
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Anuja Bokare | + 2542 word(s) | 2542 | 2021-08-30 04:37:33 | | | |
| 2 | Peter Tang | Meta information modification | 2542 | 2021-10-11 12:28:28 | | |
Video Upload Options
Graphene represents a new generation of materials which exhibit unique physicochemical properties such as high electron mobility, tunable optics, a large surface to volume ratio, and robust mechanical strength. These properties make graphene an ideal candidate for various optoelectronic, photonics, and sensing applications. . In recent years, numerous efforts have been focused on azobenzene polymers (AZO-polymers) as photochromic molecular switches and thermal sensors because of their light induced conformations and surface-relief structures. However, these polymers often exhibit drawbacks like low photon storage lifetime, energy density and aggregation. These issues can be alleviated by incorporating graphene derivatives (GDs) into AZO-polymers to form orderly arranged molecules like GD-AZO composites.
1. Introduction
2. Properties of GDs, AZO-Polymers, and GD-AZO Composites
2.1. Properties of GDs
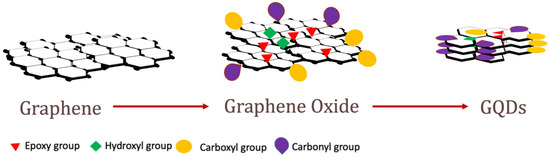
2.2. Properties of AZO-Polymers
2.3. Properties of GD-AZO Composites
3. Synthesis of GD-AZO Composites
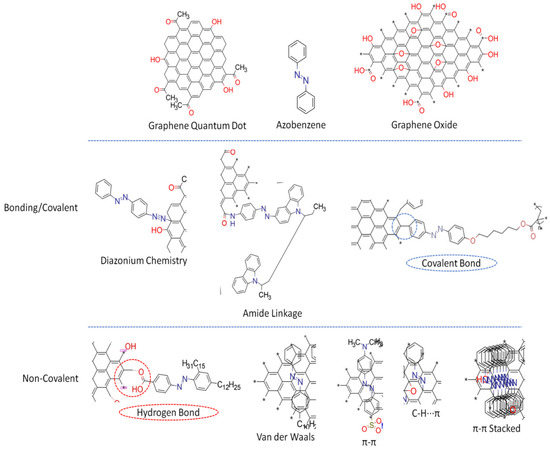
3.1. Covalent Linkages
3.2. Non-Covalent Linkage
4. Characterization Techniques
4.1. Fourier Transform Infrared Spectroscopy
|
Wavenumbers (cm−1) |
Assigned Functional Groups |
|---|---|
|
3434 |
O=H Stretching |
|
1728 |
C=O Stretching |
|
1640 |
C=C Stretching |
|
1386 |
C–O Stretching |
|
1060 |
C–O–C Stretching |
|
1581 |
N=N Stretching |
|
1297 |
C–N stretching |
|
3060 |
C–H stretching |
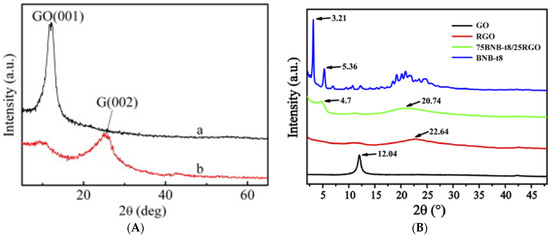
4.2. X-ray Diffraction Analysis
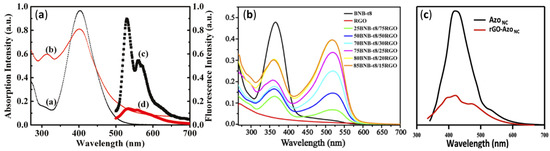
4.3. UV-Visible and Photoluminescence Spectroscopy
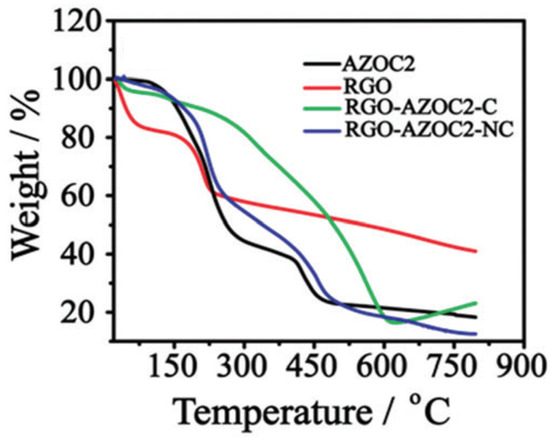
4.4. Thermogravimetric Analysis
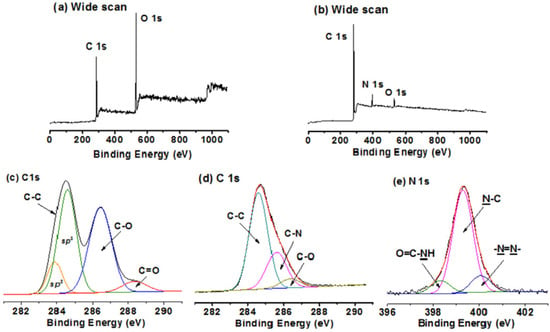
4.5. X-ray Photoelectron Spectroscopy Analysis
5. Applications
|
Graphene Derivative |
Azobenzene Derivative |
