Brief Procedure | Major Findings | Limitations | References |
|---|---|---|---|---|---|---|
| EE2/ ultra-pure water and treated wastewater |
Photocatalytic degradation using ZnO under simulated solar radiation | EE2 conc: 100–500 µg/L, photon flux: 4.93 × 10−7–5.8 × 10−7 Einstein L−1 S−1; ZnO conc: 50–500 mg/L, treatment time: 2–10 min. | Spiking of water matrix was spiked with EE2, photocatalysis of the solution. Periodic sampling and centrifugation. |
Rapid EE2 degradation occurred via first-order kinetics. | Detection of EE2 in the effluent. Retardation of EE2 degradation by organic and inorganic matter. |
[39] |
| BPA/municipal WWTP, bottled water, ultra-pure water | Solar photocatalytic degradation | pH: 6.1, catalyst: 81.3–339.2 mg, TiO2 loading: 0, 81.3, 101.8, 152.3, 339.2 mg, ZnO loading: 0.5–6.8 mg/cm2, H2O2: (25–100 mg/L), BPA initial conc.: 50–200 µg/L, treatment time: 0–90 min. | The incident radiation intensity was measured econometrically. The water matrix was spiked with the organic substance with the addition of the ZnO /TiO2 catalytic plate. Periodic sampling and analysis. |
Increasing the number of immobilized catalysts enhances BPA conversion. | Partial inhibition of BPA degradation due to the presence of EE2. Weak degradation in wastewater. |
[26] |
| E1, E2, EE2, E3, NP, BPA/artificial, and real wastewater | Enzymatic degradation using fungal laccases | pH: 1–1.5 Temperature: −20 °C Contact time: 2, 6, 24 h. |
Constant shaking as laccase uses molecular oxygen for oxidizing substrates. Acidification of enzymatic reaction at each time interval (2, 6, and 24 h). Complete inactivation of the laccase activity. Extraction via solid-phase extraction (SPE) for chemical analysis. |
Immobilized laccase removed EDCs (83% for T. Versicolor and 87% of M. thermophile), 99% removal after 24 h. Removal rates for estrogenic = 82% after 24 h. |
Formation of toxic by-products. | [40] |
| BPA | Fungal laccases degradation using oxidative enzyme | (1): 25 μM of each molecule, pH 5.0 (50 mM sodium citrate buffer), 1.5 U/mL laccase, (2): 100 μM BPA, pH 5.0 (50 mM sodium citrate buffer), 25 °C, and 1.5 U/mL laccase, reaction time: 1 h. |
Addition of methanol and Tween to the solution. Incubation of each EDC. Addition of hydrochloric acid (HCl) to the reaction mixture and centrifugation at a specific time interval at room temperature. Analysis of supernatant and BPA degradation. |
BPA was oxidized under all conditions tested. |
Complex procedure. | [41] |
| 2-chlorophenol and SMZ/municipal wastewater |
Laccase degradation | pH 7, initial SMX at 10 μM and ACE at 10 μM. Time (h): 0, 0.25,1, 24. |
NA | Excellent removal of SMZ in the absence of mediators in secondary effluent. | Poor removal of sulfamethoxazole in all buffered solutions. Not economically viable. |
[42] |
| BPA, 2,4-dichlorophenol, 4-tert OP, pentachlorophenol, and NP/aquatic plants | Enzymatic degradation | Endogenous H2O2 concentration in aquatic plants (170–590 μmol/kg-FW) | EDCs were degraded by oxidative enzymes. | Longer treatment period (>100 days). Complex procedure. |
[43] | |
| Atrazine (herbicide), phenyl phenol, BPA, and TCS/municipal wastewater | Biosorption and biodegradation. | Feed NaCl concentration (0–15 g/L). Initial MLSS = 16 g/L; HRT = 5 d; mixed liquor pH = 7 ± 0.1; temperature = 35 ± 1 °C. | Feeding the bioreactor, circulation of digested sludge. Mixing of the sludge. |
Trimethoprim, carazolol, hydroxyzine, amitriptyline, and linuron, removal rates ≤ 80%. Phenyl phenol removal = 60%. |
Relatively low removal rates of phenyl phenol, BPA, and TCS. BPA was poorly removed, from 40% to 20%. Poor removal of atrazine (6.8%). |
[44] |
| DEHP, fluoranthene, AMPA, and E1/ wastewater effluent | Filamentous fungi biodegradation. | pH 5.5, incubation period: 96 h (AT96h), degradation period: 10 days. |
Degradation test conducted in mineral medium incubated for 10 days with each fungus. | Fungi degradation of DEHP = 100%, AMPA = 69% with F. solani and T. harzianum. | E1 not degraded by all fungal isolate trials. | [35] |
3.3. Enzymatic Degradation
3.4. Removal of EDCs by Membranes
| Major Contaminants/Water | Treatment Process | Operating/Treatment Factor | Brief Procedure | Major Findings | Limitations | References |
|---|---|---|---|---|---|---|
| E1, E2, progesterone, testosterone/purified water | UF membrane | MWCO: 1–100kDa Pressure: 0.5–5 bar Pure water flux (L/m2h) 20.8–359.2 Final flux:21.9–288.5 Time: 2–40 min pH: 8 |
Stirring feed solution at 200 rpm for 16 h. Filtering of purified membrane for 30 min. Measurement of pure water flux. Collection of permeate. |
Removal via solute–solute interactions for E1 correspond to higher proportion of organic matter at 25–50 mg/L for 10 kDa (48–52%); 100 kDa (33–38%) membranes. | Poor removals of E1 and hormone contaminants (52% and 38%). | [59] |
| BPA, CBZ, IBF, and SFZ/drinking water | UF membrane | Operating speed: 50 psi. Flow rate: 0.65 L/min per cell. |
Initial partial removal of BPA. | Poor BPA removal using modified PES membranes. | [60] | |
| BPA/drinking water | UF-PS (PS) membrane. | Temp: 25 ± 0.5 °C. pH: 7–13 BPA concentration: 100–500 μg/L. pH: (3.68–10) |
Measurement of pure water flux. Filtration of BPA solution. |
Higher removal at the initial stage of the filtration. | Lower removal efficiency (20%). Fouling. |
[61] |
| BPA/pure BPA solution |
UF membrane | pH: (3–13) MWCO: 100 Da TMP: 0.1 × 106–0.3 × 106 Pa Temp: 20 ± 2 °C BPA conc.: 5 mg/L |
The UF membrane was installed and the solution was introduced into the UF cup and followed by magnetic stirring. | Both salt and acidic pH improve the transportation of BPA. | BPA rejection decreased significantly when the BPA molecule was ionized. | [62] |
