Over the years, the persistent occurrence of superfluous endocrine-disrupting compounds (EDCs) (sub µg L−1) in water has led to serious health disorders in human and aquatic lives, as well as undermined the water quality. At present, there are no generally accepted regulatory discharge limits for the EDCs to avert their possible negative impacts. Moreover, the conventional treatment processes have reportedly failed to remove the persistent EDC pollutants, and this has led researchers to develop alternative treatment methods. Comprehensive information on the recent advances in the existing novel treatment processes and their peculiar limitations is still lacking. In this regard, the various treatment methods for the removal of EDCs are critically studied and reported in this entry.
1. Introduction
The detection of endocrine-disrupting compounds (EDCs) as contaminants in the environment has drawn the significant interest of researchers during the past few years, owing to their potential human and environmental threats [
1]. Several chemicals (some illicit and some still in circulation) have been considered as EDCs [
2]. The increasing accumulation of more EDC micro-contaminants in natural waters is mainly attributable to the advancement and rapid expansion of chemical technologies for organic production and processing [
3].
These contaminants can infiltrate directly into the aquatic environment via effluent outflow and indirectly as runoff, yet the main carrier of EDC contaminants to the freshwater bodies is via treated and raw urban effluent release into water bodies [
4,
5]. Moreover, even most of the treated potable water resources may be polluted through deep-well injection of the effluent and surface water outflow [
6]. This shows that even treated water is not absolutely free from the EDC contaminants [
7,
8]. The persistence of EDCs in water even at a trace concentration is notably dangerous to the health because of its ability to cause metabolic and reproductive disorders; therefore, the need for efficient management of EDCs contained in effluent before discharge is indispensable [
9,
10].
The management of effluent discharges emanating from various sources, such as pharmaceutical compounds, pesticides, personal care products, and similar compounds, has received significant attention in many countries [
11,
12]. This ensures considerable control, even though more stringent regulations are still required for better management [
13]. Various studies have indicated that EDCs are ubiquitous and can frequently be found in almost all water sources, namely surface waters, groundwater, municipal water, treated and untreated wastewater treatment plant (WWTP) effluent, and finished drinking water, globally [
14,
15]. Primarily, the most practiced management technique is a conventional treatment. In this vein, several reports have indicated that the conventional treatment approach is inefficient in the elimination of EDC contaminants from water [
8,
16,
17,
18]. This is because several EDCs are non-biodegradable in nature or have poor biodegradability and strong chemical cohesion in the environment [
19]. For instance, about 41 and 40 EDC pollutants were found in the treated effluent and environmental waters at the downstream and upstream of wastewater treatment facilities (WWTF), respectively [
20]. Notably, among these, higher proportions of BPA (239.0 ng/L; 396.4 ng/L), diclofenac (467.7ng/L; 1461.5 ng/L), carbamazepine (157.1 ng/L; 279.5 ng/L), and ibuprofen (153.3 ng/L; 312.1 ng/L) were recorded in the effluent of both upstream and downstream of WWTF. Analogously, Mailler et al. [
21] reported that NP and BPA were detected in the treated effluents of wastewater treatment plants, ranging between 100 and 1000 ng/L, as well as higher proportions of artificial sweeteners, close to 1000–10,000 ng/L. Besides, effluent discharge from municipal wastewater was classified as the major source of EDCs in the rivers in China [
22]. Moreover, Lin [
7], in his study, established that some selected EDC contaminants such as DEET and TCEP are relatively resistant to the conventional treatment process. This trend was corroborated by Carmona et al. [
23], who established that PPCPs compounds ranging between 6.72 to 940 ng/L were discovered in an effluent discharge after the conventional wastewater treatment process.
2. Treatment Processes in Removing Endocrine Disrupting Compounds
The emergence of unregulated micro-contaminants, such as endocrine-disrupting compounds (EDCs), created the need for effectual treatment technologies to remediate the concentration level [
37]. Recently, several approaches for the elimination of EDCs from wastewater, including potable water, have been reported. These include the conventional treatment method, adsorption process, biological treatment based on enzymatic degradation, photocatalysis degradation, ozonation and oxidation processes, use of membrane filtration technique, and hybrid systems [
24,
43,
71,
72].
Conventional Treatment Process
The conventional treatment process comprises three major phases, namely primary (or mechanical), secondary, and advanced phases for the remediation of EDC contaminants from water sources. The initial primary phase is configured to eliminate the suspended, gross, and floating solids from raw wastewater from its source. It also involves screening to confine solid objects and removal of suspended solids through sedimentation by gravity [
71]. The secondary treatment contains activated sludge, which employs an aeration tank or dispersed-growth reactor containing microorganisms (consuming the organic matter and converting it into carbon dioxide (CO
2), water, and energy to enhance reproduction and development), mixed liquor, and a suspension of wastewater. The constituents of the aeration tank are agitated vigorously by the aerator, which then supplies required oxygen to the biological suspension [
72]. The trickling filters in the secondary treatment phase serve as a support media where wastewater is applied intermittently or continuously over the media, such that, as the water flows, the microbes become linked to the media and build a fixed film. In this context, the organic matter in the wastewater dissolves into the film, where it is metabolized [
73]. The conventional treatment technique has remained the most extensively utilized treatment process for decades and is widely considered to be very efficient in handling reclaimed water by eliminating the mass of microbial contaminants and chemical compounds. Hence, the efficiency of the conventional treatment process has been investigated by researchers to assess its effectiveness in removing EDC contaminants from water.