Linkage Method (Type of Linkage) |
Application |
Reference |
|---|---|---|---|---|
|
GO and RGO |
Azobenzene |
Covalent Diazotization method |
Solar Thermal storage |
[55] |
|
RGO |
Azobenzene |
Covalent Diazotization method |
Solar Thermal storage |
[56] |
|
GO |
Azobenzene |
Covalent Diazotization method |
Potential Supercapacitor electrodes |
[57] |
|
GO |
Azobenzene |
Covalent Amide linkage |
Photoswitches |
[51] |
|
GO |
Poly (N-vinylcarbazole) |
Covalent Amide linkage |
Resistive random-access memory |
[53] |
|
GO |
Azobenzene |
Covalent Amide linkage |
Photoswitches |
[49] |
|
GO |
Amino functionalized Azobenzene |
Covalent Polyimide method |
Photoswitches |
[58] |
|
RGO |
Polyazo (Bismark-Brown -Y) |
Noncovalent π–π stacking |
Chemiresistor for dissolved O2 |
[59] |
|
RGO |
Polyazo (Bismark-Brown -Y) |
Noncovalent π–π stacking |
Chemiresistor sensor for mitochondrial Oxygen consumption |
[60] |
|
RGO and GO |
Azobenzene nanocluster |
Noncovalent π–π stacking and direct immobilization |
p-type diode n-type diode |
[61] |
|
R-GO |
Azobenzene BNB-t8 |
Noncovalent π–π stacking |
Nonlinear optical material |
[50] |
|
RGO |
Azobenzene from cardanol |
Covalent And Noncovalent |
Photoswitches |
[52] |
|
RGO |
Bis-azobenzene |
Covalent bonding |
Solar thermal storage |
[62] |
|
Au-doped RGO |
Gemini Azo |
Noncovalent Photochromic stabilizers |
Photoswitches |
[63] |
|
GQDs |
Azobenzene derived from cardanol |
Noncovalent Hydrogen bonding |
Fluorescent Probe and IMPLICATION logic gate |
[64] |
|
GO |
Cationic surfactant azo |
Noncovalent Photochromic stabilizers |
Photoswitches |
[65] |
References
- Tiwari, S.K.; Sahoo, S.; Wang, N.; Huczko, A. Graphene Research and Their Outputs: Status and Prospect. J. Sci. Adv. Mater. Devices 2020, 5, 10–29.
- Sattar, T. Current Review on Synthesis, Composites and Multifunctional Properties of Graphene. Top. Curr. Chem. 2019, 377, 10.
- Ji, X.; Xu, Y.; Zhang, W.; Cui, L.; Liu, J. Review of Functionalization, Structure and Properties of Graphene/polymer Composite Fibers. Compos. Part A Appl. Sci. Manuf. 2016, 87, 29–45.