| DMP, DEP, DBP, DnOP, DEHP/water | NF membrane | pH: 4–9; pure water flux: 47.5 L/m2 h; temperature: 25–45 °C. | Preparation of a feed solution. Measurement of concentrations of PAEs in both the feed and permeate. |
Removal efficiencies of 95.4%, 95.1%, and 91.5% were recorded for DEHP, DnOP, and DBP. | Lower adsorption rates. Low rejection of sulfamides. |
[63] |
| BPA/biologically treated wastewater | MF and NF | Suspended solids = 78 ± 12 mg BPA conc.: 0.3 ± 0.14–0.7 ± 0.27 Jv(L/m2h) = 6.0–18.6 80 L/(m2h) for NF Temp = 21 °C TMP = 0.3 MPa (MF) 0.7 MPa (NF) |
Circulation of module with pure water. Determination of pure water infiltration. |
Both techniques eliminate BPA. BPA removal efficiency: 61–75% with NF. | Fouling. A decline in permeate flux in MF. |
[64] |
| BPA/model solution | NF and RO membranes | Temperature: 45–50 °C Max pressure: 31–83 bar, pH: 2–11 water permeability: 0.85–14.86 (L/m2h bar) Time: 30–360 min |
≥98% BPA rejection was achieved with polyamide-based RO membranes. | High energy demand. Too many modular units. |
[65] | |
| BPA, E2, E1, E3, EE2/synthetic wastewater | UF membrane | working pressures (25, 30, 50, 75 kPa); temp: 20 ± 2 °C; TOC = 7 mg/L; pH 7.6; conductivity = 1000 | Soaking of fresh membrane for 24 h. Removal of impurities. Determination of flux. |
EDCs removal rates of (10–76%) were achieved via a fouled membrane. | Poor removal of E3 (10–17%). | [66] |
| BPA, DMP, DBP, NP, DOP/water | Nano-functionalized membrane using polypropylene (PP) non-woven fabric | Operating pressure: 0.02–0.5 Mpa; pH: 6.5; Temp: 25 °C | The target pollutants were dissolved in deionized water and quantified. The filtration experiment was conducted. |
˃80% BPA rejection was recorded after a period of 1.3 s. | Removal of contaminants was attained at higher operating pressure of 0.5 MPa. | [67] |
| Oxybenzone and BPA/synthetic solution | Nanohybrid (CuSG) blended PES-HF membranes | Filtration time: 120 min; temp: 20 °C; pressure: 1 bar |
25 mg/L solution of oxybenzone and 5 mg/L BPA solution were filtrated via the HFM samples and the permeate was analyzed via a UV–visible spectrophotometer | Higher rejection of oxybenzone (98%) and BPA (95%) was recorded. Elevated pure water permeability (528.2 ± 44.6 Ml/m2/h/mmHg). |
Nil | [68] |
| BPA/synthetic solution | UF(TFC) immobilized with TiO2 | Preparation of feed solution. Quantification of the feed and the permeate solution. |
[69] | |||
| BPA/drinking water | Nanocomposite membrane electrospun PVDF-PVP-MnO2 | Working pressure: 0.5–2.5 bars; sampling period: 0, 5, 10, 20, and 30 min; temp: 27 °C. |
The membrane was fabricated using electrospinning technique and was applied in a filtration system to assess the removal efficiency of BPA. The concentrations of BPA were analyzed using HPLC. | Complete rejection of BPA (100%) was attained for NF2 and NF6 after 30 min. | Nil | [70] |
| BPA/synthetic solution | Photocatalytic PSF/TiO2/Fe-doped composite UF membrane | BPA concentration: 10 mg/L; specific temperature: 140–220 °C, 6–24 h; pressure: 0.1–0.2 MPa. |
Preparation of Fe-doped TiO2 photocatalysts, synthesis of photocatalytic membranes; assessment of photocatalytic performance | BPA removal rate of 90.78% was recorded. | Nil | [71] |
| BPA/water | PSF/GO nano-composite membranes | Input pressure: 1–5 bar, operating time: 10–50 min, pH: 3–11, initial BPA concentration: 1–9 mg/L. |
Synthesis of GO; preparation of GO/PSF nano-hybrid membranes; BPA concentration was analyzed using a UV–vis spectrophotometer |
BPA removal efficiency of 93% was attained. | [72] |
3.5. Removal of EDCs by Ozonation and Advanced Oxidation Processes (AOPs)
| Major Contaminants/Sources | Treatment Process | Treatment Factor | Brief Procedure | Major Findings | Limitations | References |
|---|---|---|---|---|---|---|
| Diltiazen, progesterone, BBP, E1, CBZ, acetaminophen/biological sludge |
Pulse ozonation experiment | Operating pressure = 5 bar; gas flow rate = 10–140 L/h; MLSS = 2.3–4.2 g/L; ozone period: 6–150 min. Ozone dose (mgO3/L): 1.11–18.65; pH = 6.4–7.1 |
Ozonation of the sludge samples. Continuous aeration. Analysis of the residual EDCs conc. in the samples. |
˃99% removal of target EDCs contaminants were achieved after 4 days. | Production of toxic by-products. The high cost of ozone production. |
[87] |
| BPA, E2, and EE2/wastewater | AOP (H2O2, O3, UV, UV/TiO2, UV/H2O2, and UV/O3) | NA | NA | The removal rate of pharmaceutical EDCs ≥ 96% during UV/TiO2 process. | Poor removal of caffeine. Generation of several oxidation by-products with high toxic potential. |
[88] |
| E2, EE2, BPA/wastewater treatment plant effluent matrix | Degradation by UV light/chlorine | Chlorine conc.: 0.2–2 mg/L; reaction time: 30 min; initial EDC conc.: 100 µg/L; UVC irradiance: 14.79 mW cm−2; temp.: 25; pH: 7 | Spiking of EDCs in WWTP effluent and ultrapure water. UV/Cl process. Samples collection. Addition of sodium thiosulfate followed by filtration. Disinfection evaluations. |
The combination of UVC with chlorine significantly and rapidly degrades EDCs. An upsurge in chlorine concentration yields almost 99% EDCs removal. |
Formation of chlorate by-product disinfection. UV light penetration can be obstructed by turbidity. |
[89][90] |
| E1, E2, EE2, DES, TCS, 17α- treubolone, 17 β- treubolone, 19- nortestosterone, AEDb testosterone, methyltestotesterone, 4-OHA, prednisone cortisol, cortison, 19- norethindrone, medroxyprogesterone, BPA, 4-tert-OP, 4- NP, triclocarban, ADD, 17β- boldenone, stanozolol, epi-andosterone, andosterone 5α-dihydrosteterone, preanisolone, dexamethasone, ethynyl testosterone, progesterone/secondary wastewater effluent |