Table 2 presents a summary of findings regarding the existing conventional treatment approaches.
Table 2. Treatment by conventional processes.
| Major Contaminants/Sources |
Treatment Process |
Treatment Factor |
Brief Procedure |
Major Findings |
Limitations |
References |
| TCS, NP2EO, IBF, DCF, TCS, BPA, KFN, NP, NP1EO, NPX/wastewater, and sewage sludge samples |
Conventional treatment (mesophilic anaerobic
sludge digestion) |
(HRT: 9 h; SRT: 8 d),
(SRT: 17 d). (HRT: 23 h, SRT: 18 d). |
Wastewater samples collection.
Sewage sludge samples were homogenized extractions of wastewater samples. |
The removal efficiency of DCF and IBF ranged between 39% and 100%, IBF and NPX were ˃80%. |
Higher proportions of NP
in digested sludge.
Detection of TCS and NP in treated wastewater.
Too many modular units. |
[74] |
E1, E2,
E3, EE2, BPA, and 4-NP/rural wastewater effluent |
Activated sludge.
Micro-power biofilm reactor.
Constructed wetland.
Stabilization pond. |
Temp: 30 and 70 °C;
HRT = 12–24;
24–120;
24–240;
10–16. |
Biological contact oxidation.
Subsurface flow.
Facultative pond.
Anoxic oxidation. |
Percent removal of target EDCs > 70% in summer. |
Unstable performance of decentralized processes.
Pronounced impacts of effluent discharged on the quality of receiving water.
Too many modular units. |
[63] |
| BPA, E1, E2, E3, EE2, and DES/effluent from a wastewater treatment plant |
WWTP
activated sludge treatment processes.
Oxidation ditch reversed anaerobic and sequential batch reactor SBR. |
HRT: 7.6–35.31 h
SRT: 5.8–31.9 days |
|
73.7% of BPA was removed.
High removal rates of EDCs (i.e., > 85%). |
Some concentrations of EDCs were found in the effluents and can pose potential risks to ecosystems and human health.
Longer HRT and SRT. |
[75] |
| 59 EDC contaminants/ wastewater effluents |
Fluidized powdered activated carbon (PAC) pilot (WWTP configuration). |
SRT: 5–7 days; bed depth: 1–3 m. hydraulic velocity: 6–12 m/h; contact time:
10–20 min. |
Pre-primary and biological treatments. Pre-treatment (screening). Biofiltration system, Micropollutant analyses. |
Removal of parabens and pesticides ranged between 50% and 95%, paracetamol, IBF, sulfamethoxazole 60–80% |
Artificial sweeteners (1000–10,000 ng/L), BPA and NP (100–1000 ng/L) were detected in the effluent. |
[21] |
3. Contemporary Techniques for the Removal of EDCs from Various Water Sources
3.1. Catalytic Degradation of EDCs
The catalytic degradation process involves stimulating the rate of degradation of the EDCs [
82]. The enhancement of the degradation rate may be achieved via the presence of photo radiation or organic enzymes [
83]. Primarily, the catalyst offers a choice of reaction path with lesser excitation energy compared with the non-catalysed mechanism. Typically, the catalyst reacts to build a temporary intermediate in catalysed mechanisms, which subsequently rejuvenate the original catalyst in a virtuous circle [
80].
3.2. Photo-Catalytic Degradation of EDCs
Generally, the photocatalytic degradation of EDC contaminants requires the stimulation of photoreactions under a combined influence of light (solar irradiation or UV) and a catalyst [
19,
81]. It involves multiple steps (such as diffusion and adsorption of EDCs, chemical reactions, desorption of intermediates, and removal of the product from the interface) to complete the process. The reaction products (intermediates) of these steps ultimately constitute the end products during the last stage. Usually, the desired end products of a completed photocatalytic degradation process are H
2O and CO
2. Detection of these reaction intermediates would offer additional insight into the mechanism involved in the degradation technique and would facilitate the degradation pathway. Thus, the efficacy of a successful and higher photocatalytic process is based on the generation of HO
• radicals [
19].
The performance and degradation rate of a photocatalytic process hinges on several working conditions that determine the elimination of EDCs in water. These include light intensity, wavelength, presence of organic and inorganic compounds, reaction temperature, catalyst loading, concentration and chemical structure of the contaminants, initial concentration of the substrate, solution pH, and dissolved oxygen [
19,
84]. Few studies have explored photocatalytic and enzymatic degradation processes to eliminate EDCs from different water sources (
Table 3). Furthermore, the photocatalysis process has been considered as a promising technique for degrading EDCs, with no secondary contamination, moderate reaction medium, and better energy-saving [
85].