- Georgakilas, V.; Otyepka, M.; Bourlinos, A.B.; Chandra, V.; Kim, N.; Kemp, K.C.; Hobza, P.; Zboril, R.; Kim, K.S. Functionalization of Graphene: Covalent and Non-Covalent Approaches, Derivatives and Applications. Chem. Rev. 2012, 112, 6156–6214.
- Yang, G.-H.; Bao, D.-D.; Liu, H.; Zhang, D.-Q.; Wang, N.; Li, H.-T. Functionalization of Graphene and Applications of the Derivatives. J. Inorg. Organomet. Polym. Mater. 2017, 27, 1129–1141.
- He, S.; Li, H.; Chen, H. Preparation of Light-Sensitive Polymer/graphene Composite via Molecular Recognition by β-Cyclodextrin. J. Mater. Sci. 2018, 53, 14337–14349.
- Bottari, G.; Herranz, M.Á.; Wibmer, L.; Volland, M.; Rodríguez-Pérez, L.; Guldi, D.M.; Hirsch, A.; Martín, N.; D’Souza, F.; Torres, T. Chemical Functionalization and Characterization of Graphene-Based Materials. Chem. Soc. Rev. 2017, 46, 4464–4500.
- Kumar, R.; Singh, R.K.; Dubey, P.K.; Oh, I.-K. Review on Functionalized Graphenes and Their Applications. Smart Nanosyst. Eng. Med. 2012, 1, 18.
- Peimyoo, N.; Li, J.; Shang, J.; Shen, X.; Qiu, C.; Xie, L.; Huang, W.; Yu, T. Photocontrolled Molecular Structural Transition and Doping in Graphene. ACS Nano 2012, 6, 8878–8886.
- Seo, S.; Min, M.; Lee, S.M.; Lee, H. Photo-Switchable Molecular Monolayer Anchored between Highly Transparent and Flexible Graphene Electrodes. Nat. Commun. 2013, 4, 1920.
- Liu, Z.; Wang, H.I.; Narita, A.; Chen, Q.; Mics, Z.; Turchinovich, D.; Kläui, M.; Bonn, M.; Müllen, K. Photoswitchable Micro-Supercapacitor Based on a Diarylethene-Graphene Composite Film. J. Am. Chem. Soc. 2017, 139, 9443–9446.
- Döbbelin, M.; Ciesielski, A.; Haar, S.; Osella, S.; Bruna, M.; Minoia, A.; Grisanti, L.; Mosciatti, T.; Richard, F.; Prasetyanto, E.A.; et al. Light-Enhanced Liquid-Phase Exfoliation and Current Photoswitching in Graphene-Azobenzene Composites. Nat. Commun. 2016, 7, 11090.
- Purkait, M.K.; Sinha, M.K.; Mondal, P.; Singh, R. (Eds.) Chapter 4—Photoresponsive Membranes. In Interface Science and Technology; Elsevier: Amsterdam, The Netherlands, 2018; Volume 25, pp. 115–144.
- Gonzalez, A.; Kengmana, E.S.; Fonseca, M.V.; Han, G.G.D. Solid-State Photoswitching Molecules: Structural Design for Isomerization in Condensed Phase. Mater. Today Adv. 2020, 6, 100058.
- Han, G.D.; Park, S.S.; Liu, Y.; Zhitomirsky, D.; Cho, E.; Dincă, M.; Grossman, J.C. Photon Energy Storage Materials with High Energy Densities Based on Diacetylene–azobenzene Derivatives. J. Mater. Chem. A Mater. Energy Sustain. 2016, 4, 16157–16165.
- Kumar, G.S.; Neckers, D.C. Photochemistry of Azobenzene-Containing Polymers. Chem. Rev. 1989, 89, 1915–1925.
- De Martino, S.; Mauro, F.; Netti, P.A. Photonic Applications of Azobenzene Molecules Embedded in Amorphous Polymer. Riv. Nuovo Cim. 2020, 43, 599–629.
- Wei, Y.-B.; Tang, Q.; Gong, C.-B.; Lam, M.H.-W. Review of the Recent Progress in Photoresponsive Molecularly Imprinted Polymers Containing Azobenzene Chromophores. Anal. Chim. Acta 2015, 900, 10–20.
- Yager, K.G.; Barrett, C.J. Novel Photo-Switching Using Azobenzene Functional Materials. J. Photochem. Photobiol. A Chem. 2006, 182, 250–261.