Fe (VI) treatment process | Temp. = 23 ± 2 °C micropollutants = 100 µg/L−1; Fe (VI) = 10 mgFeL−1; pH: 6.88–7.09; Fe (VI) dosage = 0, 2.5, 5, and 10 mgFe L−1; DOC. = 5.0 mgCl−. |
Application of Fe (VI) to secondary effluent. Dosing of solid Fe (VI) in the effluent. Stirring of the solution. Addition of methanol and H2SO4. |
Fe (VI) treatment could achieve both oxidative eliminations of detected EDCs as a tertiary treatment technology. | It failed to react with triclocarban, three androgens. Low ferrate (VI) production rate. |
[91][90] |
| EE2/synthetic secondary effluent | Ozonation | Ozone conc.: 2, 4, 9 mg/L; NOM conc.: 0–80 mg/L; pH: 6–10 O3: TOC: 0.2–1.0 |
Spiking different conc. of ozone into the stock solution. Removal of residual ozone and radicals. Testing of blank controls. |
The initial concentration of ozone and natural organic substance adversely affect degradation efficiency. Effective degradation of EE2 by ozonation at pH 6 resulted in higher degradation of EE2. | Generation of toxic by-products. Production of solid by-products. High operating costs. |
[92] |
| BPA/aqueous solution | Microwave-enhanced Mn-Fenton process | BPA initial concentration = 100.0 mg/L; reaction time = 6 min | Addition of BPA solution with Fenton reagents followed by heating. Determination of BPA conc. |
BPA removal = 99.7% and total organic carbon (TOC) (53.1%). | Generation of complicated secondary sludge. A narrow range of optimal pH (2.5–4.0). |
[93] |
3.6. Removal of Endocrine-Disrupting Compounds via Adsorption Process
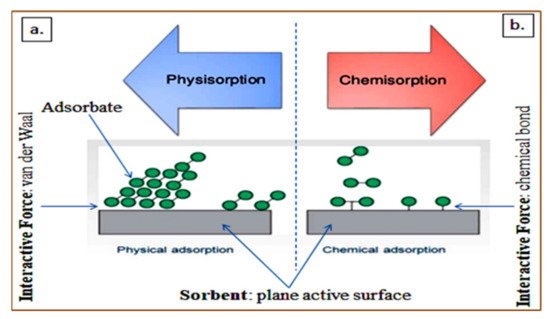
| Major Contaminants/Sources | Treatment Process | Treatment Factor | Brief Procedure | Major Findings | Limitations | References |
|---|---|---|---|---|---|---|
| EE2/water | Adsorption (polyamide adsorbent) | pH: 4.8–9.1; constant dosage of 0.2 g/L; contact time: 24 h; agitation rate: 250 rpm; temp.: 25 °C. | Dilution of EE2 working solutions from EE2 stock solutions. Addition of adsorbent into EE2 aqueous solutions. Agitation of mixed solutions. |
Maximum adsorption capacity = 25.4 mg g/L. Adsorption rates ranged between 5.3- and 22.4-fold. |
A molecule-level investigation. | [107] |
| BPA, NP BP3, TCS/aerobically treated greywater | Adsorption (PAC) | 29.0 g/70.6 mL bed volume; initial compound proportion: 100–1600 µg/L; dose: 1.25 g/L; contact time: 5 min. | NA | TCS removal = 95%. BPA removal = 99%. NP removal = 84%. |
The exorbitant cost of PAC. | [108][78] |
| TCS, E1, E2, and EE2, clofibric acid, CBZ, clofibrate methyl ester, clofibrate/water, and treated wastewater | Batch adsorption using crosslinked polymer adsorbent and activated carbon | Polymer sorbent dosage: 0.2–1.2 g/L; AC: 0.05–0.2 g/L; retention time: 5.7–24.2 min, temp.: 21 ± 2 °C. | Removal of selected EDCs from ultrapure water. Introduction of polymer adsorbents in solutions of EDCs and agitation. |
TCS = 92%, CBZ = 90.5%, E1 = 71.4%, EE2 = 71.3% removals. | Poor contaminants removal using AC when treated municipal wastewater was used. | [109] |
| BPA/DI water | Batch Adsorption (nano-magnetite) |
Adsorption time: 0–120 min; pH: 2–12; adsorbent dose: 0.04–0.22 g; BPA conc: 10–75 ppm; temperature: 30, 35, 40, 45, 50, 55, and 60 °C. | Introduction of 0.1 g of magnetite into different conc. of BPA. Solutions agitation for 45 min at 30 °C. Measurement of residual BPA conc. |
Synthetized magnetite offers great potential for the remediation of BPA-contaminated media. | Low adsorption capacity. Longer treatment period. |
[110] |
| BPA, E2, EE2/sediment | Adsorption (aquatic colloids and sediment in a single and binary system). | Equilibrium conc.: 0.40–2.00 mg/L; aquatic colloids: 42.0 mg/L, 103.5 mg/L; initial concentration of EDCs: 0.5–2.5; pH: 8.24–8.37. |
NA | Sediments enhance contaminants. sorption process by colloids in a binary system. | [111] | |
| BPA, EE2, CytR, 5-Fu, diazinon, cytrabine, caffeine, phenazone, atrazine, 4-NP/hospital wastewater | Adsorption (PAC) | Dosage: 8, 23, 43 (mg/L); PAC doses: 10, 20, and 40 mg/L; initial conc.: 20, 40, and 80 mg/L. Retention time = 2 days. |
The effluent of the PAC reactor was filtered via a flat sheet UF membrane. | Removal efficiencies of diclofenac and carbamazepine and propranolol were 99%, 100%, and ˃94%. | PAC could not remove antibiotic resistance and failed to deactivate pathogens. Energy-intensive. |
[106] |
| Tonalide, BPA, TCS, metolachlor, ketoprofen, and E3/aqueous solutions | Adsorption using PVP-coated magnetite nanoparticles sorbent | pH: 7.5; contact time: 5–40 min; adsorbent dose: 0.75 to 2.5 mg/L; stirring speed: 150 rpm. | NPs were added to the solution followed by sonication. Vials were agitated at 150 rpm. Sample analysis. | The maximum adsorption capacities of BPA and ketoprofen were 90.91 and 83.33 µg/mg, respectively. | NA | [112] |
| PFOA, PFOS, ACE, DIF, and CHL/eenvironmental water | Batch adsorption (magnetic nanoparticles-attached fluorographene-based sorbent) | Initial conc. of adsorbate: 180 µg/L; adsorbent dose: 400 mg/L; speed: 220 rpm; contact time: 10, 30 min |
Solution stirring with developed sorbents and PAC, followed by separation. Measurement of residual EDCs conc. |
DIF, ACE, and CHL (97–99%), PFOA removal ranged between 92% and 95%, PFOS (94–97%). | NA | [113] |
References
- Omar, T.F.T.; Aris, A.Z.; Yusoff, F.M.; Mustafa, S. Occurrence, distribution, and sources of emerging organic contaminants in tropical coastal sediments of anthropogenically impacted Klang River estuary, Malaysia. Mar. Pollut. Bull. 2018, 131, 284–293.