Table 3. Removals of some EDCs during photocatalytic and enzymatic degradation processes.
| Major Contaminants/Source |
Treatment Process |
Treatment Factor |
Brief Procedure |
Major Findings |
Limitations |
References |
EE2/
ultra-pure water and treated wastewater |
Photocatalytic degradation using ZnO under simulated solar radiation |
EE2 conc: 100–500 µg/L, photon flux: 4.93 × 10−7–5.8 × 10−7 Einstein L−1 S−1; ZnO conc: 50–500 mg/L, treatment time: 2–10 min. |
Spiking of water matrix was spiked with EE2,
photocatalysis of the solution.
Periodic sampling and centrifugation. |
Rapid EE2 degradation occurred via first-order kinetics. |
Detection of EE2 in the effluent.
Retardation of EE2 degradation by organic and inorganic matter. |
[86] |
| BPA/municipal WWTP, bottled water, ultra-pure water |
Solar photocatalytic degradation |
pH: 6.1, catalyst: 81.3–339.2 mg, TiO2 loading: 0, 81.3, 101.8, 152.3, 339.2 mg, ZnO loading: 0.5–6.8 mg/cm2, H2O2: (25–100 mg/L), BPA initial conc.: 50–200 µg/L, treatment time: 0–90 min. |
The incident radiation intensity was measured econometrically.
The water matrix was spiked with the organic substance with the addition of the ZnO /TiO2 catalytic plate.
Periodic sampling and analysis. |
Increasing the number of immobilized catalysts enhances BPA conversion. |
Partial inhibition of BPA degradation due to the presence of EE2.
Weak degradation in wastewater. |
[43] |
| E1, E2, EE2, E3, NP, BPA/artificial, and real wastewater |
Enzymatic degradation using fungal laccases |
pH: 1–1.5
Temperature: −20 °C
Contact time: 2, 6, 24 h. |
Constant shaking as laccase uses molecular oxygen for oxidizing substrates.
Acidification of enzymatic reaction at each time interval (2, 6, and 24 h).
Complete inactivation of the laccase activity.
Extraction via solid-phase extraction (SPE) for chemical analysis. |
Immobilized laccase removed EDCs (83% for T. Versicolor and 87% of M. thermophile), 99% removal after 24 h.
Removal rates for estrogenic = 82% after 24 h. |
Formation of toxic by-products. |
[87] |
| BPA |
Fungal laccases degradation using oxidative enzyme |
(1): 25 μM
of each molecule, pH 5.0 (50 mM sodium citrate buffer), 1.5 U/mL laccase,
(2): 100 μM BPA, pH 5.0 (50 mM sodium citrate buffer), 25 °C, and 1.5 U/mL laccase, reaction time: 1 h. |
Addition of methanol and Tween to the solution.
Incubation of each EDC.
Addition of hydrochloric acid (HCl) to the reaction mixture and centrifugation at a specific time interval at room temperature.
Analysis of supernatant and BPA degradation. |
BPA was oxidized
under all conditions tested. |
Complex procedure. |
[88] |
2-chlorophenol and
SMZ/municipal wastewater |
Laccase degradation |
pH 7, initial SMX at 10 μM and ACE at 10 μM.
Time (h): 0, 0.25,1, 24. |
NA |
Excellent removal of SMZ in the absence of mediators in secondary effluent. |
Poor removal of sulfamethoxazole in all buffered solutions.
Not economically viable. |
[89] |
| BPA, 2,4-dichlorophenol, 4-tert OP, pentachlorophenol, and NP/aquatic plants |
Enzymatic degradation |
Endogenous H2O2 concentration in aquatic plants (170–590 μmol/kg-FW) |
|
EDCs were degraded by oxidative enzymes. |
Longer treatment period (>100 days).
Complex procedure. |
[90] |
| Atrazine (herbicide), phenyl phenol, BPA, and TCS/municipal wastewater |
Biosorption and biodegradation. |
Feed NaCl concentration (0–15 g/L). Initial MLSS = 16 g/L; HRT = 5 d; mixed liquor pH = 7 ± 0.1; temperature = 35 ± 1 °C. |
Feeding the bioreactor,
circulation of digested sludge.
Mixing of the sludge. |
Trimethoprim, carazolol, hydroxyzine, amitriptyline, and linuron, removal rates ≤ 80%.
Phenyl phenol removal = 60%. |
Relatively low removal rates of phenyl phenol, BPA, and TCS.
BPA was poorly removed, from 40% to 20%.
Poor removal of atrazine (6.8%). |
[91] |
| DEHP, fluoranthene, AMPA, and E1/ wastewater effluent |
Filamentous fungi biodegradation. |
pH 5.5, incubation period: 96 h (AT96h), degradation period:
10 days. |
Degradation test conducted in mineral medium incubated for 10 days with each fungus. |
Fungi degradation of DEHP = 100%, AMPA = 69% with F. solani and T. harzianum. |
E1 not degraded by all fungal isolate trials. |
[80] |
3.3. Enzymatic Degradation
Phytoremediation (enzymatic degradation) is another novel remediation and a promising technique for the elimination of EDCs and other similar chemical compounds in wastewater. Researchers have identified several micro-organisms as critical factors to proceed with the EDC phytoremediation process, and the most widely applied ones are fungal, bacterial, and algal strains, as well as mixed cultures [
92]. Enzymatic degradation also depends on the microorganism activities, although the degree of degradation has a strong correlation with several environmental factors, such as pH, nutrient, and temperature [
93]. Some of these bio-enzymes include oxidoreductases: laccases, tyrosinases, polyphenol oxidases, manganese peroxidase, lignin peroxidase, horseradish peroxidase, and bitter gourd peroxidase. Studies have collectively indicated that apart from the environmental factors, quite a few redox mediators, additives, and surfactants could better enhance the enzymatic oxidation process [
94].