- Tang, J.; Feng, Y.; Feng, W. Photothermal Storage and Controllable Release of a Phase-Change Azobenzene/aluminum Nitride Aerogel Composite. Compos. Commun. 2021, 23, 100575.
- Sun, S.; Liang, S.; Xu, W.-C.; Xu, G.; Wu, S. Photoresponsive Polymers with Multi-Azobenzene Groups. Polym. Chem. 2019, 10, 4389–4401.
- Yuan, K.; Guo, Y.-J.; Zhao, X. A Novel Photo-Responsive Azobenzene-Containing Nanoring Host for Fullerene-Guest Facile Encapsulation and Release. Phys. Chem. Chem. Phys. 2014, 16, 27053–27064.
- Jintoku, H.; Matsuzawa, Y.; Yoshida, M. Dual Use of Anionic Azobenzene Derivative as Dispersant and Dopant for Carbon Nanotubes for Enhanced Thermal Stability of Transparent Conductive Films. Carbon 2019, 152, 247–254.
- Romi, S.; Fanetti, S.; Alabarse, F.; Mio, A.M.; Bini, R. Synthesis of Double Core Chromophore-Functionalized Nanothreads by Compressing Azobenzene in a Diamond Anvil Cell. Chem. Sci. 2021, 12, 7048–7057.
- Feng, W.; Luo, W.; Feng, Y. Photo-Responsive Carbon Nanomaterials Functionalized by Azobenzene Moieties: Structures, Properties and Application. Nanoscale 2012, 4, 6118–6134.
- Li, M.; Feng, Y.; Liu, E.; Qin, C.; Feng, W. Azobenzene/graphene Hybrid for High-Density Solar Thermal Storage by Optimizing Molecular Structure. Sci. China Tech. Sci. 2016, 59, 1383–1390.
- Luo, W.; Feng, Y.; Qin, C.; Li, M.; Li, S.; Cao, C.; Long, P.; Liu, E.; Hu, W.; Yoshino, K.; et al. High-Energy, Stable and Recycled Molecular Solar Thermal Storage Materials Using AZO/graphene Hybrids by Optimizing Hydrogen Bonds. Nanoscale 2015, 7, 16214–16221.
- Al-Bataineh, Q.M.; Ahmad, A.A.; Alsaad, A.M.; Telfah, A. New Insight on Photoisomerization Kinetics of Photo-Switchable Thin Films Based on Azobenzene/Graphene Hybrid Additives in Polyethylene Oxide. Polymers 2020, 12, 2954.
- Mousaabadi, K.Z.; Ensafi, A.A.; Hadadzadeh, H.; Rezaei, B. Reduced Graphene Oxide and Carbon Nanotubes Composite Functionalized by Azobenzene, Characterization and Its Potential as a Curcumin Electrochemical Sensor. J. Electroanal. Chem. 2020, 873, 114418.
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric Field Effect in Atomically Thin Carbon Films. Science 2004, 306, 666–669.
- Campuzano, S.; Yáñez-Sedeño, P.; Pingarrón, J.M. Carbon Dots and Graphene Quantum Dots in Electrochemical Biosensing. Nanomaterials 2019, 9, 634.
- Gao, X.-G.; Cheng, L.-X.; Jiang, W.-S.; Li, X.-K.; Xing, F. Graphene and Its Derivatives-Based Optical Sensors. Front. Chem. 2021, 9, 615164.
- Jin, X.; Feng, C.; Ponnamma, D.; Yi, Z.; Parameswaranpillai, J.; Thomas, S.; Salim, N.V. Review on Exploration of Graphene in the Design and Engineering of Smart Sensors, Actuators and Soft Robotics. Chem. Eng. J. Adv. 2020, 4, 100034.
- Xie, S.; Natansohn, A.; Rochon, P. Recent Developments in Aromatic Azo Polymers Research. Chem. Mater. 1993, 5, 403–411.
- Benkhaya, S.; M’rabet, S.; El Harfi, A. Classifications, Properties, Recent Synthesis and Applications of Azo Dyes. Heliyon 2020, 6, e03271.