- Frye, C.; Bo, E.; Calamandrei, G.; Calzà, L.; Dessì-Fulgheri, F.; Fernández, M.; Fusani, L.; Kah, O.; Kajta, M.; Le Page, Y.; et al. Endocrine disrupters: A review of some sources, effects, and mechanisms of actions on behaviour and neuroendocrine systems. J. Neuroendocrinol. 2012, 24, 144–159.
- Segneanu, A.E.; Orbeci, C.; Lazau, C.; Sfirloaga, P.; Vlazan, P.; Bandas, C.; Grozescu, I. Waste water treatment methods. Water Treat. 2013, 53–80.
- Falconer, I.R.; Chapman, H.F.; Moore, M.R.; Ranmuthugala, G. Endocrine-Disrupting Compounds: A Review of Their Challenge to Sustainable and Safe Water Supply and Water Reuse. Environ. Toxicol. Int. J. 2006, 21, 181–191.
- Taylor, P.; Sei, K.; Takeda, T.; Soda, S.O.; Fujita, M.; Ike, M. Removal characteristics of endocrine-disrupting chemicals by laccase from white-rot fungi. J. Environ. Sci. Health Part A 2015, 43, 53–60.
- Mompelat, S.; Le Bot, B.; Thomas, O. Occurrence and fate of pharmaceutical products and by-products, from resource to drinking water. Environ. Int. 2009, 35, 803–814.
- Lin, J. Conventional Water Treatment Processes for Removing Pharmaceutical and Endocrine Disrupting Compounds. Environ. Water Resour. Eng. Mast. Proj. 2011, 46, 1–11.
- Ferreiro, C.; Iker, G.; Lombraña, I.; De Luis, A.; Villota, N.; Ros, O.; Etxebarria, N. Contaminants of Emerging Concern Removal in an E ffl uent of Wastewater Treatment Plant under Biological and Continuous Mode Ultrafiltration Treatment. Sustainability 2020, 12, 1–19.
- Gomes, R.; Lester, J. Endocrine Disrupters in Receiving Waters; CRC Press: Boca Raton, FL, USA, 2002; ISBN 9781420032185.
- Garrido-Cardenas, J.A.; Esteban-García, B.; Agüera, A.; Sánchez-Pérez, J.A.; Manzano-Agugliaro, F. Wastewater treatment by advanced oxidation process and their worldwide research trends. Int. J. Environ. Res. Public Health 2020, 17, 170.
- Hua Liu, Z.; Kanjo, Y.; Mizutani, S. Removal mechanisms for endocrine disrupting compounds (EDCs) in wastewater treatment—Physical means, biodegradation, and chemical advanced oxidation: A review. Sci. Total Environ. 2009, 407, 731–748.
- Ribeiro, A.R.; Nunes, O.C.; Pereira, M.F.R.; Silva, A.M.T. An overview on the advanced oxidation processes applied for the treatment of water pollutants defined in the recently launched Directive 2013/39/EU. Environ. Int. 2015, 75, 33–51.
- Barbosa, M.; Moreira, N.F.F.; Ribeiro, A.R.; Pereira, M.F.R.; Silva, A.M.T. Occurrence and removal of organic micropollutants: An overview of the watch list of EU Decision 2015/495. Water Res. 2016.
- Zhang, Z.; Rhind, S.M.; Kerr, C.; Osprey, M.; Kyle, C.E. Selective pressurized liquid extraction of estrogenic compounds in soil and analysis by gas chromatography-mass spectrometry. Anal. Chim. Acta 2011, 685, 29–35.
- Rivera-Utrilla, J.; Sánchez-Polo, M.; Ferro-García, M.Á.; Prados-Joya, G.; Ocampo-Pérez, R. Pharmaceuticals as emerging contaminants and their removal from water. A review. Chemosphere 2013, 93, 1268–1287.
- Balabanič, D.; Hermosilla, D.; Blanco, A.; Merayo, N.; Klemenčič, A.K. The possibility of removal of endocrine disrupters from paper mill waste waters using anaerobic and aerobic biological treatment, membrane bioreactor, ultra-filtration, reverse osmosis and advanced oxidation processes. WIT Trans. Ecol. Environ. 2010, 132, 33–44.
- Tijani, J.O.; Fatoba, O.O.; Petrik, L.F. A Review of Pharmaceuticals and Endocrine-Disrupting Compounds: Sources, Effects, Removal, and Detections. Water Air Soil Pollut. 2013.
- Coday, B.D.; Yaffe, B.G.M.; Xu, P.; Cath, T.Y. Rejection of trace organic compounds by forward osmosis membranes: A literature review. Environ. Sci. Technol. 2014, 48, 3612–3624.
- Canle, M.; Fernández Pérez, M.I.; Santaballa, J.A. Photocatalyzed degradation/abatement of endocrine disruptors. Curr. Opin. Green Sustain. Chem. 2017, 6, 101–138.