Table 3 presents the recent findings on the use of enzymes and their treatment conditions for removing EDCs.
Macellaro et al. [
88] examined the degradation of five different EDCs using four distinct fungal laccases, subject to the availability of both synthetic and natural mediators. The results obtained from this study revealed that all laccases could oxidize different EDCs, with bisphenol A (BPA) exclusively oxidized under all conditions tested. In addition, mediators remarkably increase the performance of enzymatic treatment and enhance the degradation of substrates refractory to laccases oxidation. Two main possible limitations of this study were the tedious nature of the experiment procedure and challenges in adapting enzymes capable of eliminating the target compounds with an affinity constant of the same order of magnitude concerning the typical proportions of EDCs in the surroundings.
3.4. Removal of EDCs by Membranes
Membrane technology is the most extensively applied physicochemical separation technology for the removal of salt and microbes from water [
100,
101,
102]. Membrane processes have been productively utilized in difficulties relating to unavailability of fresh and clean water and could remove EDCs and natural organic matter (NOM) from both wastewater reuse and drinking water [
103,
104]. This could be achieved due to its unique characteristics, including energy efficiency, compactness, high throughput, and cost-effectiveness [
105].
Essentially, pressure-operated membrane processes are described and classified into four major classes, mostly based on the pore size and operating pressure exerted: microfiltration, ultrafiltration, nanofiltration, and reverse osmosis [
106,
107].
Moreover, polyvinylidene fluoride (PVDF), polyacrylonitrile (PAN), polyethersulfone (PES), cellulose acetate (CA), and polysulfone (PSF) are the most frequently applied polymer materials in membrane purification for water treatment [
108]. Among these, PVDF is the most favored and broadly employed polymeric membrane and has drawn growing interest in recent years from manufacturers and researchers. This is because PVDF polymer has exhibited unique and promising characteristics that make it an effectual and superior candidate to reject EDC contaminants from water. These include exceptional aging resistance, outstanding mechanical strength and thermal stability, and good chemical resistance, which are central for the practical application of membrane technology [
109]. In addition, PVDF shows acceptable processability for fabricating flat sheet, hollow fibre (HF), and tubular membranes, and it is dissolvable in numerous conventional solvents, such as N, N-dimethyl acetamide (DMAC), dimethylformamide (DMF), and N-methyl-2-pyrrolidone (NMP) [
110]. The chemical and physical characteristics of the material could strongly affect membrane performance [
100], since the ideal membrane is one that can yield a high flux with zero fouling or clogging and that is chemically stable and resistant, physically durable, nonbiodegradable, and low cost.
Table 4 presents a summary of some research findings on the application of membrane treatment technique in eliminating EDCs pollutants.
Table 4. Removal of EDCs by Membranes.
| Major Contaminants/Water |
Treatment Process |
Operating/Treatment Factor |
Brief Procedure |
Major Findings |
Limitations |
References |
| E1, E2, progesterone, testosterone/purified water |
UF membrane |
MWCO: 1–100kDa
Pressure: 0.5–5 bar
Pure water flux (L/m2h)
20.8–359.2
Final flux:21.9–288.5
Time: 2–40 min
pH: 8 |
Stirring feed solution at 200 rpm for 16 h.
Filtering of purified membrane for 30 min.
Measurement of pure water flux.
Collection of permeate. |
Removal via solute–solute interactions for E1 correspond to higher proportion of organic matter at 25–50 mg/L for 10 kDa (48–52%); 100 kDa (33–38%) membranes. |
Poor removals of E1 and hormone contaminants (52% and 38%). |
[111] |
| BPA, CBZ, IBF, and SFZ/drinking water |
UF membrane |
Operating speed: 50 psi.
Flow rate: 0.65 L/min per cell. |
|
Initial partial removal of BPA. |
Poor BPA removal using modified PES membranes. |
[113] |
| BPA/drinking water |
UF-PS (PS) membrane. |
Temp: 25 ± 0.5 °C.
pH: 7–13
BPA concentration: 100–500 μg/L.
pH: (3.68–10) |
Measurement of pure water flux.
Filtration of BPA solution. |
Higher removal at the initial stage of the filtration. |
Lower removal efficiency (20%).
Fouling. |
[120] |
BPA/pure BPA
solution |
UF membrane |
pH: (3–13)
MWCO: 100 Da
TMP: 0.1 × 106–0.3 × 106 Pa
Temp: 20 ± 2 °C
BPA conc.: 5 mg/L |
The UF membrane was installed and the solution was introduced into the UF cup and followed by magnetic stirring. |
Both salt and acidic pH improve the transportation of BPA. |
BPA rejection decreased significantly when the BPA molecule was ionized. |
[114] |
| DMP, DEP, DBP, DnOP, DEHP/water |
NF membrane |
pH: 4–9; pure water flux: 47.5 L/m2 h; temperature: 25–45 °C. |
Preparation of a feed solution.