- Spencer Hartley, G. 113. The Cis-Form of Azobenzene and the Velocity of the Thermal Cis→trans-Conversion of Azobenzene and Some Derivatives. J. Chem. Soc. 1938, 633–642.
- Krollpfeiffer, F.; Mühlhausen, C.; Wolf, G. Zur Kenntnis der Lichtempfindlichkeit von Aryl-β-naphtylamin-azofarbstoffen. Justus Liebigs Ann. Chem. 1934, 508, 39–51.
- Merino, E.; Ribagorda, M. Control over Molecular Motion Using the Cis-Trans Photoisomerization of the Azo Group. Beilstein J. Org. Chem. 2012, 8, 1071–1090.
- Layek, R.K.; Nandi, A.K. A Review on Synthesis and Properties of Polymer Functionalized Graphene. Polymer 2013, 54, 5087–5103.
- Bujak, K.; Nocoń, K.; Jankowski, A.; Wolińska-Grabczyk, A.; Schab-Balcerzak, E.; Janeczek, H.; Konieczkowska, J. Azopolymers with Imide Structures as Light-Switchable Membranes in Controlled Gas Separation. Eur. Polym. J. 2019, 118, 186–194.
- Cardano, F.; Frasconi, M.; Giordani, S. Photo-Responsive Graphene and Carbon Nanotubes to Control and Tackle Biological Systems. Front. Chem. 2018, 6, 102.
- Park, M.; Kim, N.; Lee, J.; Gu, M.; Kim, B.-S. Versatile Graphene Oxide Nanosheets via Covalent Functionalization and Their Applications. Mater. Chem. Front. 2021, 5, 4424–4444.
- Fu, J.; Wang, X.; Wang, T.; Zhang, J.; Guo, S.; Wu, S.; Zhu, F. Covalent Functionalization of Graphene Oxide with a Presynthesized Metal-Organic Framework Enables a Highly Stable Electrochemical Sensing. ACS Appl. Mater. Interfaces 2019, 11, 33238–33244.
- Smith, A.T.; LaChance, A.M.; Zeng, S.; Liu, B.; Sun, L. Synthesis, Properties, and Applications of Graphene Oxide/reduced Graphene Oxide and Their Nanocomposites. Nano Mater. Sci. 2019, 1, 31–47.
- Ran, J.; Chu, C.; Pan, T.; Ding, L.; Cui, P.; Fu, C.-F.; Zhang, C.-L.; Xu, T. Non-Covalent Cross-Linking to Boost the Stability and Permeability of Graphene-Oxide-Based Membranes. J. Mater. Chem. A Mater. Energy Sustain. 2019, 7, 8085–8091.
- Vapaavuori, J.; Geraldine Bazuin, C.; Priimagi, A. Supramolecular Design Principles for Efficient Photoresponsive Polymer–azobenzene Complexes. J. Mater. Chem. 2018, 6, 2168–2188.
- Ahmed, J.; Tabish, T.A.; Zhang, S.; Edirisinghe, M. Porous Graphene Composite Polymer Fibres. Polymers 2020, 13, 76.
- Teng, Z.; Wang, B.; Hu, Y.; Xu, D. Light-Responsive Nanocomposites Combining Graphene Oxide with POSS Based on Host-Guest Chemistry. Chin. Chem. Lett. 2019, 30, 717–720.
- Zhang, X.; Feng, Y.; Huang, D.; Li, Y.; Feng, W. Investigation of Optical Modulated Conductance Effects Based on a Graphene Oxide–azobenzene Hybrid. Carbon 2010, 48, 3236–3241.
- Ran, X.; Li, Y.; Wei, Z.; Huo, X.; He, Y.; Wang, X.; Kuang, Y.; Guo, L. Highly Enhanced Nonlinear Optical Absorption with Ultrafast Charge Transfer of Reduced Graphene Oxide Hybridized by an Azobenzene Derivative. Opt. Express 2021, 29, 5213–5225.
- Zhang, X.; Feng, Y.; Lv, P.; Shen, Y.; Feng, W. Enhanced Reversible Photoswitching of Azobenzene-Functionalized Graphene Oxide Hybrids. Langmuir 2010, 26, 18508–18511.