- Archer, E.; Petrie, B.; Kasprzyk-Hordern, B.; Wolfaardt, G.M. The fate of pharmaceuticals and personal care products (PPCPs), endocrine disrupting contaminants (EDCs), metabolites and illicit drugs in a WWTW and environmental waters. Chemosphere 2017, 174, 437–446.
- Mailler, R.; Gasperi, J.; Coquet, Y.; Deshayes, S.; Zedek, S.; Cren-Olivé, C.; Cartiser, N.; Eudes, V.; Bressy, A.; Caupos, E.; et al. Study of a large scale powdered activated carbon pilot: Removals of a wide range of emerging and priority micropollutants from wastewater treatment plant effluents. Water Res. 2015, 72, 315–330.
- Peng, X.; Yu, Y.; Tang, C.; Tan, J.; Huang, Q.; Wang, Z. Occurrence of steroid estrogens, endocrine-disrupting phenols, and acid pharmaceutical residues in urban riverine water of the Pearl River Delta, South China. Sci. Total. Environ. 2008, 7, 1–9.
- Carmona, E.; Andreu, V.; Picó, Y. Occurrence of acidic pharmaceuticals and personal care products in Turia River Basin: From waste to drinking water. Sci. Total Environ. 2014, 484, 53–63.
- Ismail, N.A.H.; Wee, S.Y.; Kamarulzaman, N.H.; Aris, A.Z. Quantification of multi-classes of endocrine-disrupting compounds in estuarine water. Environ. Pollut. 2019, 249, 1019–1028.
- Grassi, M.; Kaykioglu, G.; Belgiorno, V.; Lofrano, G. Emerging Compounds Removal from Wastewater. Green Chem. Sustain. 2012, 15–38.
- WHO; UNEP; ILO. International Programme on Chemical Safety, W./ U./ I. Report of the Joint Ipcs-Japan Workshop on Endocrine Disruptors: Research Needs and Future Directions; WHO: Geneva, Switzerland, 2004; pp. 1–52.
- Mozo, I.; Lesage, N.; Sperandio, M.; Bessiere, Y. Impact of sonication on activated sludge properties and consequences on PAH partitioning. Can. J. Chem. Eng. 2016, 94, 244–252.
- Wilson, S.P.; Ouki, S.K.; Saroj, D.P.; Pearce, P.A.; Bancroft, L.; Germain, E. Adopting Primary Plastic Trickling Filters as a Solution for Enhanced Nitrification. Water Environ. Res. 2014, 87, 80–87.
- Samaras, V.G.; Stasinakis, A.S.; Mamais, D.; Thomaidis, N.S.; Lekkas, T.D. Fate of selected pharmaceuticals and synthetic endocrine disrupting compounds during wastewater treatment and sludge anaerobic digestion. J. Hazard. Mater. 2013, 244–245, 259–267.
- Ye, X.; Guo, X.; Cui, X.; Zhang, X.; Zhang, H.; Wang, M.K.; Qiu, L.; Chen, S. Occurrence and removal of endocrine-disrupting chemicals in wastewater treatment plants in the Three Gorges Reservoir area, Chongqing, China. J. Environ. Monit. 2012, 14, 2204–2211.
- López Fernández, R.; Coleman, H.M.; Le-Clech, P. Impact of operating conditions on the removal of endocrine disrupting chemicals by membrane photocatalytic reactor. Environ. Technol. 2014, 35, 2068–2074.
- Zhang, D.; Niu, H.; Zhang, X.; Meng, Z.; Cai, Y. Strong adsorption of chlorotetracycline on magnetite nanoparticles. J. Hazard. Mater. 2011, 192, 1088–1093.
- Sin, J.C.; Lam, S.M.; Lee, K.T.; Mohamed, A.R. Degrading two endocrine-disrupting chemicals from water by UV irradiation with the presence of nanophotocatalysts. Desalin. Water Treat. 2013, 51, 3505–3520.
- Bouchiat, R.; Veignie, E.; Grizard, D.; Soebert, C.; Vigier, M.; Rafin, C. Ability of filamentous fungi to degrade four emergent water priority pollutants. Desalin. Water Treat. 2016, 57, 6740–6746.
- Kim, S.; Cho, H.; Joo, H.; Her, N.; Han, J.; Yi, K.; Kim, J.O.; Yoon, J. Evaluation of performance with small and scale-up rotating and flat reactors; photocatalytic degradation of bisphenol A, 17Β–estradiol, and 17A–ethynyl estradiol under solar irradiation. J. Hazard. Mater. 2017, 336, 21–32.
- Sin, J.C.; Lam, S.M.; Mohamed, A.R.; Lee, K.T. Degrading endocrine disrupting chemicals from wastewater by tiO 2 photocatalysis: A review. Int. J. Photoenergy 2012, 2012.
- Wang, R.; Ma, X.; Liu, T.; Li, Y.; Song, L.; Tjong, S.C.; Cao, L.; Wang, W.; Yu, Q.; Wang, Z. Degradation aspects of endocrine disrupting chemicals: A review on photocatalytic processes and photocatalysts. Appl. Catal. A Gen. 2020, 597, 117547.
- Frontistis, Z.; Fatta-kassinos, D.; Xekoukoulotakis, N.P. Photocatalytic degradation of 17 α-ethynylestradiol in environmental samples by ZnO under simulated solar radiation. J. Chem. Technol. Biotechnol. 2012.
- Becker, D.; Rodriguez-mozaz, S.; Insa, S.; Schoevaart, R.; Barcelo, D.; De Cazes, M.; Belleville, M.P.; Marcano, J.S.; Misovic, A.; Oehlmann, J.; et al. Removal of endocrine disrupting chemicals in wastewater by enzymatic treatment with fungal laccases. Org. Process. Res. Dev. 2017, 21, 480–491.
- Macellaro, G.; Pezzella, C.; Cicatiello, P.; Sannia, G.; Piscitelli, A. Fungal laccases degradation of endocrine disrupting compounds. Biomed Res. Int. 2014, 2014.
- Haugland, J.O.; Kinney, K.A.; Johnson, W.H.; Camino, M.M.A.; Whitman, C.P.; Lawler, D.F. Laccase removal of 2-chlorophenol and sulfamethoxazole in municipal wastewater. Water Environ. Res. 2019, 1–11.
- Reis, A.R.; Sakakibara, Y. Enzymatic degradation of endocrine-disrupting chemicals in aquatic plants and relations to biological Fenton reaction. Water Sci. Technol. 2012, 775–782.