Measurement of concentrations of PAEs in both the feed and permeate. |
Removal efficiencies of 95.4%, 95.1%, and 91.5% were recorded for DEHP, DnOP, and DBP. |
Lower adsorption rates.
Low rejection of sulfamides. |
[131] |
| BPA/biologically treated wastewater |
MF and NF |
Suspended solids = 78 ± 12 mg BPA conc.: 0.3 ± 0.14–0.7 ± 0.27
Jv(L/m2h) = 6.0–18.6
80 L/(m2h) for NF
Temp = 21 °C
TMP = 0.3 MPa (MF)
0.7 MPa (NF) |
Circulation of module with pure water.
Determination of pure water infiltration. |
Both techniques eliminate BPA. BPA removal efficiency: 61–75% with NF. |
Fouling.
A decline in permeate flux in MF. |
[53] |
| BPA/model solution |
NF and RO membranes |
Temperature: 45–50 °C
Max pressure: 31–83 bar, pH: 2–11
water permeability: 0.85–14.86 (L/m2h bar)
Time: 30–360 min |
|
≥98% BPA rejection was achieved with polyamide-based RO membranes. |
High energy demand.
Too many modular units. |
[121] |
| BPA, E2, E1, E3, EE2/synthetic wastewater |
UF membrane |
working pressures (25, 30, 50, 75 kPa); temp: 20 ± 2 °C; TOC = 7 mg/L; pH 7.6; conductivity = 1000 |
Soaking of fresh membrane for 24 h.
Removal of impurities.
Determination of flux. |
EDCs removal rates of (10–76%) were achieved via a fouled membrane. |
Poor removal of E3 (10–17%). |
[119] |
| BPA, DMP, DBP, NP, DOP/water |
Nano-functionalized membrane using polypropylene (PP) non-woven fabric |
Operating pressure: 0.02–0.5 Mpa; pH: 6.5; Temp: 25 °C |
The target pollutants were dissolved in deionized water and quantified.
The filtration experiment was conducted. |
˃80% BPA rejection was recorded after a period of 1.3 s. |
Removal of contaminants was attained at higher operating pressure of 0.5 MPa. |
[132] |
| Oxybenzone and BPA/synthetic solution |
Nanohybrid (CuSG) blended PES-HF membranes |
Filtration time: 120 min;
temp: 20 °C; pressure: 1 bar |
25 mg/L solution of oxybenzone and 5 mg/L BPA solution were filtrated via the HFM samples and the permeate was analyzed via a UV–visible spectrophotometer |
Higher rejection of oxybenzone (98%) and BPA (95%) was recorded.
Elevated pure water permeability (528.2 ± 44.6 Ml/m2/h/mmHg). |
Nil |
[133] |
| BPA/synthetic solution |
UF(TFC) immobilized with TiO2 |
Preparation of feed solution.
Quantification of the feed and the permeate solution. |
|
|
|
[115] |
| BPA/drinking water |
Nanocomposite membrane electrospun PVDF-PVP-MnO2 |
Working pressure: 0.5–2.5 bars; sampling period: 0, 5,
10, 20, and 30 min; temp: 27 °C. |
The membrane was fabricated using electrospinning technique and was applied in a filtration system to assess the removal efficiency of BPA. The concentrations of BPA were analyzed using HPLC. |
Complete rejection of BPA (100%) was attained for NF2 and NF6 after 30 min. |
Nil |
[128] |
| BPA/synthetic solution |
Photocatalytic PSF/TiO2/Fe-doped composite UF membrane |
BPA
concentration: 10 mg/L; specific temperature: 140–220 °C, 6–24 h; pressure: 0.1–0.2 MPa. |
Preparation of Fe-doped TiO2 photocatalysts, synthesis of photocatalytic membranes; assessment of photocatalytic performance |
BPA removal rate of 90.78% was recorded. |
Nil |
[129] |
| BPA/water |
PSF/GO nano-composite membranes |
Input pressure: 1–5 bar,
operating time: 10–50 min, pH: 3–11, initial BPA concentration:
1–9 mg/L. |
Synthesis of GO; preparation of GO/PSF nano-hybrid membranes; BPA concentration
was analyzed using a UV–vis spectrophotometer |
BPA removal efficiency of 93% was attained. |
|
[130] |
3.5. Removal of EDCs by Ozonation and Advanced Oxidation Processes (AOPs)
Advanced oxidation processes (AOPs) are generally applied for the elimination of persistent and recalcitrant EDC constituents from municipal and industrial wastewater. In this context, AOP techniques can become very favourable methods for purifying wastewater comprising hardly biodegradable or non-biodegradable organic compounds with excessive poisonousness [
3]. The AOPs can be successfully applied in wastewater purification to destroy the persistent EDC contaminants, the oxidation procedure being controlled by the very strong oxidative potential of the HO
• radicals produced into the reaction medium by various mechanisms [
134]. AOPs are extensively identified as techniques that employ strong radical oxidants (such as
·OH, SO
4–) to fast-track or accelerate the removal of several organic pollutants from different water matrices [
135]. Notably,
•OH is one of the most exceedingly non-selective and reactive radical species existing in AOPs, with a standard reduction potential of 2.8 V vs. standard hydrogen electrode (SHE) [
136]. These processes involve the generation of strongly reactive oxidizing hydroxyl radicals (HO
−) species, such that the generation of
•OH could be enhanced in the presence of H
2O
2, ultraviolet, and Fenton reagent [
137]. AOPs can be employed to oxidize contaminants partly or completely, typically via several oxidants. Photocatalytic and photo-chemical advanced oxidation processes including UV/TiO
2, UV/H
2O
2, UV/H
2O
2/O
3, UV/H
2O
2/Fe
2+(Fe
3+), UV/O
3, and UV/H
2O
2/TiO
2 can be utilized for oxidative degradation of EDC contaminants. A complete mineralization of the EDC contaminants is not essential, as it is more valuable to convert them into biodegradable aliphatic carboxylic acids succeeded by a biological process [
138]. The preferential utilization of H
2O
2 (oxidative agent) and HO radicals producer is evidenced by the fact that the hydrogen peroxide is simple to store, transport, and utilize, with an efficient and safe procedure [
134].