- Kizhisseri, D.R.; Venugopal, G.; Lalitha Lekshmi, C.; Joseph, K.; Mahesh, S. Photoresponse Modulation of Reduced Graphene Oxide by Surface Modification with Cardanol Derived Azobenzene. New J. Chem. 2018, 42, 18182–18188.
- Zhang, B.; Liu, L.; Wang, L.; Liu, B.; Tian, X.; Chen, Y. Covalent Modification of Graphene Oxide with Poly(N-Vinylcarbazole) Containing Pendant Azobenzene Chromophores for Nonvolatile Ternary Memories. Carbon 2018, 134, 500–506.
- Zhang, X.; Hou, L.; Samorì, P. Coupling Carbon Nanomaterials with Photochromic Molecules for the Generation of Optically Responsive Materials. Nat. Commun. 2016, 7, 11118.
- Pang, W.; Xue, J.; Pang, H. A High Energy Density Azobenzene/Graphene Oxide Hybrid with Weak Nonbonding Interactions for Solar Thermal Storage. Sci. Rep. 2019, 9, 5224.
- Feng, Y.; Liu, H.; Luo, W.; Liu, E.; Zhao, N.; Yoshino, K.; Feng, W. Covalent Functionalization of Graphene by Azobenzene with Molecular Hydrogen Bonds for Long-Term Solar Thermal Storage. Sci. Rep. 2013, 3, 3260.
- Teng, Y.; Li, S.; Xue, C.; Zhang, H.; Zhu, L.; Tang, Y. Synthesis of Polyaniline/Graphene Oxide/Azobenzene Composite and Its Adjustable Photoelectric Properties. Adv. Polym. Technol. 2020, 2020, 8730852.
- Cao, T.; Zhao, F.; Da, Z.; Qiu, F.; Yang, D.; Guan, Y.; Cao, G.; Zhao, Z.; Li, J.; Guo, X. Synthesis of Amino-Functionalized Graphene Oxide/Azobenzene Polyimide and Its Simulation of Optical Switches. Z. Phys. Chem. 2017, 231, 1797–1814.
- Olean-Oliveira, A.; Teixeira, M.F.S. Development of a Nanocomposite Chemiresistor Sensor Based on π-Conjugated Azo Polymer and Graphene Blend for Detection of Dissolved Oxygen. Sens. Actuators B Chem. 2018, 271, 353–357.
- Olean-Oliveira, A.; Olean-Oliveira, T.; Moreno, A.C.R.; Seraphim, P.M.; Teixeira, M.F.S. A Chemiresistor Sensor Based on Azo-Polymer and Graphene for Real-Time Monitoring of Mitochondrial Oxygen Consumption. ACS Sens. 2019, 4, 118–125.
- Deka, M.J.; Sahoo, S.K.; Chowdhury, D. P-Type and N-Type Azobenzene Nanocluster Immobilized Graphene Oxide Nanocomposite. J. Photochem. Photobiol. A Chem. 2019, 372, 131–139.
- Zhao, X.; Feng, Y.; Qin, C.; Yang, W.; Si, Q.; Feng, W. Controlling Heat Release from a Close-Packed Bisazobenzene-Reduced-Graphene-Oxide Assembly Film for High-Energy Solid-State Photothermal Fuels. ChemSusChem 2017, 10, 1395–1404.
- Chen, S.; Wang, W.; Xu, W. A Photoresponsive Azobenzene-Graphene-Gold Nanocomposite Using Cationic Azobenzene-Surfactant as Stabilizers and Its Electrochemical Behavior. Mater. Lett. 2016, 180, 196–199.
- Renuka, K.D.; Lekshmi, C.L.; Joseph, K.; Mahesh, S. Sustainable Bioresource-Derived Components for Molecular Keypad Lock and IMPLICATION Logic Gate Construction. ChemistrySelect 2017, 2, 11615–11619.
- Chen, S.; Bao, L.; Ou, E.; Peng, C.; Wang, W.; Xu, W. A Cationic Azobenzene-Surfactant-Modified Graphene Hybrid: Unique Photoresponse and Electrochemical Behavior. Nanoscale 2015, 7, 19673–19686.