- Song, X.; Mcdonald, J.; Price, W.E.; Khan, S.J.; Hai, F.I.; Ngo, H.H.; Guo, W.; Nghiem, L.D. Bioresource Technology Effects of salinity build-up on the performance of an anaerobic membrane bioreactor regarding basic water quality parameters and removal of trace organic contaminants. Bioresour. Technol. 2016, 216, 399–405.
- Garcia-Rodríguez, A.; Matamoros, V.; Fontàs, C.; Salvadó, V. The ability of biologically based wastewater treatment systems to remove emerging organic contaminants—A review. Environ. Sci. Pollut. Res. 2014, 21, 11708–11728.
- Husain, Q.; Qayyum, S. iological and enzymatic treatment of bisphenol A and other endocrine disrupting compounds: A review. Crit. Rev. Biotechnol. 2012, 1–33.
- Ali, M.; Hussain, Q.; Ishaqi, H.M. Fungal Bioremediation: Fundamentals and Applications-Google Books; CRC Press: Boca Raton, FL, USA, 2019; ISBN 9781138636408.
- Schröder, P. Exploiting Plant Metabolism for the Phytoremediation of Organic Xenobiotics; Humana Press: Totowa, NJ, USA, 2007; Volume 23, pp. 251–263.
- Zheng, H.; Wang, Z.; Deng, X.; Zhao, J.; Luo, Y.; Novak, J.; Herbert, S.; Xing, B. Characteristics and nutrient values of biochars produced from giant reed at different temperatures. Bioresour. Technol. 2013, 130, 463–471.
- Zhan, C.; Sharma, P.R.; He, H.; Sharma, S.K.; McCauley-Pearl, A.; Wang, R.; Hsiao, B.S. Rice husk based nanocellulose scaffolds for highly efficient removal of heavy metal ions from contaminated water. Environ. Sci. Water Res. Technol. 2020, 6, 3080–3090.
- Zhu, Y.; Wei, J.; Zhang, H.; Liu, K.; Kong, Z.; Dong, Y.; Jin, G.; Tian, J.; Qin, Z. Fabrication of composite membrane with adsorption property and its application to the removal of endocrine disrupting compounds during filtration process. Chem. Eng. J. 2018, 352, 53–63.
- Yoon, Y.; Westerhoff, P.; Snyder, S.A.; Wert, E.C. Nanofiltration and ultrafiltration of endocrine disrupting compounds, pharmaceuticals and personal care products. J. Memb. Sci. 2006, 270, 88–100.
- Yuan, H.; He, Z. Integrating membrane filtration into bioelectrochemical systems as next generation energy-efficient wastewater treatment technologies for water reclamation: A review. Bioresour. Technol. 2015, 195, 202–209.
- Peters, R.E.M.; Courtenay, S.C.; Hewitt, L.M.; MacLatchy, D.L. Effects of 17α-ethynylestradiol on early-life development, sex differentiation and vitellogenin induction in mummichog (Fundulus heteroclitus). Mar. Environ. Res. 2010, 69, 178–186.
- Zhang, J.; Chua, H.C.; Zhou, J.; Fane, A.G. Factors affecting the membrane performance in submerged membrane bioreactors. J. Memb. Sci. 2006, 284, 54–66.
- Lin, H.; Peng, W.; Zhang, M.; Chen, J.; Hong, H.; Zhang, Y. A review on anaerobic membrane bioreactors: Applications, membrane fouling and future perspectives. Desalination 2013, 314, 169–188.
- Kang, G.D.; Cao, Y. ming Application and modification of poly(vinylidene fluoride) (PVDF) membranes—A review. J. Memb. Sci. 2014, 463, 145–165.
- Liu, F.; Hashim, N.A.; Liu, Y.; Abed, M.R.M.; Li, K. Progress in the production and modification of PVDF membranes. J. Memb. Sci. 2011, 375, 1–27.
- Neale, P.A.; Schäfer, A.I. Quantification of solute-solute interactions in steroidal hormone removal by ultrafiltration membranes. Sep. Purif. Technol. 2012, 90, 31–38.
- Hu, Z.; Si, X.; Zhang, Z.; Wen, X. Enhanced EDCs removal by membrane fouling during the UF process. DES 2014, 336, 18–23.
- Zhao, F.B.; Tang, C.C.; Liu, X.Y.; Shi, F.J.; Song, X.R.; Tian, Y.; Li, Z.S. Transportation characteristics of bisphenol A on ultrafiltration membrane with low molecule weight cut-off. Desalination 2015, 362, 18–25.
- Al-Rifai, J.H.; Khabbaz, H.; Schäfer, A.I. Removal of pharmaceuticals and endocrine disrupting compounds in a water recycling process using reverse osmosis systems. Sep. Purif. Technol. 2011, 77, 60–67.
- Syahida, N.; Anan, M.; Jaafar, J.; Hafiz, M.; Othman, D.; Rahman, M.A.; Aziz, F.; Shahrodin, N.S. Titanium dioxide incorporated thin film composite membrane for bisphenol A removal. Malays. J. Fundam. Appl. 2019, 15, 755–760.
- Pera-Titus, M.; García-Molina, V.; Baños, M.A.; Giménez, J.; Esplugas, S. Degradation of chlorophenols by means of advanced oxidation processes: A general review. Appl. Catal. B Environ. 2004, 47, 219–256.
- Hu, X.; Shi, W.; Cao, F.; Hu, G.; Hao, Y.; Wei, S.; Wang, X.; Yu, H. Chemosphere Bioanalytical and instrumental analysis of estrogenic activities in drinking water sources from Yangtze River Delta. Chemosphere 2013, 90, 2123–2128.
- Al-husaini, I.S.; Rahim, A.; Yusoff, M.; Lau, W.; Fauzi, A.; Al-abri, M.Z.; Dzul, M.; Wirzal, H. Chemical Engineering Research and Design Iron oxide nanoparticles incorporated polyethersulfone electrospun nanofibrous. Chem. Eng. Res. Des. 2019, 8, 142–154.
- Yüksel, S.; Kabay, N.; Yüksel, M. Removal of bisphenol A (BPA) from water by various nanofiltration (NF) and reverse osmosis (RO) membranes. J. Hazard. Mater. 2013, 263, 307–310.