Ozonation and AOPs are powerful redox techniques which exhibit remarkable advantages over the conventional treatment process, particularly small footprint, higher degradation rates, and non-selective removal of non-biodegradable persistent refractory compounds that could not be treated by the conventional treatment process [
10].
Besides, these processes allow decontaminating effects which are crucial for water reusability applications due to direct human contact, such as household reclamation applications [
139]. Ozone can degrade organic pollutants directly and indirectly through the generation of a reactive oxidizing agent (
•OH). The aim of AOPs as a pre-treatment process, either singularly or with supplementary processes, is to enhance the qualities of the conventionally treated effluent and to achieve deactivation of pathogens not treatable by conventional approach [
10]. However, several EDC pollutants are susceptible to both ozone and AOPs (particularly carbamazepine and naproxen), while some are simply dependent on
•OH (namely meprobamate and atrazine) [
140].
Notably, the most frequently applied AOPs to eliminate EDCs from various water matrices comprise of ozonation (catalytic), heterogenous photocatalysis using UV light source, Fenton and photo-Fenton processes, electrochemical oxidation, or a combination of any of the processes [
141].
Different catalysts have been identified for catalytic processes subject to the reaction procedure, involving metal oxides (Zn, Mn, Ti, Bi, Cu, and Co, etc.), noble metals (such as Pd, Ir, Pt, Rh, and Ru), or metal-free carbonaceous material (viz., activated carbons, graphite, carbon fibres and foams, carbon nanotubes, and carbon xerogels) [
12]. However, the most widely utilized catalyst in ozonation and other advanced oxidation processes is titanium dioxide (TiO
2) [
10]. Amongst the various photocatalysts, TiO
2 has been demonstrated to be a promising and favourable semiconductor photocatalyst in advanced oxidation processes and heterogeneous photocatalysis due to its low cost, availability, stability, non-toxicity, unique photocatalytic efficacy, and its potential applications in water and wastewater management [
142]. Comparatively, degradation of EDCs via a solar photocatalytic approach has not been sufficiently explored, despite it being a promising technique with unique characteristics such as zero secondary contamination, benign reaction condition, facile procedure, and low energy demand [
143,
144,
145,
146,
147].
Table 5 presents a summary of the recent advances in AOPs applications for remediating EDCs.
Table 5. Removals of EDCs during ozonation and advanced oxidation processes.
| Major Contaminants/Sources |
Treatment Process |
Treatment Factor |
Brief Procedure |
Major Findings |
Limitations |
References |
Diltiazen, progesterone,
BBP, E1, CBZ, acetaminophen/biological sludge |
Pulse ozonation experiment |
Operating pressure = 5 bar; gas flow rate = 10–140 L/h; MLSS = 2.3–4.2 g/L; ozone period: 6–150 min.
Ozone dose (mgO3/L):
1.11–18.65; pH = 6.4–7.1 |
Ozonation of the sludge samples. Continuous aeration.
Analysis of the residual EDCs conc. in the samples. |
˃99% removal of target EDCs contaminants were achieved after 4 days. |
Production of toxic by-products.
The high cost of ozone production. |
[148] |
| BPA, E2, and EE2/wastewater |
AOP (H2O2, O3, UV, UV/TiO2, UV/H2O2, and UV/O3) |
NA |
NA |
The removal rate of pharmaceutical EDCs ≥ 96% during UV/TiO2 process. |
Poor removal of caffeine.
Generation of several oxidation by-products with high toxic potential. |
[149] |
| E2, EE2, BPA/wastewater treatment plant effluent matrix |
Degradation by UV light/chlorine |
Chlorine conc.: 0.2–2 mg/L; reaction time: 30 min; initial EDC conc.: 100 µg/L; UVC irradiance: 14.79 mW cm−2; temp.: 25; pH: 7 |
Spiking of EDCs in WWTP effluent and ultrapure water.
UV/Cl process.
Samples collection.
Addition of sodium thiosulfate followed by filtration. Disinfection evaluations. |
The combination of UVC with chlorine significantly and rapidly degrades EDCs.