- Sun, B.; Guan, X.; Fang, J.; Tratnyek, P.G. Activation of Manganese Oxidants with Bisulfite for Enhanced Oxidation of Organic Contaminants: The Involvement of Mn(III). Environ. Sci. Technol. 2015, 49, 12414–12421.
- Brillas, E.; Sirés, I.; Oturan, M.A. Electro-fenton process and related electrochemical technologies based on fenton’s reaction chemistry. Chem. Rev. 2009, 109, 6570–6631.
- Igunnu, E.T.; Chen, G.Z. Produced water treatment technologies. Int. J. Low-Carbon Technol. 2014, 9, 157–177.
- Nasseri, S.; Ebrahimi, S.; Abtahi, M.; Saeedi, R. Synthesis and characterization of polysulfone/graphene oxide nano-composite membranes for removal of bisphenol A from water. J. Environ. Manag. 2018, 205, 174–182.
- Wei, X.; Shi, Y.; Fei, Y.; Chen, J.; Lv, B.; Chen, Y.; Zheng, H.; Shen, J.; Zhu, L. Removal of trace phthalate esters from water by thin-film composite nanofiltration hollow fiber membranes. Chem. Eng. J. 2016, 292, 382–388.
- Modi, A.; Bellare, J. Copper sulfide nanoparticles/carboxylated graphene oxide nanosheets blended polyethersulfone hollow fiber membranes: Development and characterization for efficient separation of oxybenzone and bisphenol A from water. Polymer 2019, 163, 57–67.
- Gmurek, M.; Olak-Kucharczyk, M.; Ledakowicz, S. Photochemical decomposition of endocrine disrupting compounds—A review. Chem. Eng. J. 2017, 310, 437–456.
- Wang, H.; Wang, J. Electrochemical degradation of 4-chlorophenol using a novel Pd/C gas-diffusion electrode. Appl. Catal. B Environ. 2007, 77, 58–65.
- Hernandez-LeahZeeman, G.; Buisman, C.J.N. Removal of micropollutants from aerobically treated grey water via ozone and activated carbon. Water Res. 2011, 5, 1–10.
- Gerrity, D.; Stanford, B.D.; Trenholm, R.A.; Snyder, S.A. An evaluation of a pilot-scale nonthermal plasma advanced oxidation process for trace organic compound degradation. Water Res. 2010, 44, 493–504.
- Gernjak, W.; Krutzler, T.; Glaser, A.; Malato, S.; Caceres, J.; Bauer, R.; Fernández-Alba, A.R. Photo-fenton treatment of water containing natural phenolic pollutants. Chemosphere 2003, 50, 71–78.
- Shi, F.; Ma, Y.; Ma, J.; Wang, P.; Sun, W. Preparation and characterization of PVDF/TiO 2 hybrid membranes with different dosage of nano-TiO 2. J. Memb. Sci. 2012, 389, 522–531.
- Shindume L, H.; Zhao, Z.; Wang, N.; Liu, H.; Umar, A.; Zhang, J.; Wu, T.; Guo, Z. Enhanced Photocatalytic Activity of B, N-Codoped TiO 2 by a New Molten Nitrate Process. J. Nanosci. Nanotechnol. 2018, 19, 839–849.
- Fernández-Domene, R.M.; Sánchez-Tovar, R.; Lucas-Granados, B.; Muñoz-Portero, M.J.; Ramírez-Grau, R.; García-Antón, J. Visible-light photoelectrodegradation of diuron on WO3 nanostructures. J. Environ. Manag. 2018, 226, 249–255.
- Wei, N.; Cui, H.; Wang, C.; Zhang, G.; Song, Q.; Sun, W.; Song, X.; Sun, M.; Tian, J. Bi2O3 nanoparticles incorporated porous TiO2 films as an effective p-n junction with enhanced photocatalytic activity. J. Am. Ceram. Soc. 2017, 100, 1339–1349.
- Tian, J.; Hu, X.; Yang, H.; Zhou, Y.; Cui, H.; Liu, H. High yield production of reduced TiO 2 with enhanced photocatalytic activity. Appl. Surf. Sci. 2016, 360, 738–743.
- Hu, X.; Zhao, H.; Tian, J.; Gao, J.; Li, Y.; Cui, H. Synthesis of few-layer MoS2 nanosheets-coated TiO2 nanosheets on graphite fibers for enhanced photocatalytic properties. Sol. Energy Mater. Sol. Cells 2017, 172, 108–116.
- Muz, M.; Ak, M.S.; Komesli, O.T.; Gökçay, C.F. An ozone assisted process for treatment of EDC’s in biological sludge. Chem. Eng. J. 2013, 217, 273–280.
- Dudziak, M.; Kudlek, E.; Burdzik-niemiec, E. Decomposition of micropollutants and changes in the toxicity of water matrices subjected to various oxidation processes. Desalination Water Treat. 2018, 117, 22233.
- Saggioro, E.M.; Chaves, F.P.; Felix, L.C.; Gomes, G.; Bila, D.M. Endocrine Disruptor Degradation by UV/Chlorine and the Impact of Their Removal on Estrogenic Activity and Toxicity. Int. J. Photoenergy 2019, 2019, 1–9.
- Talaiekhozani, A.; Bagheri, M.; Talaei, M.R.; Jaafarzadeh, N. An Overview on Production and Applications of Ferrate(VI). Jundishapur J. Heal. Sci. 2016, 8.
- Zhang, Z.; Zhu, H.; Wen, X.; Si, X. Degradation behavior of 17 α-ethinylestradiol by ozonation in the synthetic secondary e ffl uent. J. Environ. Sci. 2012, 24, 228–233.
- Li, S.; Zhang, G.; Wang, P.; Zheng, H.; Zheng, Y. Microwave-enhanced Mn-Fenton process for the removal of BPA in water. Chem. Eng. J. 2016, 294, 371–379.
- Pérez-González, A.; Urtiaga, A.M.; Ibáñez, R.; Ortiz, I. State of the art and review on the treatment technologies of water reverse osmosis concentrates. Water Res. 2012, 46, 267–283.
- Bai, X.; Acharya, K. Removal of seven endocrine disrupting chemicals (EDCs) from municipal wastewater effluents by a freshwater green alga. Environ. Pollut. 2019, 247, 534–540.
- Bernal, V.; Giraldo, L.; Moreno-Piraján, J.C.; Balsamo, M.; Erto, A. Mechanisms of methylparaben adsorption onto activated carbons: Removal tests supported by a calorimetric study of the adsorbent–adsorbate interactions. Molecules 2019, 24, 413.
- Sharma, P.R.; Sharma, S.K.; Antoine, R.; Hsiao, B.S. Efficient Removal of Arsenic Using Zinc Oxide Nanocrystal-Decorated Regenerated Microfibrillated Cellulose Scaffolds. ACS Sustain. Chem. Eng. 2019, 7, 6140–6151.
- Adeleke, O.A.; Latiff, A.A.A.; Saphira, M.R.; Daud, Z.; Ismail, N.; Ahsan, A.; Ab Aziz, N.A.; Al-Gheethi, A.; Kumar, V.; Fadilat, A.; et al. Principles and Mechanism of Adsorption for the Effective Treatment of Palm Oil Mill Effluent for Water Reuse; Elsevier: Amsterdam, The Netherlands, 2018; ISBN 9780128139028.
- Sharma, P.R.; Chattopadhyay, A.; Sharma, S.K.; Geng, L.; Amiralian, N.; Martin, D.; Hsiao, B.S. Nanocellulose from Spinifex as an Effective Adsorbent to Remove Cadmium(II) from Water. ACS Sustain. Chem. Eng. 2018, 6, 3279–3290.
- Khan, M.A.; Ngabura, M.; Choong, T.S.Y.; Masood, H.; Chuah, L.A. Biosorption and desorption of Nickel on oil cake: Batch and column studies. Bioresour. Technol. 2012, 103, 35–42.
- Malhotra, M.; Suresh, S.; Garg, A. Tea waste derived activated carbon for the adsorption of sodium diclofenac from wastewater: Adsorbent characteristics, adsorption isotherms, kinetics, and thermodynamics. Environ. Sci. Pollut. Res. 2018, 25, 32210–32220.
- Adeleke, A.A.; Latiff, A.A.A.; Daud, Z.; Mohamed, R.M.S.R.; Aziz, N.A.A.; Ismail, N.; Rafatullah, M.; Ahmad, A.; Hossain, K. Adsorption of pollutants from palm oil mill effluent using natural adsorbents: Optimization and isotherm studies. Desalin. Water Treat. 2019, 169, 181–190.
- Lobzenko, I.; Shiihara, Y.; Sakakibara, A.; Uchiyama, Y.; Umeno, Y.; Todaka, Y. Chemisorption enhancement of single carbon and oxygen atoms near the grain boundary on Fe surface: Ab initio study. Appl. Surf. Sci. 2019, 493, 1042–1047.
- Dickenson, E.R.V.; Drewes, J.E. Quantitative structure property relationships for the adsorption of pharmaceuticals onto activated carbon. Water Sci. Technol. 2010, 62, 2270–2276.
- Mailler, R.; Gasperi, J.; Coquet, Y.; Derome, C.; Buleté, A.; Vulliet, E.; Bressy, A.; Varrault, G.; Chebbo, G.; Rocher, V. Removal of emerging micropollutants from wastewater by activated carbon adsorption: Experimental study of different activated carbons and factors influencing the adsorption of micropollutants in wastewater. J. Environ. Chem. Eng. 2016, 4, 1102–1109.
- Tagliavini, M.; Engel, F.; Weidler, P.G.; Scherer, T.; Schäfer, A.I. Adsorption of steroid micropollutants on polymer-based spherical activated carbon (PBSAC). J. Hazard. Mater. 2017, 337, 126–137.
- Adebayo, B.O.; Orimolade, F.A.; Adekola, G.B. Adsorptive removal of bisphenol A using synthesized magnetite nanoparticles. Appl. Water Sci. 2018, 8, 1–8.
- Sun, W.; Zhou, K. Adsorption of three selected endocrine disrupting chemicals by aquatic colloids and sediments in single and binary systems. J. Soils Sediments 2015, 15, 456–466.
- Alizadeh Fard, M.; Vosoogh, A.; Barkdoll, B.; Aminzadeh, B. Using polymer coated nanoparticles for adsorption of micropollutants from water. Colloids Surfaces A Physicochem. Eng. Asp. 2017, 531, 189–197.
- Kovalova, L.; Siegrist, H.; Von. Gunten, U.; Eugster, J.; Hagenbuch, M.; Wittmer, A.; Moser, R.; Mcardell, C.S. Elimination of Micropollutants during Post-Treatment of Hospital Wastewater with Powdered Activated Carbon, Ozone, and UV. Environ. Sci. Technol. 2013, 47, 7899–7908.
- Mannie, N.; Bower, A. Challenges in Determining the Correct Waste Disposal Solutions for Local Municipalities—A South African Overview. In Proceedings of the 20th WasteCon Conference, Somerset West, South Africa, 6–10 October 2014; pp. 17–22.
- Luo, Y.; Guo, W.; Hao, H.; Duc, L.; Ibney, F.; Zhang, J.; Liang, S.; Wang, X.C. Science of the Total Environment A review on the occurrence of micropollutants in the aquatic environment and their fate and removal during wastewater treatment. Sci. Total Environ. 2014, 473–474, 619–641.
- Komesli, O.T.; Muz, M.; Ak, M.S.; Bakirdere, S.; Gokcay, C.F. Occurrence, fate and removal of endocrine disrupting compounds (EDCs) in Turkish wastewater treatment plants. Chem. Eng. J. 2015, 277, 202–208.
- Zhang, Z.; Feng, Y.; Gao, P.; Wang, C.; Ren, N. Occurrence and removal efficiencies of eight EDCs and estrogenicity in a STP. J. Environ. Monit. 2011, 13, 1366–1373.
- Zhang, M.; Zhou, Q.; Li, A.; Shuang, C.; Wang, W.; Wang, M. A magnetic sorbent for the efficient and rapid extraction of organic micropollutants from large-volume environmental water samples. J. Chromatogr. A 2013, 1316, 44–52.
- Grover, D.P.; Zhou, J.L.; Frickers, P.E.; Readman, J.W. Improved removal of estrogenic and pharmaceutical compounds in sewage effluent by full scale granular activated carbon: Impact on receiving river water. J. Hazard. Mater. 2011, 185, 1005–1011.
- Qiang, Z.; Dong, H.; Zhu, B.; Qu, J.; Nie, Y. A comparison of various rural wastewater treatment processes for the removal of endocrine-disrupting chemicals (EDCs). Chemosphere 2013, 92, 986–992.