An upsurge in chlorine concentration yields almost 99% EDCs removal. |
Formation of chlorate by-product disinfection.
UV light penetration can be obstructed by turbidity. |
[150,151] |
E1, E2, EE2, DES, TCS,
17α- treubolone, 17 β- treubolone, 19- nortestosterone, AEDb
testosterone, methyltestotesterone,
4-OHA, prednisone
cortisol, cortison,
19- norethindrone,
medroxyprogesterone,
BPA, 4-tert-OP, 4- NP,
triclocarban, ADD,
17β- boldenone, stanozolol, epi-andosterone, andosterone
5α-dihydrosteterone, preanisolone,
dexamethasone,
ethynyl testosterone,
progesterone/secondary wastewater effluent |
Fe (VI) treatment process |
Temp. = 23 ± 2 °C
micropollutants = 100 µg/L−1; Fe (VI) = 10 mgFeL−1; pH: 6.88–7.09; Fe (VI) dosage = 0, 2.5, 5, and 10 mgFe L−1;
DOC. = 5.0 mgCl−. |
Application of Fe (VI) to secondary effluent.
Dosing of solid Fe (VI) in the effluent.
Stirring of the solution.
Addition of methanol and H2SO4. |
Fe (VI) treatment could achieve both oxidative eliminations of detected EDCs as a tertiary treatment technology. |
It failed to react with triclocarban, three androgens.
Low ferrate (VI) production rate. |
[59,151] |
| EE2/synthetic secondary effluent |
Ozonation |
Ozone conc.: 2, 4, 9 mg/L; NOM conc.: 0–80 mg/L; pH: 6–10
O3: TOC: 0.2–1.0 |
Spiking different conc. of ozone into the stock solution.
Removal of residual ozone and radicals. Testing of blank controls. |
The initial concentration of ozone and natural organic substance adversely affect degradation efficiency. Effective degradation of EE2 by ozonation at pH 6 resulted in higher degradation of EE2. |
Generation of toxic by-products.
Production of solid by-products.
High operating costs. |
[152] |
| BPA/aqueous solution |
Microwave-enhanced Mn-Fenton process |
BPA initial concentration = 100.0 mg/L; reaction time = 6 min |
Addition of BPA solution with Fenton reagents followed by
heating.
Determination of BPA conc. |
BPA removal = 99.7% and total organic carbon (TOC) (53.1%). |
Generation of complicated secondary sludge.
A narrow range of optimal pH (2.5–4.0). |
[153] |
3.6. Removal of Endocrine-Disrupting Compounds via Adsorption Process
Adsorption is one of the most effective methods for treating wastewater, and it essentially depends on the availability of active sites on the sorbent, surface chemistry, and also the chemical (sorbate pKa, basicity or acidity of the sorbent, etc.) and physical properties (such as the sorbate molecular size, sorbent pore density, contact area, etc.), and the specific interactions between adsorbent–adsorbate [
102,
155]. However, the adsorbent and adsorbate may have distinct properties based on their constituents, and this is the key determinant of the type of adsorption [
156,
157]. Generally, the adsorption process may be considered as physisorption and chemisorption [
102,
158]. The processes may occur in different interfaces such as solid–liquid and/or solid–gas in the presence of interactive forces between the surfaces [
159,
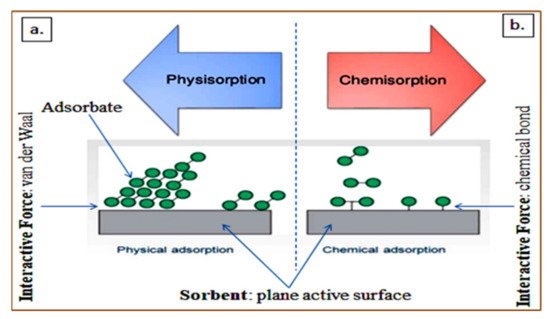
160]. The physical interaction between the adsorbed compounds and the solid surface due to weak van der Waals force of attraction results in the reversible process called physisorption. The fundamental interaction of permanent and temporary electric dipoles generates the van der Waal forces. Principally, the adsorbate is at a distance from the interacting active plane surface but entrapped due to the binding energy, and this allows multiple layers or a single layer of adsorption [
158].
As a result of the weak binding energy, a lower temperature is required for the desorption process. The activation energy usually ranges between 20 and 40 kJ, which implies that the tendency of the active sorbent in an aqueous medium to dissolve is high [
161]. This could subvert the overall adsorption capacity, though the mechanism correlates with the treatment factors, particularly pH, dosage, particle size, temperature, contact time, and agitation speed [
158]. Moreover, most adsorbents have an excellent potential for regenerating adsorption capacity and the release of quality and safe effluent suitable for discharge [
162].
Figure 3a,b depict a schematic physisorption and chemisorption mechanism, respectively. In the chemisorption mechanism, the chemical bonding results in the breakage and formation of a new bond between the active plane surface of the sorbent and adsorbate [
158]. This signifies that higher adsorption energy and temperature is required, which is usually in the range between 200 and 400 kJ/mol [
161]. Distinctly, single layer adsorbate occurs in chemisorption, and the mechanism is influenced by the aforementioned treatment factors [
163].
Adsorption technique for the removal of EDCs from various water sources using AC has received extensive efforts, which have yielded considerable progress in the last two decades [
164,
165,
166]. For example, Temmink et al. [
139] and Kovalova et al. [
25] in their separate studies reported excellent EDCs removal using PAC, ranging between 84% and 99% under different operating adsorption conditions. The summary of findings regarding the removal of EDCs via adsorption is presented in
Table 6.
Table 6. Removals of some EDCs during the adsorption process.
| Major Contaminants/Sources |
Treatment Process |
Treatment Factor |
Brief Procedure |
Major Findings |
Limitations |
References |
| EE2/water |
Adsorption (polyamide adsorbent) |
pH: 4.8–9.1; constant dosage of 0.2 g/L; contact time: 24 h; agitation rate: 250 rpm; temp.: 25 °C. |
Dilution of EE2 working solutions from EE2 stock solutions.
Addition of adsorbent into EE2 aqueous solutions.
Agitation of mixed solutions. |
Maximum adsorption capacity = 25.4 mg g/L.
Adsorption rates ranged between 5.3- and 22.4-fold. |
A molecule-level investigation. |
[70] |
| BPA, NP BP3, TCS/aerobically treated greywater |
Adsorption (PAC) |
29.0 g/70.6 mL bed volume; initial compound proportion: 100–1600 µg/L; dose: 1.25 g/L; contact time: 5 min. |
NA |
TCS removal = 95%.
BPA removal = 99%.
NP removal = 84%. |
The exorbitant cost of PAC. |
[26,139] |
| TCS, E1, E2, and EE2, clofibric acid, CBZ, clofibrate methyl ester, clofibrate/water, and treated wastewater |
Batch adsorption using crosslinked polymer adsorbent and activated carbon |
Polymer sorbent dosage: 0.2–1.2 g/L; AC: 0.05–0.2 g/L; retention time: 5.7–24.2 min, temp.: 21 ± 2 °C. |
Removal of selected EDCs from ultrapure water.
Introduction of polymer adsorbents in solutions of EDCs and agitation. |
TCS = 92%, CBZ = 90.5%, E1 = 71.4%, EE2 = 71.3% removals. |
Poor contaminants removal using AC when treated municipal wastewater was used. |
[41] |
| BPA/DI water |
Batch Adsorption
(nano-magnetite) |
Adsorption time: 0–120 min; pH: 2–12; adsorbent dose: 0.04–0.22 g; BPA conc: 10–75 ppm; temperature: 30, 35, 40, 45, 50, 55, and 60 °C. |
Introduction of 0.1 g of magnetite into different conc. of BPA.
Solutions agitation for 45 min at 30 °C.
Measurement of residual BPA conc. |
Synthetized magnetite offers great potential for the remediation of BPA-contaminated media. |
Low adsorption capacity.
Longer treatment period. |
[167] |
| BPA, E2, EE2/sediment |
Adsorption (aquatic colloids and sediment in a single and binary system). |
Equilibrium conc.: 0.40–2.00 mg/L; aquatic colloids: 42.0 mg/L, 103.5 mg/L;
initial concentration of EDCs: 0.5–2.5; pH: 8.24–8.37. |
NA |
Sediments enhance contaminants. sorption process by colloids in a binary system. |
|
[168] |
| BPA, EE2, CytR, 5-Fu, diazinon, cytrabine, caffeine, phenazone, atrazine, 4-NP/hospital wastewater |
Adsorption (PAC) |
Dosage: 8, 23, 43 (mg/L);
PAC doses: 10, 20, and 40 mg/L; initial conc.: 20,
40, and 80 mg/L. Retention time = 2 days. |
The effluent of the PAC reactor was filtered via a flat sheet UF membrane. |
Removal efficiencies of diclofenac and carbamazepine and propranolol were 99%, 100%, and ˃94%. |
PAC could not remove antibiotic resistance and failed to deactivate pathogens.
Energy-intensive. |
[25] |
| Tonalide, BPA, TCS, metolachlor, ketoprofen, and E3/aqueous solutions |
Adsorption using PVP-coated magnetite nanoparticles sorbent |
pH: 7.5; contact time: 5–40 min; adsorbent dose: 0.75 to 2.5 mg/L; stirring speed: 150 rpm. |
NPs were added to the solution followed by sonication. Vials were agitated at 150 rpm. Sample analysis. |
The maximum adsorption capacities of BPA and ketoprofen were 90.91 and 83.33 µg/mg, respectively. |
NA |
[169] |
| PFOA, PFOS, ACE, DIF, and CHL/eenvironmental water |
Batch adsorption (magnetic nanoparticles-attached fluorographene-based sorbent) |
Initial conc. of adsorbate: 180 µg/L;
adsorbent dose: 400 mg/L;
speed: 220 rpm;
contact time: 10, 30 min |
Solution stirring with developed sorbents and PAC, followed by separation.
Measurement of residual EDCs conc. |
DIF, ACE, and CHL (97–99%), PFOA removal ranged between 92% and 95%, PFOS (94–97%). |
NA |
[62] |
This entry is adapted from the peer-reviewed paper 10.3390/polym13193229